MRI-Based Phenotyping for Osteosarcopenic Adiposity in Subjects from a Population-Based Cohort
Abstract
STUDY IMPORTANCE QUESTIONS
- Osteosarcopenic adiposity (OSA) is defined as the hazardous triad of osteopenia, sarcopenia, and adiposity causing impaired functionality and metabolic dysregulation.
- Ectopic adipogenesis and fat redistribution into the viscera, bone, and muscle tissue have been described as the key components in the co-development and progression of OSA.
- The clinical significance of OSA syndrome has been increasingly recognized in recent years, presenting with a higher overall health risk compared to the sum of its individual component traits.
- Biomarkers of bone, muscle, and fat observed by magnetic resonance imaging may depict the conditions of OSA and may therefore be feasible for body composition phenomapping and cardio-metabolic risk stratification.
- Subjects with an OSA phenotype were significantly older, showed the highest grades of skeletal muscle fat, and together with the three other adiposity-containing subgroups also showed the highest BMIs.
- The highest prevalence of an impaired glucose tolerance and significantly higher blood pressure, blood dyslipidemia, and hepatic steatosis were found in the OSA subgroup.
- Given the high rate of their comorbidity and high risk of complications, clinical suspicion or diagnosis of either component of the OSA triad should prompt the targeted examination of the other ones.
- Recognizing any of the three traits of the OSA complex (e.g., by MRI) may help to guide further diagnostics and induce early holistic and effective intervention and treatments, which might improve health outcomes.
- MRI-based phenotyping using biomarkers of bone, muscle, and fat may be feasible especially for body composition phenotyping and may therefore allow for targeted risk stratification and further cardio-metabolic risk assessment in suspected OSA syndrome.
Abstract
1. Introduction
2. Methods
2.1. Study Design
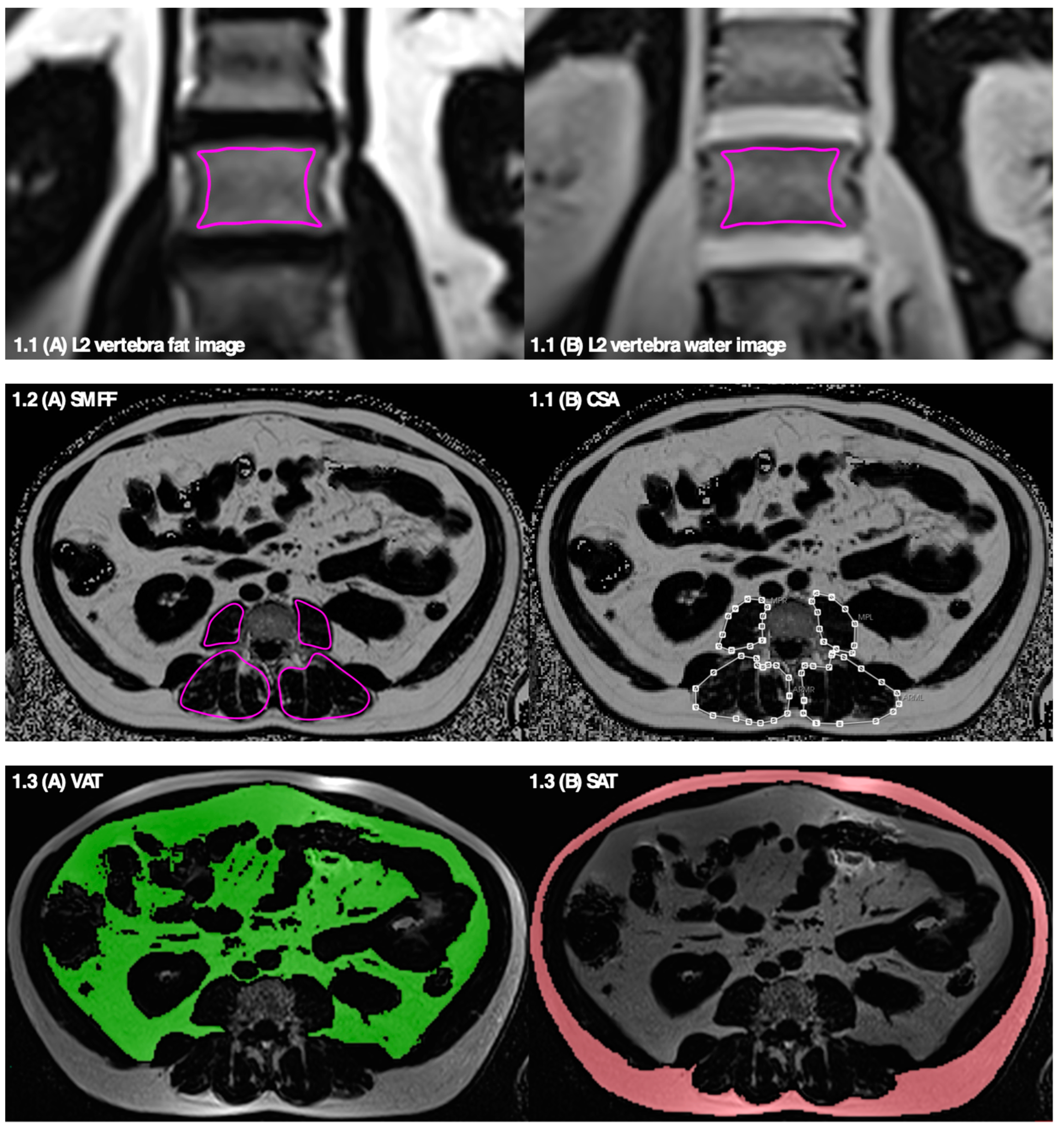
2.2. MR Imaging Protocol and Biomarkers of Osteosarcopenic Adiposity
2.2.1. Bone Marrow Fat Fraction—Osteopenia
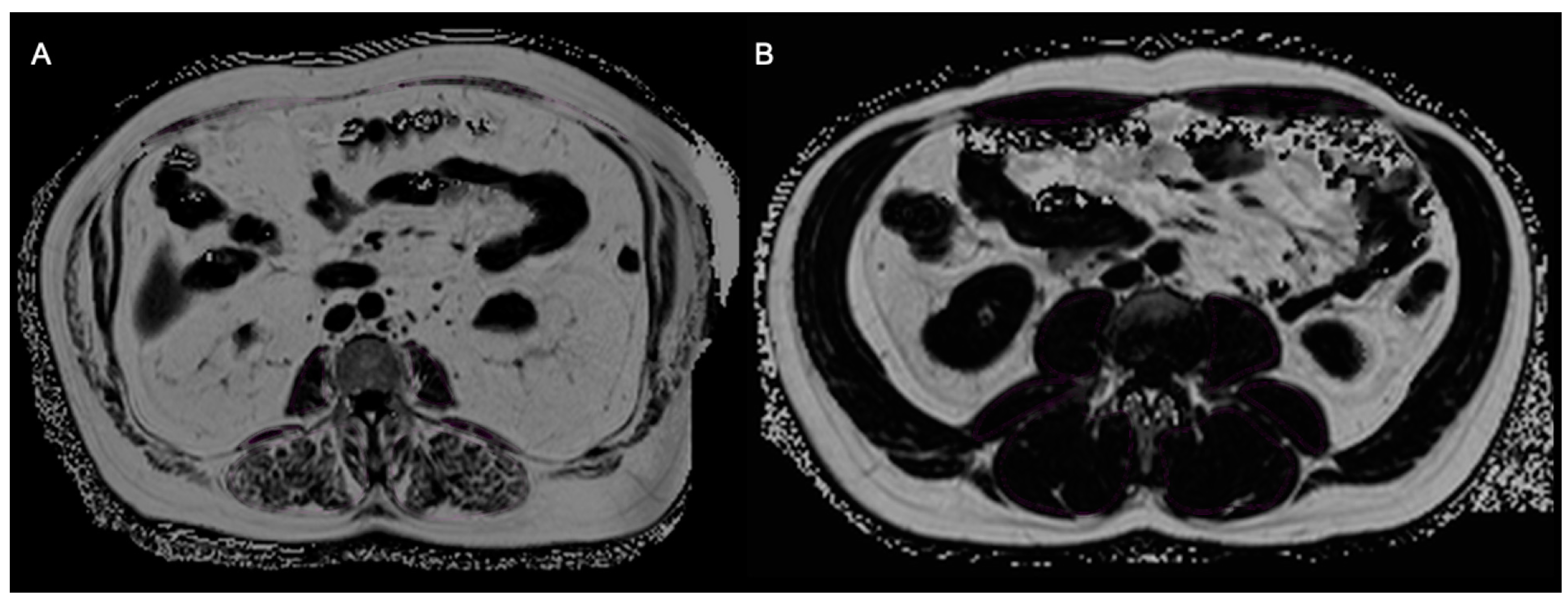
2.2.2. Skeletal Muscle Fat Fraction and Muscle Mass—Sarcopenia
2.2.3. Total, Visceral, and Subcutaneous Adipose Tissue—Adiposity
2.2.4. Phenotypic Subgroups of the OSA Complex
2.3. Health Assessment and Covariates
2.3.1. Anthropometry and BIA
2.3.2. Glucose Tolerance, Lipid Metabolism, Vitamin D
2.3.3. Physical Activity
2.3.4. Other Covariates
2.4. Statistical Analysis
3. Results
3.1. Adipose Phenotype
3.2. Sarcopenic Phenotype
3.3. Osteopenic Phenotype
3.4. Correlations
4. Discussion
4.1. Limitations
