Abstract
Background: Physical activity can have positive effects on motor and non-motor symptoms in Parkinson’s disease, but its benefits in terms of quality of life and function are uncertain and vary based on the specific forms of activities and interventions. Objective: We sought to assess the current evidence on the positive effects of physical activity in people with Parkinson’s disease and more specifically in relation to its potential benefits for quality of life. Methods: This systematic review was conducted between January and April 2024 via the PubMed, Medline, and Scopus databases. Predetermined search criteria were used that included the following terms: “Parkinson’s disease”, “quality of life” and “physical activity”. Results: A total of 1669 articles were identified. After utilizing predetermined criteria, a total of fifteen articles met the selection criteria. Statistically significant improvements in quality of life were found in seven studies. Seven studies demonstrated a significant improvement in non-motor symptoms, while nine studies showed an improvement in motor symptoms. Conclusions: Despite heterogeneity in the study designs, interventions and clinical assessments, the articles identified in this review yielded mostly positive results in relation to physical activities. The findings reflect an improvement in motor and non-motor symptoms may translate to a better quality of life in people with Parkinson’s disease.
1. Introduction
Parkinson’s disease (PD) is a common neurodegenerative disorder typically characterized by motor symptoms and now increasingly recognized according to a myriad of complex non-motor symptoms (NMS). The latter are often subtle at onset and can occur many years prior to diagnosis [1,2,3,4]. With a wide array of symptomatology and the inevitable progression of the disease due to the loss of the dopaminergic neurons residing in the substantia nigra and non-dopaminergic dysfunction, quality of life (QoL) is predictably impacted in PD [5,6,7]. Early identification and treatment of symptoms can promote an improvement in the QoL of these individuals [8,9] and whilst management is often individualized as part of best practice, it can be challenging and ideally should encompass both pharmacological and non-pharmacological modalities [10,11].
When examining any purported treatment or procedure and its subsequent benefits or risks in terms of the outcomes for patients, QoL is an established construct used and universally understood to reflect the overall subjective well-being of an individual. It is not only pathophysiology and the manifestation of disease but also an individual’s function, satisfaction and contentment experienced in life which form the construct of QoL [12,13]. QoL outcomes are useful for attaining evidence of meaningful benefit, enhancing the significance of variables of interest and allowing for more holistic decision-making [12]. The link between QoL and physical activity (PA) has increasingly been consolidated in the literature [13], including in PD, whereby many studies have assessed various non-pharmacological interventions. Examples include exercise programs [14], Tai Chi Quan [15] and aquatic physiotherapy [16], which demonstrated an improvement in QoL and well-being in individuals with PD. PD-specific instruments have also been developed to assess QoL in this patient group [17,18,19,20,21].
Growing evidence indicates PA can offer tangible improvements in QoL measures for people with PD. The multisystem effects of PD require trials that examine PA and any reported benefits to account for its potential mechanisms, ranging from the simplistic approach of feeling more connected to others to its impact on neuroplasticity [22].
It is thought that exercise may complement standard pharmacological approaches by enhancing neuroplasticity in PD, such as regeneration and the survival of pars compacta neurons [23,24]. It is postulated that many neurotrophic factors are influenced by PA. For example, brain-derived neurotrophic factor (BDNF) is stimulated during exercise to mediate neuroprotective effects and is thought to improve cognition and mood [25,26,27]. Evidence suggests exercise can positively affect neuroplasticity via various mechanisms that include the up-titration of binding in the dopaminergic pathways [28,29] and inhibition of Lewy body formation in rat models [29]. Reducing neurodegeneration via the regulation of autophagy and apoptosis have also been proposed as mechanisms of the benefits of PA [25].
With feasible improvements in both, NMS (in terms of anxiety, cognitive functions and depression) and activities of daily living, exercise has the potential to significantly benefit the overall QoL in people with PD [30]. In this review, our defined objective was to assess the current evidence on the positive effects of physical activity in people with Parkinson’s disease and, more specifically, in relation to its potential benefit in terms of quality of life.
2. Materials and Methods
2.1. Literature Search and Selection Criteria
This systematic review was conducted between January and April 2024. The literature search used the guiding question of “Does physical activity positively affect quality of life in people with Parkinson’s disease?”. Based on a PICO approach, the population of interest comprised people with Parkinson’s disease with an interest in the positive effects of physical activity in the context of quality of life.