4.2. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ilich, J.Z.; Kelly, O.J.; Inglis, J.E.; Panton, L.B.; Duque, G.; Ormsbee, M.J. Interrelationship among muscle, fat, and bone: Connecting the dots on cellular, hormonal, and whole body levels. Ageing Res. Rev. 2014, 15, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Ilich, J.Z.; Kelly, O.J.; Inglis, J.E. Osteosarcopenic Obesity Syndrome: What Is It and How Can It Be Identified and Diagnosed? Curr. Gerontol. Geriatr. Res. 2016, 1, 7325973. [Google Scholar] [CrossRef]
- Kirk, B.; Zanker, J.; Duque, G. Osteosarcopenia: Epidemiology, diagnosis, and treatment—Facts and numbers. J. Cachexia Sarcopenia Muscle 2020, 11, 609–618. [Google Scholar] [CrossRef] [PubMed]
- Ormsbee, M.J.; Prado, C.M.; Ilich, J.Z.; Purcell, S.; Siervo, M.; Folsom, A.; Panton, L. Osteosarcopenic obesity: The role of bone, muscle, and fat on health. J. Cachexia Sarcopenia Muscle 2014, 5, 183–192. [Google Scholar] [CrossRef]
- Joglekar, S.; Asghar, A.; Mott, S.L.; Johnson, B.E.; Button, A.M.; Clark, E.; Mezhir, J.J. Sarcopenia Is an Independent Predictor of Complications Following Pancreatectomy for Adenocarcinoma. J. Surg. Oncol. 2015, 111, 771–775. [Google Scholar] [CrossRef]
- Vetrano, D.L.; Landi, F.; Volpato, S.; Corsonello, A.; Meloni, E.; Bernabei, R.; Onder, G. Association of sarcopenia with short- and long-term mortality in older adults admitted to acute care wards: Results from the CRIME study. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2014, 69, 1154–1161. [Google Scholar] [CrossRef] [PubMed]
- Filippin, L.I.; Teixeira, V.N.D.O.; da Silva, M.P.M.; Miraglia, F.; da Silva, F.S. A predictor of mortality and the need for early diagnosis and intervention. Aging Clin. Exp. Res. 2015, 27, 249–254. [Google Scholar] [CrossRef]
- Perna, S.; Spadaccini, D.; Nichetti, M.; Avanzato, I.; Faliva, M.A.; Rondanelli, M. Osteosarcopenic Visceral Obesity and Osteosarcopenic Subcutaneous Obesity, Two New Phenotypes of Sarcopenia: Prevalence, Metabolic Profile, and Risk Factors. J. Aging Res. 2018, 2018, 6147426. [Google Scholar] [CrossRef]
- Kelly, O.J.; Gilman, J.C.; Boschiero, D.; Ilich, J.Z. Osteosarcopenic obesity: Current knowledge, revised identification criteria and treatment principles. Nutrients 2019, 11, 747. [Google Scholar] [CrossRef]
- Lee, S.Y.; Gallagher, D. Assessment methods in human body composition. Curr. Opin. Clin. Nutr. Metab. Care 2008, 11, 566–572. [Google Scholar] [CrossRef]
- Bertheau, R.C.; Lorbeer, R.; Nattenmüller, J.; Wintermeyer, E.; Machann, J.; Linkohr, B.; Peters, A.; Bamberg, F.; Schlett, C.L. Bone marrow fat fraction assessment in regard to physical activity: KORA FF4–3-T MR imaging in a population-based cohort. Eur. Radiol. 2020, 30, 3417–3428. [Google Scholar] [CrossRef] [PubMed]
- Borga, M.; West, J.; Bell, J.D.; Harvey, N.C.; Romu, T.; Heymsfield, S.B.; Leinhard, O.D. Advanced body composition assessment: From body mass index to body composition profiling. J. Investig. Med. 2018, 66, 887–895. [Google Scholar] [CrossRef] [PubMed]
- Bamberg, F.; Hetterich, H.; Rospleszcz, S.; Lorbeer, R.; Auweter, S.D.; Schlett, C.L.; Schafnitzel, A.; Bayerl, C.; Schindler, A.; Saam, T.; et al. Subclinical Disease Burden as Assessed by Whole-Body MRI in Subjects with Prediabetes, Subjects with Diabetes, and Normal Control Subjects From the General Population: The KORA-MRI Study. Diabetes 2017, 66, 158–169. [Google Scholar] [CrossRef]
- Cordes, C. MR-Based Assessment of Bone Marrow Fat in Osteoporosis, Diabetes, and Obesity. Front. Endocrinol. 2016, 7, 74. [Google Scholar] [CrossRef] [PubMed]
- Rosen, C.J.; Bouxsein, M.L. Mechanisms of Disease: Is osteoporosis the obesity of bone? Nat. Clin. Pract. Rheumatol. 2006, 2, 35–43. [Google Scholar] [CrossRef]
- He, J.; Fang, H.; Li, X. Vertebral bone marrow fat content in normal adults with varying bone densities at 3T magnetic resonance imaging. Acta Radiol. 2019, 60, 509–515. [Google Scholar] [CrossRef]
- Meunier, P.; Aaron, J.; Edouard, C.; VlGNON, G. Osteoporosis and the Replacement of Cell Populations of the Marrow by Adipose Tissue: A quantitative study of 84 iliac bone biopsies. Clin. Orthop. Relat. Res. 1971, 80, 147–154. [Google Scholar] [CrossRef]
- Li, C.-w.; Yu, K.; Shyh-Chang, N.; Jiang, Z.; Liu, T.; Ma, S.; Luo, L.; Guang, L.; Liang, K.; Ma, W.; et al. Pathogenesis of sarcopenia and the relationship with fat mass: Descriptive review. J. Cachexia. Sarcopenia Muscle 2022, 13, 781–794. [Google Scholar] [CrossRef]
- Kiefer, L.S.; Fabian, J.; Rospleszcz, S.; Lorbeer, R.; Machann, J.; Storz, C.; Kraus, M.S.; Schlett, C.L.; Roemer, F.; Wintermeyer, E.; et al. Assessment of the degree of abdominal myosteatosis by magnetic resonance imaging in subjects with diabetes, prediabetes and healthy controls from the general population. Eur. J. Radiol. 2018, 105, 261–268. [Google Scholar] [CrossRef]
- Kiefer, L.S.; Fabian, J.; Lorbeer, R.; Machann, J.; Storz, C.; Kraus, M.S.; Wintermeyer, E.; Schlett, C.; Roemer, F.; Nikolaou, K.; et al. Inter- and intra-observer variability of an anatomical landmark-based, manual segmentation method by MRI for the assessment of skeletal muscle fat content and area in subjects from the general population. Br. J. Radiol. 2018, 91, 20180019. [Google Scholar] [CrossRef]
- Storz, C.; Heber, S.D.; Rospleszcz, S.; Machann, J.; Sellner, S.; Nikolaou, K.; Lorbeer, R.; Gatidis, S.; Elser, S.; Petters, A.; et al. The role of visceral and subcutaneous adipose tissue measurements and their ratio by magnetic resonance imaging in subjects with prediabetes, diabetes and healthy controls from a general population without cardiovascular disease. Br. J. Radiol. 2018, 91, 20170808. [Google Scholar] [CrossRef] [PubMed]
- Würslin, C.; Machann, J.; Rempp, H.; Claussen, C.; Yang, B.; Schick, F. Topography Mapping of Whole Body Adipose Tissue Using A Fully Automated and Standardized Procedure. J. Magn. Reson. Imaging 2010, 31, 430–439. [Google Scholar] [CrossRef] [PubMed]
- WHO. Definition and Diagnosis of Diabetes Mellitus and Intermediate Hyperglycemia; WHO: Geneva, Switzerland, 2006; ISBN 9241594934.