The terms in the present review, “Parkinson’s disease”, “quality of life” and “physical activity”, were used as search terms in three databases, PubMed, Medical Literature Analysis and Retrieval System Online (Medline) and Scopus, and were cross-checked with the use of Boolean AND. All terms are included in the Medical Subject Headings (MeSH) and Health Sciences Descriptors (DeCS).
The inclusion criteria were as follows: (i) articles published between 1991 and 2024 written in English, Spanish or Portuguese; (ii) randomized interventional studies (level of evidence II) [31], which was specifically chosen to ensure the reliability and validity of the outcomes by minimizing bias; (iii) studies with 10 or more participants diagnosed with idiopathic PD in the intervention group, as well as appropriate evaluation of the effects of physical activity and quality of life. The exclusion criteria comprised the following: (i) studies in animals, letters to the editor or systematic or integrative reviews and (ii) repeated articles in different databases. The different phases of the systematic review are summarized in Figure 1.
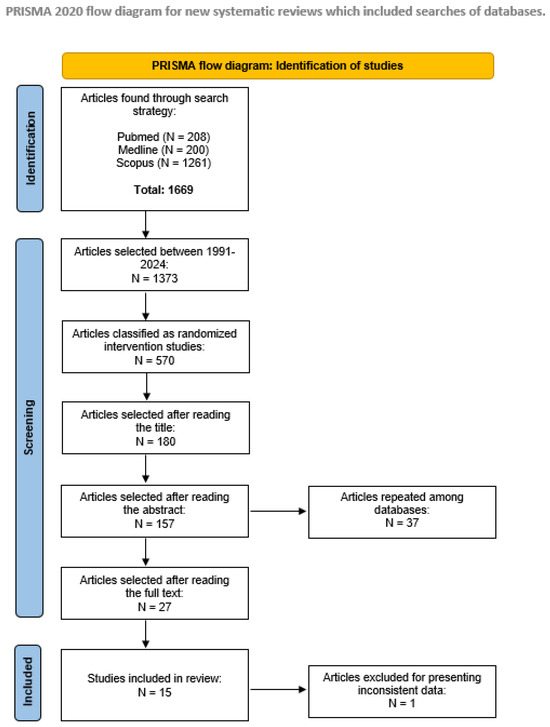
Figure 1.
PRISMA 2020 systematic review flowchart of the selection of studies.
Articles were blindly and independently selected by two reviewers, after which the abstracts were read. After that, two more reviewers were added, and the JADAD scale was applied. The JADAD consists of 5 questions that assess the following aspects of clinical trials: randomization, blinding and description of losses to follow-up [32]. These measures ensured the reliability and validity of the studies in question.
The protocol for this systematic review was registered with PROSPERO on 25 October 2021.
2.2. Outcomes of Interest
The primary outcomes related to QoL were assessed using the following instruments: Parkinson’s disease questionnaire-8 (PDQ-8), Parkinson’s disease questionnaire-39 (PDQ-39), Parkinson’s disease Quality of Life Questionnaire (PDQL), the 33-item Parkinson’s disease quality of life questionnaire (PDQUALIF), EuroQoL five-dimension (EQ-5D) and the Short Form Health Survey (SF-36).
As QoL has a global scope, its associated effects on motor symptoms and NMS were considered as secondary outcomes. A diverse array of instruments for assessing the latter were used based on the respective study objectives and variables of interest. Therefore, the following instruments were utilized:
- Assessment of motor symptoms: Two-Minute Walk Test (2MWT), 6-Minute Walk Test (6MWT), Berg Balance Scale (BBS); Continuous-Scale Physical Functional Performance Test (CS-PFP), Falls Efficacy Scale International (FES-I), Freezing of Gait Questionnaire (FOG, Functional Reach Test (FRT), Physical Activity Questionnaire (IPAQ), Mini-Balance Evaluation Systems Test (MBEST), Movement Disorder Society Unified Parkinson’s Disease Rating Scale (MDS-UPDRS), Sit-to-Stand Test (STS), Test of Attentional Performance Flexibility (TAPF), Timed Up and Go (TUG).
- Assessment of non-motor symptoms: Fear of Falling Avoidance Behavior Questionnaire (FFABQ), Parkinson’s Disease Non-Motor Symptom Questionnaire (N-MSQ), Parkinson’s Disease Sleep Scale (PDSS), Scales for Outcomes in Parkinson’s Disease (SCOPA)—sleep and gastrointestinal, Parkinson Fatigue Scale (PFS).
- Assessment of affective symptoms: Beck Depression Inventory (BDI), Fatigue Severity Scale (FSS), Geriatric Depression Scale (GDS), Hospital Anxiety and Depression Scale (HADS), State-Trait Anxiety Inventory (STAI).
- Cognitive assessment: Mini Mental State Examination (MMSE), Montreal Cognitive Assessment (MoCA), Test of Attentional Performance (TAP), Trail Making Test (TMT).
2.3. Data Extraction
Data extraction was conducted by one author and confirmed by the co-authors. After applying the inclusion and exclusion criteria, each article was reviewed in detail by two nominated members, with particular interest in the participant demographics, type of PA, frequency, duration and mode of delivery, as well as the effect on QoL.
3. Results
The selection process identified 1669 articles, of which 980 were excluded due to lack of randomization or because they had not been published between 1991 and 2024. A further 502 articles were excluded following title and abstract screening. The remaining 150 articles were reviewed and resulted in 14 articles meeting the inclusion criteria (see Figure 1—flow diagram).
The selected articles were in their entirety randomized interventional studies published between 2012 and 2024. With respect to the location of the respective studies, five studies were from North America [33,34,35,36,37]; two were from South America: Brazil [38,39]; five were from Europe: Hungary [40], Italy [41], the United Kingdom [42], Germany [43]; The Netherlands [44]; and three were from Asia: the Republic of Korea [30] and Hong Kong [45,46]. All of the articles were published in English.
3.1. Participants
The number of participants in each study ranged from 20 to 230, with an overall total of 1200 participants (see Table 1). There was a higher male-to-female ratio in all studies apart from three, which had a higher female-to-male ratio [30,45,46]. The duration of PD since diagnosis ranged from 1 to 15 years in the selected sample, with the exception of two studies that reported this information at baseline but did not specify disease duration [45,46]. Though duration of disease was considered as a variable, the researchers did not present these data in the latter study.

Table 1.
Cohort demographics, including intervention and control groups.
3.2. Medication
Two studies did not mention whether the participants were analyzed in an ON or OFF state [30,40,43]. Three studies did not detail medication use [30,39,43]. The use of dopaminergic medications (levodopa and/or dopamine agonist) was defined as the inclusion criterion for only one study [44].
In contrast, seven studies assessed participants in the ON state [34,35,36,37,38,39,45]. Only two studies assessed participants in both the ON and OFF states [33,44].
The mean levodopa-equivalent daily dose (LEDD) with a standard deviation (range) was presented in only four studies, these being 725.0 ± 234 mg/day (PD Irish dance) and 645.0 ± 216 mg/day (control) [41], 843.4 ± 308.8 mg/day (high-intensity agility program) and 884.8 ± 332.0 mg/day (control group) [40], 419.3 ± 389.2 mg/day (high-intensity multimodal exercise boot camp) and 476.7 ± 300.0 mg/day (control) [33] and 766.4 ± 607.2 (mindfulness meditation) and 518.5 ± 562.3 mg/day (control) [45]. Two studies detailed the various types of levodopa replacement therapy utilized [38,41].
3.3. Intervention and Activity Type
A variety of interventions were used, including a high-intensity multimodal boot camp [33], yoga [34,35,46], aerobic exercise [37,40,44], flexibility and function training [37], stretching and resistance training exercises [39,45,46], mindfulness meditation-based exercise [30,45], high-intensity agility training [40], Brazilian dance [38], deep-water exercise [38], Nordic walking [38,41], Irish dancing [41], physiotherapy exercise [41,43], multimodal Parkinson’s complex treatment [43], gym-based exercises [45], free weight exercise [39] and power weight training [36] (see Table 2). The duration of the intervention ranged from 3 weeks to 16 months. The frequency of the intervention varied from 1 to 5 sessions per week and encompassed a minimum of 30 min and a maximum of 120 min per session. Two studies had a relatively short intervention duration of between 3 and 8 weeks [33,40,46], whilst seven studies had a median duration of 3 to 9 months [34,35,36,41,42,43,44]. One study had a longer duration of 16 months [37].