- Maurer, E.; Klinger, C.; Lorbeer, R.; Rathmann, W.; Peters, A.; Schlett, C.L.; Nikolaou, K.; Bamberg, F.; Notohamiprodjo, M.; Walter, S.S. Long-term effect of physical inactivity on thoracic and lumbar disc degeneration—An MRI-based analysis of 385 individuals from the general population. Spine J. 2020, 20, 1386–1396. [Google Scholar] [CrossRef] [PubMed]
- Meisinger, C.; Heier, M.; Völzke, H.; Löwel, H.; Mitusch, R.; Hense, H.-W.; Lüdemann, J. Regional disparities of hypertension prevalence and management within Germany. J. Hypertens. 2006, 24, 293–299. [Google Scholar] [CrossRef]
- Walter, S.S.; Wintermeyer, E.; Klinger, C.; Lorbeer, R.; Rathmann, W.; Peters, A.; Schlett, C.L.; Thorand, B.; Gatidis, S.; Nikolaou, K.; et al. Association between metabolic syndrome and hip osteoarthritis in middle-aged men and women from the general population. PLoS ONE 2020, 15, e0230185. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). Clinical Practice Guidelines EASL–EASD–EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease q. J. Hepatol. 2016, 64, 1388–1402. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Obesity and Overweight—Key Facts. 2021. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 11 November 2024).
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.-P.; Rolland, Y.; Schneider, S.M.; et al. Sarcopenia—European consensus on definition and diagnosis, Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef]
- Abidin, N.Z.; Mitra, S.R. Determination of Cutoff Values for the Screening of Osteosarcopenia in Obese Postmenopausal Women. Curr. Gerontol. Geriatr. Res. 2021, 2021, 6634474. [Google Scholar] [CrossRef]
- Ponti, F.; Santoro, A.; Mercatelli, D.; Gasperini, C.; Conte, M.; Martucci, M.; Sangiorgi, L.; Franceschi, C.; Bazzocchi, A. Aging and Imaging Assessment of Body Composition: From Fat to Facts. Front. Endocrinol. 2020, 10, 861. [Google Scholar] [CrossRef]
- Smith, S.R.; Lovejoy, J.C.; Greenway, F.; Ryan, D.; Dejonge, L.; de la Bretonne, J.; Volafova, J.; Bray, G.A. Contributions of total body fat, abdominal subcutaneous adipose tissue compartments, and visceral adipose tissue to the metabolic complications of obesity. Metabolism 2001, 50, 425–435. [Google Scholar] [CrossRef]
- Kiefer, L.S.; Fabian, J.; Rospleszcz, S.; Lorbeer, R.; Machann, J.; Kraus, M.S.; Fischer, M.; Roemer, F.; Rathmann, W.; Meisinger, C.; et al. Population-based cohort imaging: Skeletal muscle mass by magnetic resonance imaging in correlation to bioelectrical-impedance analysis. J. Cachexia Sarcopenia Muscle 2011, 13, 976–986. [Google Scholar] [CrossRef] [PubMed]