Table 2.
Interventions and methods of identified studies.
Different professionals were involved in the facilitation of PA in the respective studies, and as such, the nature of the PA varied accordingly. Several professionals were involved in delivering and facilitating PA as an intervention, such as physiotherapists, personal trainers, dance teachers and yoga teachers/instructors.
3.4. Measurement Tools
The most commonly used instrument to assess QoL was the 39-item Parkinson’s Disease Questionnaire (PDQ-39), which was utilized in seven studies [33,35,38,39,40,41,44] (see Table 3).

Table 3.
Effects of physical activities on quality of life and functional well-being.
A variety of measures and instruments were used to evaluate motor symptoms. Seven studies used the Movement Disorder Society—Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) [33,38,40,42,44,45,46], and five studies used the UPDRS [34,36,37,39,41]. Most of the studies utilized part III of the MDS-UPDRS or the UPDRS for motor assessment [33,34,36,37,38,39,41,42,44,45,46]. Only one study used part II (motor aspects of experience of daily living) [40] and IV (motor complications—dyskinesia and fluctuation) of the MDS-UPDRS [44]. One study used the UPDRS total score [37]. Two studies did not use the UPDRS [30,43].
In terms of non-motor symptom assessment, the studies identified utilized various instruments, with only one study using the Non-Motor Symptoms Questionnaire (N-MSQ) [42]. The non-motor symptom assessments are further summarized in Table 4, and one of the fourteen studies did not evaluate any NMS [35]. In addition, part I of the UPDRS was used to assess the non-motor aspects of experiences of daily living in only one study [37].

Table 4.
Effects of physical activities on motor and non-motor symptoms.
3.5. Methodology Quality
Based on the level of evidence and selection criteria, all 15 studies comprised randomized clinical trials (Cochrane Level of Evidence II). Nine studies had a single-blinded component [33,34,37,39,40,41,42,45,46], whilst one study was double-blinded [44]. The other five studies had no blinding [30,35,36,38,43]. In the majority of studies, the control group involved a smaller active component of home-based exercise.
3.6. Positive Effects on Outcomes of Interest
Statistically significant improvements in QoL were found in seven studies using the EQ-5D, PDQL, PDQ-8 and PDQ-39 s [30,35,36,39,40,43,46]. The remainder of the articles either had no statistically significant improvement in QoL or did not specify p-values for analysis. Seven studies demonstrated a statistically significant improvement in NMS [30,33,38,40,43,45,46], whilst nine studies showed an improvement in motor symptoms [30,33,38,39,40,41,44,45,46].
Analysis of the type of intervention performed in the studies showed significant benefits in the QoL scores for yoga practice [35,46] and the Mindfulness Mediation-Based Complex Exercise Program (MMBCEP) [30]. Another study that applied PWT as a different form of exercise also had a positive impact on QoL [36]. The study by Son et al. (2018) included stretching and complex strength exercises [30]. As such, it could also be compared to the PA intervention performed in the previous study [36]. The frequency and duration varied among studies.
Meditation performed as part of the intervention in one study focused on different subjects, such as respiration, loving and imagery training. This study demonstrated effects not only on physical aspects but also psychological/mental aspects with a reduction in negative self-images of oneself [30]. A similar approach of mind-body exercises was also adopted by Kwok et al. [46]. Yoga showed QoL improvements in the mobility domain and the overall PDQ-39 score [35,46]. Some interventions focused on specific aspects of motor function such as muscular endurance [30]; general strength [33]; upper extremity muscular strength [30]; lower extremity muscular strength [35]; balance [30,41,44]; mobility [41,44]; gait [33,34,41,44]; bradykinesia [33,34,41,44]; posture [33,34,41,44] as well as reaching and grasping [41]. Studies that yielded positive effects on QoL and NMS assessed depression, anxiety, cognitive function [30] and sleep disturbance [30]. Studies by Ni et al. also showed positive effects on QoL, although these studies did not evaluate NMS [35,36].