- Hamrick, M.W.; McGee-Lawrence, M.E.; Frechette, D.M. Fatty Infiltration of Skeletal Muscle: Mechanisms and Comparisons with Bone Marrow Adiposity. Front. Endocrinol. 2016, 7, 69. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.H.; Hwang, H.J.; Shin, H.Y.; Han, C.H. Association between Sarcopenic Obesity and Bone Mineral Density in Middle-Aged and Elderly Korean. Ann. Nutr. Metab. 2016, 8, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Ilich, J.Z.; IInglis, J.E.; Kelly, O.J.; McGee, D.L. Osteosarcopenic obesity is associated with reduced handgrip strength, walking abilities, and balance in postmenopausal women. Osteoporos. Int. 2015, 26, 2587–2595. [Google Scholar] [CrossRef] [PubMed]
- Hita-Contreras, F.; Martínez-Amat, A.; Cruz-Díaz, D.; Pérez-López, F.R. Osteosarcopenic obesity and fall prevention strategies. Maturitas 2015, 80, 126–132. [Google Scholar] [CrossRef]
- Chen, X.; Kong, C.; Yu, H.; Gong, J.; Lan, L.; Zhou, L.; Gong, J.; Liu, P.; Xu, L.; Deng, Q. Association between osteosarcopenic obesity and hypertension among four minority populations in China: A cross-sectional study. BMJ Open 2019, 9, e026818. [Google Scholar] [CrossRef]
- Mo, D.; Hsieh, P.; Yu, H.; Zhou, L.; Gong, J.; Xu, L.; Liu, P.; Chen, G.; Chen, Z.; Deng, Q. Osteosarcopenic obesity and its relationship with dyslipidemia in women from different ethnic groups of China. Arch. Osteoporos. 2018, 13, 65. [Google Scholar] [CrossRef]




| BMFF | SMFF | TAT | |
| sex-specific median: women men | 55.8% 55.4% | 13.2% 10.4% | 10.8 L 12.9 L |
| “Normal” phenotype | ≤sex-specific median | ≤sex-specific median | ≤sex-specific median |
| isolated osteopenia | >sex-specific median | ≤sex-specific median | ≤sex-specific median |
| isolated sarcopenia | ≤sex-specific median | >sex-specific median | ≤sex-specific median |
| osteopenic sarcopenia | >sex-specific median | >sex-specific median | ≤sex-specific median |
| isolated adiposity | ≤sex-specific median | ≤sex-specific median | >sex-specific median |
| osteopenic adiposity | >sex-specific median | ≤sex-specific median | >sex-specific median |
| sarcopenic adiposity | ≤sex-specific median | >sex-specific median | >sex-specific median |
| osteosarcopenic adiposity | >sex-specific median | >sex-specific median | >sex-specific median |
| Whole Sample | “Normal” Phenotype | Isolated Osteopenia | Isolated Sarcopenia | Osteopenic Sarcopenia | Isolated Adiposity | Osteopenic Adiposity | Sarcopenic Adiposity | Osteosarcopenic Adiposity | ||
|---|---|---|---|---|---|---|---|---|---|---|
| n = 363 | n = 88 (24.2%) | n = 40 (11.0%) | n = 22 (6.1%) | n = 34 (9.4%) | n = 28 (7.7%) | n = 28 (7.7%) | n = 42 (11.6%) | n = 81 (22.3%) | p-Value | |
| Age in Years | 56.0 ± 9.1 | 49.0 ± 7.3 | 55.3 ± 6.8 | 60.0 ± 8.9 | 62.2 ± 7.0 | 49.5 ± 6.0 | 55.6 ± 9.0 | 56.7 ± 7.7 | 62.4 ± 6.9 | <0.005 |
| Male Gender | 209 (57.6%) | 45 (51.1%) | 23 (57.5%) | 13 (59.1%) | 23 (67.6%) | 18 (64.3%) | 20 (71.4%) | 28 (66.7%) | 39 (48.1%) | 0.19 |
| Female Gender | 154 (42.4%) | 43 (48.9%) | 17 (42.5%) | 9 (40.9%) | 11 (32.4%) | 10 (35.7%) | 8 (28.6%) | 14 (33.3%) | 42 (51.9%) | |
| Body Composition by Anthropometry | ||||||||||
| Body Weight in kg | 82.4 ± 15.8 | 74.7 ± 11.8 | 71.6 ± 12.6 | 76.0 ± 13.8 | 72.2 ± 10.7 | 96.7 ± 14.4 | 92.5 ± 12.2 | 97.2 ± 15.3 | 86.0 ± 12.1 | <0.005 |