Two studies measured PA with the 31-item Longitudinal Aging Study Amsterdam Physical Activity Questionnaire (LAPAQ) [34,44], whilst one study each used the International Physical Activity Questionnaire (IPAQ) [33] and the Physical Activity Scale for the Elderly [42], respectively.
In terms of activities of daily living and QoL, meaningful results were shown in two studies that utilized differing instruments, namely the Activities of Daily Living scale, the Schwab and England Activities of Daily Living Scale and MDS-UPDRS-ADL part II [40,41]. In addition, falls were evaluated in two studies via questionnaires and considered as complications that can compromise motor function [33,44].
4. Discussion
4.1. Physical Activity in Parkinson’s Disease and Its Impact in Quality of Life
Adherence to PA may be perceived as challenging for individuals affected by neurodegenerative diseases such as PD, whereby the symptom burden increases with disease progression [47]. The barriers to initiating and maintaining regular exercise routines are multifaceted, including (1) body structure and function, of which PD motor and non-motor symptoms are part, (2) activities and participation, (3) personal and (4) environmental. Factors such as advancing age, comorbidities and frailty, alongside varying responses to treatment, can impede adherence to PA regimes. The detriment of reduced PA and a lack of mobility are postulated to accelerate frailty, fall risks, immobility and reduced QoL. When combined, a lack of PA may contribute towards an increased risk of hospitalization and the need for long-term care.
This complex interplay between physical activity and health outcomes necessitates a robust method for evaluating its effect on QoL, whereby the latter is considered a broad concept that encompasses biopsychosocial and spiritual well-being and should not be solely considered an absence of disease [48]. In assessing QoL, homogeneity was observed across studies that utilized the PDQ-39.
Enhancements in QoL across different PA modalities that comprise individual and group forms of intervention, as well as facility- and home-based programs, highlight the potential of tailored physical activities to mitigate the barriers to implementation with the intention of improving overall health outcomes. In this review, we found one study that involved individual exercise programs at designated facilities and home-based regimes had yielded positive results in the outcomes measured, which included improvements in daily living activities and social support [36].
4.2. Motor Symptom Benefit
Physical therapy and specialized exercise programs have shown significant benefits in PD. A sensorimotor agility boot camp, involving activities like Tai Chi, boxing, lunges, kayaking, agility courses and Pilates, notably improved gait measures [33]. Irish dance, although not initially sought after by individuals with PD, presents itself as a strategy that can improve mobility, thereby contributing to an enhanced quality of life [41]. Additionally, it is an activity that can also be enjoyable and performed together with other family members. Conversely, the control group, engaging in a physiotherapy program, reported less benefits.
Resistance exercises and power resistance training (PWT) were particularly effective, showing more significant improvements in mobility [40,42] and muscle strength [30,36], as well as upper and lower limb bradykinesia [36].Furthermore, high-intensity exercise programs in non-demented individuals with mild to moderate stage PD may prove to be beneficial in terms of mobility and balance, thus facilitating the maintenance of independence and functional well-being [40]. Moreover, improvements were also noted in areas related to endurance, coordination, agility, and balance [30,40], which further supports the role of varied and targeted exercise regimens in the management of PD.
Benefits related to dance therapy were seen in gains in the “Timed Up and Go test” and reductions in the Freezing of Gait Questionnaire scores given that rapid movements and step routines that are crucial for minimizing motor symptoms and enhancing balance and flexibility, potentially increasing the independence of individuals [41].
4.3. Non-Motor Symptom Benefits
It has been postulated that individuals in the early stages of PD, particularly those who retain cognitive abilities, may derive more significant benefits from PA. This advantage is likely due to the dependency of such activities on executive functions, which include attention and processing speed [49].
The effectiveness of home exercise programs was thought to be notably influenced by effect modifiers such as depression and cognitive impairment; importantly, age did not contribute significantly to the study findings [50]. A study that compared a high-intensity multimodal exercise boot camp with the usual care found that the former significantly enhanced intrinsic motivation [33]. Additionally, adherence to regular physical activity not only reduced fatigue [47] but also provided broader neurological health benefits, thereby improving overall quality of life [39].