| BMI in kg/m2 | 27.9 ± 4.6 | 24.8 ± 2.7 | 24.1 ± 2.2 | 25.8 ± 3.3 | 24.8 ± 2.5 | 31.6 ± 3.4 | 30.4 ± 2.9 | 32.4 ± 4.9 | 30.4 ± 3.7 | <0.005 |
| WHO Overweight: BMI 25–30 kg/m2 | 156 (43.0%) | 40 (45.5%) | 15 (37.5%) | 13 (59.0%) | 17 (50.0%) | 9 (32.1%) | 12 (42.9%) | 15 (35.7%) | 35 (43.2%) | <0.005 |
| WHO Obesity: BMI ≥ 30 kg/m2 | 106 (29.2%) | 2 (2.3%) | 0 (0%) | 2 (9.1%) | 0 (0%) | 19 (67.9%) | 16 (57.1%) | 26 (61.9%) | 41 (50.6%) | <0.005 |
| Waist Circumference in cm | 98.0 ± 13.7 | 87.3 ± 9.1 | 87.4 ± 9.8 | 92.6 ± 10.8 | 91.2 ± 9.4 | 109.1 ± 11.2 | 107.5 ± 7.2 | 111.1 ± 10.2 | 105.1 ± 10.2 | <0.005 |
| Body Composition by BIA | ||||||||||
| Total Body Fat in % | 32.0 ± 6.6 | 28.4 ± 5.5 | 27.9 ± 5.1 | 29.7 ± 4.0 | 28.1 ± 5.4 | 35.0 ± 5.5 | 33.7 ± 5.3 | 36.4 ± 5.9 | 36.4 ± 5.8 | <0.005 |
| Lean Body Mass Index in kg/m2 | 18.8 ± 2.5 | 17.7 ± 2.1 | 17.4 ± 2.1 | 18.1 ± 2.6 | 17.9 ± 2.2 | 20.5 ± 2.1 | 20.1 ± 1.8 | 20.4 ± 2.5 | 19.2 ± 2.4 | <0.005 |
| Appendicular Muscle Mass Index in kg/m2 | 7.8 ± 1.2 | 7.4 ± 1.1 | 7.2 ± 1.1 | 7.5 ± 1.3 | 7.4 ± 1.1 | 8.7 ± 1.1 | 8.5 ± 0.9 | 8.6 ± 1.2 | 8.0 ± 1.2 | <0.005 |
| EWGSOP Moderate Sarcopenia: SMI 8.51–10.75 kg/m2 (men) or 5.76–6.75 kg/m2 (women) | 158 (43.5%) | 36 (40.9%) | 20 (50.0%) | 10 (45.5%) | 10 (29.4%) | 26 (57.1%) | 15 (53.6%) | 19 (45.2%) | 32 (39.5%) | 0.373 |
| EWGSOP Severe Sarcopenia: SMI ≤8.50 kg/m2 (men) or ≤5.75 kg/m2 (women) | 119 (32.8%) | 34 (38.6%) | 20 (50%) | 10 (45.5%) | 22 (67.4%) | 2 (7.1%) | 6 (21.4%) | 10 (23.6%) | 15 (18.5%) | <0.005 |
| Whole Sample | “Normal” Phenotype | Isolated Osteopenia | Isolated Sarcopenia | Osteopenic Sarcopenia | Isolated Adiposity | Osteopenic Adiposity | Sarcopenic Adiposity | Osteosarcopenic Adiposity | ||
|---|---|---|---|---|---|---|---|---|---|---|
| n = 363 | n = 88 (24.2%) | n = 40 (11.0%) | n = 22 (6.1%) | n = 34 (9.4%) | n = 28 (7.7%) | n = 28 (7.7%) | n = 42 (11.6%) | n = 81 (22.3%) | p-Value | |
| Body Composition by MRI 1. Bone Marrow | ||||||||||
| Bone Marrow Fat Fraction in % | 54.3 ± 10.2 | 43.2 ± 8.5 | 60.9 ± 4.2 | 49.5 ± 4.0 | 63.7 ± 5.6 | 49.5 ± 4.2 | 61.4 ± 4.5 | 48.8 ± 6.3 | 62.6 ± 4.9 | <0.005 |
| Body Composition by MRI 2. Adipose Tissue | ||||||||||
| TAT in Liters | 12.5 ± 5.3 | 7.8 ± 2.1 | 8.2 ± 2.1 | 8.7 ± 2.2 | 9.4 ± 1.8 | 17.0 ± 3.8 | 16.3 ± 2.9 | 18.2 ± 5.0 | 16.2 ± 3.2 | <0.005 |
| VAT in Liters | 4.5 ± 2.7 | 2.2 ± 1.4 | 2.9 ± 1.6 | 3.0 ± 1.6 | 3.6 ± 1.5 | 5.9 ± 2.5 | 6.6 ± 1.7 | 6.6 ± 2.7 | 6.2 ± 2.4 | <0.005 |
| SAT in Liters | 8.0 ± 3.5 | 5.7 ± 1.6 | 5.4 ± 1.2 | 5.7 ± 1.2 | 5.8 ± 1.3 | 11.1 ± 3.2 | 9.7 ± 2.6 | 11.6 ± 3.8 | 10.0 ± 2.9 | <0.005 |
| Hepatic Fat Fraction in % | 8.7 ± 7.9 | 4.3 ± 5.6 | 4.8 ± 3.1 | 5.1 ± 4.6 | 5.5 ± 4.3 | 14.0 ± 8.3 | 13.4 ± 9.1 | 10.5 ± 7.4 | 13.4 ± 8.8 | <0.005 |
| Hepatic Fat Fraction > 5.6%, Total and in % of Corresponding SubGroup | 183 (50.4%) | 17 (19.3%) | 12 (30.0%) | 7 (31.8%) | 12 (35.3%) | 22 (78.6%) | 24 (85.7%) | 30 (71.4%) | 59 (74.7%) | <0.005 |