Incorporating mindfulness meditation into complex exercise routines demonstrated substantial benefits. These activities enhance cognitive function and emotional well-being, which is thought to be highly relevant in the context of non-motor symptoms [45]. The effectiveness of combining mindfulness-based stress reduction practices with PA, particularly in managing non-motor symptoms such as depression and anxiety, has also been evaluated positively [30,46]. This combined approach has led to reduced anxiety and improvements in concentration, memory and performance in ADLs.
4.4. Benefits of Integrative and Synergistic Therapy
While traditional PA has long been validated within therapeutic contexts, emerging evidence underscores the efficacy of integrative therapies such as meditation and yoga. Studieshave demonstrated substantial improvements in depressive symptoms, mindfulness and cognitive performance among participants engaged in these practices [45,46]. Additionally, these modalities have been shown to enhance psychospiritual outcomes, which directly mitigate symptoms of depression and anxiety [30,45].
Furthermore, the impact of such therapies extends to motor function improvements. Son et al. 2018 observed significant enhancements in physical performance measures, including the chair stand test, shoulder flexibility and walking tests such as the Six-Minute Walk Test [30]. Meditation has been associated with increased joint flexibility, a decrease in resting tremor and general improvements in motor muscle function [30,34]. These improvements are crucial, as they directly enhance performance in daily activities and the quality of life of both participants and their carers.
A significant decrease in bradykinesia scores and stiffness in both the upper and lower limbs following yoga interventions again suggests that an integrative and synergistic approach to physical activity interventions in PD may help maximize its therapeutic benefits [35].
4.5. Potential Therapeutic Mechanisms
An increase in BDNF following PA has been relatively well investigated by numerous studies that suggest its role in epigenetic processes that contribute towards synaptic neuroplasticity [51,52,53]. Furthermore, exercise and its effects on cardiovascular-related microRNAs are likely to result in broader beneficial neurovascular effects which may contribute towards neuronal health [54,55]. The expression levels of specific microRNAs have also been described in PD-specific studies that demonstrated exercise was associated with an improvement in cognition [56]. The neurophysiological effects of exercise are further elucidated by studies that support changes in and the modulation of neural networks and oscillations, as reflected by functional neuroimaging (such as the upregulation of resting state networks) and electroencephalography, thus highlighting the state of local and inter-regional neural synchrony as crucial to appreciate in relation to an individual’s function in the setting of neurodegenerative diseases such as PD [57,58,59]. In addition, growing interest of the glymphatic system in PD and its beneficial modulatory effects on protein clearance and cerebrovascular indicators further strengthens the far-reaching effects of PA on brain health [60,61,62,63].
4.6. Limitations
The articles identified in this review indicate various forms of PA may be beneficial; however, comparison between the studies is challenging due to the differences in the PA interventions and overall study heterogeneity. Studies of this nature are often subject to a proportion of selection and participation bias, as well as potential Hawthorne effects. Another challenge perceived by the researchers was the performance of PA in “non-exercisers”, which may be subject to the latter factors.
5. Conclusions
Despite differences in the study interventions and constructs, studies to date on the effects of PA on PD suggest tangible benefits in terms of both motor symptoms and NMS. Although a consensus recommendation on the best form of exercise as an intervention in PD is not currently available, it is likely that PA will be beneficial with a risk of minimal harm if patients are selected appropriately and the intervention is conducted in a safe environment. The positive impact of PA on QoL may be more significant in individuals who are able to consistently adhere and engage. Individualized PA interventions may provide better outcomes and are likely required with disease progression. Further research on PA is required to determine the best forms of therapy in people with PD and across the spectrum of its symptom burden. This would be further supported by studies that indicate the positive association of PA and neural function with the strong potential of this therapeutic modality to be better translated to and applied in the management of PD.
Author Contributions
Conceptualization, T.K.K. and D.P.C.F.B.; methodology, T.K.K. and D.P.C.F.B.; literature review, D.P.C.F.B., T.K.K., C.C.S.A.L., K.L.H. and S.T.; writing—original draft preparation, T.K.K. and D.P.C.F.B.; writing—review and editing, T.K.K. and D.P.C.F.B.; supervision, T.K.K. and D.P.C.F.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data will be made available upon request (t.khoo@griffith.edu.au).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Durcan, R.; Wiblin, L.; Lawson, R.A.; Khoo, T.K.; Yarnall, A.J.; Duncan, G.W.; Brooks, D.J.; Pavese, N.; Burn, D.J.; ICICLE-PD Study Group. Prevalence and duration of non-motor symptoms in prodromal Parkinson’s disease. Eur. J. Neurol. 2019, 26, 979–985. [Google Scholar] [CrossRef] [PubMed]
- De Pablo-Fernandez, E.; Lees, A.J.; Holton, J.L.; Warner, T.T. Prognosis and neuropathologic correlation of clinical subtypes of Parkinson disease. JAMA Neurol. 2019, 76, 470–479. [Google Scholar] [CrossRef]
- Poewe, W.; Seppi, K.; Tanner, C.N.; Halliday, G.M.; Brundin, P.; Volkmann, J.; Schrag, A.; Lang, A.E. Parkinson disease. Nat. Rev. Dis. Primers 2017, 3, 17013. [Google Scholar] [CrossRef] [PubMed]
- Schapira, A.H.V.; Chaudhuri, K.R.; Jenner, P. Non-motor features of Parkinson disease. Nat. Rev. Neurosci. 2017, 18, 435–450. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, P.G.; Domingues, D.D.; De Carvalho, L.A.; Allodi, S.; Correa, C.L. Neurotrophic factors in Parkinson’s disease are regulated by exercise: Evidence-based practice. J. Neurol. Sci. 2016, 363, 5–15. [Google Scholar] [CrossRef]
- Kadastik-Eerme, L.; Rosenthal, M.; Paju, T.; Muldmaa, M.; Taba, P. Health-related quality of life in Parkinson’s disease: A cross-sectional study focusing on non-motor symptoms. Health Qual. Life Outcomes 2015, 13, 83. [Google Scholar] [CrossRef] [PubMed]
- DeMaagd, G.; Philip, A. Parkinson’s Disease and Its Management: Part 1: Disease Entity, Risk Factors, Pathophysiology, Clinical Presentation, and Diagnosis. Pharm. Ther. 2015, 40, 504–532. [Google Scholar]
- Huang, X.; Ng, S.Y.E.; Chia, N.S.; Setiawan, F.; Tay, K.Y.; Au, W.L.; Tan, E.K.; Tan, L.C.S. Non-motor symptoms in early Parkinson’s disease with different motor subtypes and their associations with quality of life. Eur. J. Neurol. 2019, 26, 400–406. [Google Scholar] [CrossRef]
- Seppi, K.; Ray Chaudhuri, K.; Coelho, M.; Fox, S.H.; Katzenschlager, R.; Lloret, S.P.; Weintraub, D.; Sampaio, C.; the Collaborators of the Parkinson’s Disease Update on Non-Motor Symptoms Study Group on behalf of the Movement Disorders Society Evidence-Based Medicine Committee. Update on treatments for nonmotor symptoms of Parkinson’s disease—An evidence-based medicine review. Mov. Disord. 2019, 34, 180–198. [Google Scholar] [CrossRef]
- Kriebel-Gasparro, A. Parkinson’s disease: Update on medication management. J. Nurse Pract. 2016, 12, e81–e89. [Google Scholar] [CrossRef]
- Fox, S.H.; Katzenschlager, R.; Lim, S.Y.; Ravina, B.; Seppi, K.; Coelho, M.; Poewe, W.; Rascol, O.; Goetz, C.G.; Sampaio, C. The Movement Disorder Society Evidence-Based Medicine Review Update: Treatments for the motor symptoms of Parkinson’s disease. Mov. Disord. 2011, 26, S2–S41. [Google Scholar] [CrossRef] [PubMed]
- Haraldstad, K.; Wahl, A.; Andenæs, R.; Andersen, J.R.; Andersen, M.H.; Beisland, E.; Borge, C.R.; Engebretsen, E.; Eisemann, M.; Halvorsrud, L.; et al. A systematic review of quality of life research in medicine and health sciences. Qual. Life Res. 2019, 28, 2641–2650. [Google Scholar] [CrossRef] [PubMed]