| Body Composition by MRI 3. Muscle | ||||||||||
| Fat Fraction Psoas Muscle in % | 6.6 [5.1, 8.9] | 5.0 [4.2, 5.7] | 5.3 [4.5, 6.6] | 7.7 [6.1, 8.6] | 8.4 [7.3, 10.0] | 5.7 [4.9, 6.3] | 6.0 [4.9, 7.2] | 9.0 [7.5, 10.8] | 9.3 [7.4, 11.2] | <0.005 |
| Fat Fraction Autochthonous Back Muscles in % | 16.2 [11.3, 21.5] | 10.5 [8.5, 13.1] | 12.1 [9.2, 14.8] | 19.8 [17.1, 23.1] | 21.5 [16.5, 24.8] | 11.8 [9.7, 14.1] | 11.1 [9.6, 13.3] | 21.0 [18.4, 26.6] | 22.4 [19.2, 28.3] | <0.005 |
| Cross-sectional Area Psoas Muscle in mm2 | 1667.4 [1293.4, 2142.8] | 1720.1 [1391.3, 2281.9] | 1572.9 [1268.3, 2251.0] | 1714.5 [1412.3, 2350.4] | 1697.2 [1209.1, 2061.8] | 1813.3 [1543.1, 2136.6] | 1623.5 [1251.9, 2081.1] | 1619.9 [1260.7, 1987.2] | 1562.8 [1263.6, 1951.4] | 0.13 |
| Cross-sectional Area Autochthonous Back Muscles in mm2 | 4914.1 [4153.6, 5688.3] | 4812.6 [4155.1, 5609.2] | 4609.2 [3948.3, 5333.8] | 5249.9 [4184.5, 5625.4] | 4489.4 [3934.7, 5105.4] | 5611.7 [4508.2, 6337.5] | 5534.0 [5044.2, 6160.1] | 5404.9 [4645.3, 6474.3] | 4539.5 [3972.8, 5387.5] | <0.005 |
| Whole Sample | “Normal” Phenotype | Isolated Osteopenia | Isolated Sarcopenia | Osteopenic Sarcopenia | Isolated Adiposity | Osteopenic Adiposity | Sarcopenic Adiposity | Osteosarcopenic Adiposity | ||
|---|---|---|---|---|---|---|---|---|---|---|
| n = 363 | n = 88 (24.2%) | n = 40 (11.0%) | n = 22 (6.1%) | n = 34 (9.4%) | n = 28 (7.7%) | n = 28 (7.7%) | n = 42 (11.6%) | n = 81 (22.3%) | p-Value | |
| Glycemic Status | ||||||||||
| HbA1c in % | 5.6 ± 0.7 | 5.3 ± 0.4 | 5.5 ± 0.4 | 5.4 ± 0.3 | 5.6 ± 0.6 | 5.6 ± 0.9 | 5.8 ± 1.0 | 5.7 ± 1.3 | 5.8 ± 0.7 | <0.005 |
| Glycemic status | <0.005 | |||||||||
| - Normoglycemia | 226 (62.3%) | 74 (84.1%) | 33 (82.5%) | 17 (77.3%) | 27 (79.4%) | 13 (46.4%) | 14 (50.0%) | 18 (42.9%) | 30 (37.0%) | |
| - Prediabetes | 87 (24.0%) | 10 (11.4%) | 6 (15.0%) | 4 (18.2%) | 4 (11.8%) | 13 (46.4%) | 6 (21.4%) | 17 (40.5%) | 27 (33.3%) | |
| - Diabetes | 50 (13.8%) | 4 (4.5%) | 1 (2.5%) | 1 (4.5%) | 3 (8.8%) | 2 (7.1%) | 8 (28.6%) | 7 (16.7%) | 24 (29.6%) | |
| Blood Pressure (BP) | ||||||||||
| Systolic BP in mmHg | 120.6 ± 16.2 | 113.5 ± 14.5 | 114.7 ± 12.4 | 118.6 ± 14.5 | 121.8 ± 18.4 | 124.0 ± 15.6 | 125.9 ± 13.9 | 125.5 ± 19.1 | 125.8 ± 15.1 | <0.005 |
| Diastolic BP in mmHg | 75.4 ± 9.9 | 72.2 ± 9.6 | 72.8 ± 7.4 | 72.5 ± 9.4 | 73.2 ± 8.3 | 80.4 ± 8.9 | 80.9 ± 9.1 | 77.7 ± 9.6 | 77.1 ± 10.8 | <0.005 |
| Antihypertensive Medication | 86 (23.7%) | 9 (10.2%) | 6 (15.0%) | 4 (18.2%) | 8 (23.5%) | 5 (17.9%) | 11 (39.3%) | 14 (33.3%) | 29 (35.8%) | <0.005 |
| Blood Lipids and Vitamin D | ||||||||||
| HDL in mg/dL | 61.9 ± 17.5 | 64.3 ± 19.6 | 67.5 ± 16.1 | 68.4 ± 17.1 | 67.1 ± 16.0 | 50.5 ± 13.3 | 49.2 ± 11.0 | 60.0 ± 15.6 | 62.0 ± 17.1 | <0.005 |
| LDL in mg/dL | 139.3 ± 32.4 | 130.4 ± 29.4 | 140.9 ± 36.1 | 135.3 ± 29.6 | 146.8 ± 30.2 | 145.8 ± 29.1 | 148.4 ± 34.4 | 134.8 ± 32.4 | 143.1 ± 34.1 | 0.05 |
| Triglycerides in mg/dL | 131.4 ± 86.1 | 100.9 ± 61.7 | 101.5 ± 52.5 | 89.3 ± 30.3 | 118.5 ± 96.4 | 178.0 ± 76.7 | 185.2 ± 98.3 | 142.7 ± 82.2 | 155.6 ± 103.9 | <0.005 |
| Lipid-Lowering Medication | 37 (10.2%) | 3 (3.4%) | 1 (2.5%) | 0 (0.0%) | 6 (17.6%) | 1 (3.6%) | 5 (17.9%) | 6 (14.3%) | 15 (18.5%) | <0.005 |
| Vitamin D (Calciferol) in ng/mL | 23.5 ± 11.3 | 26.4 ± 13.4 | 26.9 ± 9.7 | 22.2 ± 8.5 | 26.4 ± 10.4 | 20.2 ± 11.1 | 20.5 ± 11.4 | 20.5 ± 8.6 | 21.5 ± 10.5 | <0.005 |
| Physical Activity | ||||||||||
| Physical Activity | <0.005 | |||||||||
| Regularly >2 h/week | 106 (29.2%) | 41 (46.6%) | 11 (27.5%) | 4 (18.2%) | 14 (41.2%) | 7 (25.0%) | 3 (10.7%) | 10 (23.8%) | 16 (19.8%) | |
| Regularly 1–2 h/week | 112 (30.9%) | 29 (33.0%) | 13 (32.5%) | 7 (31.8%) | 9 (26.5%) | 10 (35.7%) | 11 (39.3%) | 12 (28.6%) | 21 (25.9%) | |
| Sporadically <1 h/week | 55 (15.2%) | 10 (11.4%) | 5 (12.5%) | 2 (9.1%) | 4 (11.8%) | 7 (25.0%) | 4 (14.3%) | 7 (16.7%) | 16 (19.8%) | |
| Inactive | 90 (24.8%) | 8 (9.1%) | 11 (27.5%) | 9 (40.9%) | 7 (20.6%) | 4 (14.3%) | 10 (35.7%) | 13 (31.0%) | 28 (34.6%) | |
| Musculo-skeletal measurements | ||||||||||
| Pfirrmann Grade > 2, Disk Protrusion, or Hernia (n = 351) | 270 (74.4%) | 56 (63.6%) | 34 (85.0%) | 18 (81.8%) | 29 (85.3%) | 20 (71.4%) | 18 (64.3%) | 28 (66.7%) | 67 (82.7%) | <0.005 |
| Hip Cartilage Degeneration | 90 (24.8%) | 20 (22.7%) | 2 (5.0%) | 6 (27.3%) | 10 (29.4%) | 5 (17.9%) | 8 (28.6%) | 14 (33.3%) | 25 (30.9%) | 0.07 |
| Somatic Pain Symptoms | 140 (38.6%) | 30 (34.1%) | 9 (22.5%) | 9 (40.9%) | 11 (32.4%) | 13 (46.4%) | 9 (32.1%) | 18 (42.9%) | 41 (50.6%) | 0.09 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maurer, E.; Rospleszcz, S.; Rathmann, W.; Thorand, B.; Peters, A.; Schlett, C.L.; Bamberg, F.; Kiefer, L.S. MRI-Based Phenotyping for Osteosarcopenic Adiposity in Subjects from a Population-Based Cohort. Geriatrics 2024, 9, 150. https://doi.org/10.3390/geriatrics9060150
Maurer E, Rospleszcz S, Rathmann W, Thorand B, Peters A, Schlett CL, Bamberg F, Kiefer LS. MRI-Based Phenotyping for Osteosarcopenic Adiposity in Subjects from a Population-Based Cohort. Geriatrics. 2024; 9(6):150. https://doi.org/10.3390/geriatrics9060150
Chicago/Turabian StyleMaurer, Elke, Susanne Rospleszcz, Wolfgang Rathmann, Barbara Thorand, Annette Peters, Christopher L. Schlett, Fabian Bamberg, and Lena Sophie Kiefer. 2024. "MRI-Based Phenotyping for Osteosarcopenic Adiposity in Subjects from a Population-Based Cohort" Geriatrics 9, no. 6: 150. https://doi.org/10.3390/geriatrics9060150
APA StyleMaurer, E., Rospleszcz, S., Rathmann, W., Thorand, B., Peters, A., Schlett, C. L., Bamberg, F., & Kiefer, L. S. (2024). MRI-Based Phenotyping for Osteosarcopenic Adiposity in Subjects from a Population-Based Cohort. Geriatrics, 9(6), 150. https://doi.org/10.3390/geriatrics9060150