- Marquez, D.X.; Aguiñaga, S.; Vásquez, P.M.; Conroy, D.E.; Erickson, K.I.; Hillman, C.; Stillman, C.M.; Ballard, R.M.; Sheppard, B.B.; Petruzzello, S.J.; et al. A systematic review of physical activity and quality of life and well-being. Transl. Behav. Med. 2020, 10, 1098–1109. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Choi, M.; Yoo, Y. A Meta-Analysis of Nonpharmacological Interventions for People With Parkinson’s Disease. Clin. Nurs. Res. 2017, 26, 608–631. [Google Scholar] [CrossRef]
- Song, R.; Grabowska, W.; Park, M.; Osypiuk, K.; Vergara-Diaz, G.P.; Bonato, P.; Hausdorff, J.M.; Fox, M.; Sudarsky, L.R.; Macklin, E.; et al. The impact of Tai Chi and Qigong mind-body exercises on motor and non-motor function and quality of life in Parkinson’s disease: A systematic review and meta-analysis. Park. Relat. Disord. 2017, 41, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Terrens, A.F.; Soh, S.E.; Morgan, P.E. The efficacy and feasibility of aquatic physiotherapy for people with Parkinson’s disease: A systematic review. Disabil. Rehabil. 2018, 40, 2847–2856. [Google Scholar] [CrossRef] [PubMed]
- Jenkinson, C.; Fitzpatrick, R.; Peto, V.; Greenhall, R.; Hyman, N. The Parkinson’s Disease Questionnaire (PDQ-39): Development and validation of a Parkinson’s disease summary index score. Age Ageing 1997, 26, 353–357. [Google Scholar] [CrossRef]
- Jenkinson, C.; Fitzpatrick, R.; Peto, V.; Greenhall, R.; Hyman, N. The PDQ-8: Development and validation of a short-form Parkinson’s disease questionnaire. Psychol. Health 1997, 12, 805–814. [Google Scholar] [CrossRef]
- De Boer, A.G.; Wijker, W.; Speelman, J.D.; De Haes, J.C. Quality of life in patients with Parkinson’s disease: Development of a questionnaire. J. Neurol. Neurosurg. Psychiatry 1996, 61, 70–74. [Google Scholar] [CrossRef]
- Ware, J.E.J.; Sherbourne, C.D. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med. Care 1992, 30, 473–483. [Google Scholar] [CrossRef]
- EuroQol Group. EuroQol--a new facility for the measurement of health-related quality of life. Health Policy 1990, 16, 199–208. [Google Scholar] [CrossRef]
- Ellis, T.; Rochester, L. Mobilizing Parkinson’s Disease: The Future of Exercise. J. Park. Dis. 2018, 8, S95–S100. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, M.A.; van Wegen, E.; Newman, M.A.; Heyn, P.C. Exercise-induced increase in brain-derived neurotrophic factor in human Parkinson’s disease: A systematic review and meta-analysis. Transl. Neurodegener. 2018, 7, 7. [Google Scholar] [CrossRef] [PubMed]
- Bhalsing, K.S.; Abbas, M.M.; Tan, L. Role of Physical Activity in Parkinson’s Disease. Ann Indian Acad. Neurol. 2018, 21, 242–249. [Google Scholar]
- Feng, Y.S.; Yang, S.D.; Tan, Z.X.; Wang, M.M.; Xing, Y.; Dong, F.; Zhang, F. The benefits and mechanisms of exercise training for Parkinson’s disease. Life Sci. 2020, 245, 117345. [Google Scholar] [CrossRef]
- Palasz, E.; Wysocka, A.; Gasiorowska, A.; Chalimoniuk, M.; Niewiadomski, W.; Niewiadomska, G. BDNF as a Promising Therapeutic Agent in Parkinson’s Disease. Int. J. Mol. Sci. 2020, 21, 1170. [Google Scholar] [CrossRef] [PubMed]
- Szuhany, K.L.; Bugatti, M.; Otto, M.W. A meta-analytic review of the effects of exercise on brain-derived neurotrophic factor. J. Psychiatr. Res. 2015, 60, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.C.; Hwang, D.J.; Koo, J.H.; Um, H.S.; Lee, N.H.; Yeom, D.C.; Lee, Y.; Cho, J.Y. Association of exercise-induced autophagy upregulation and apoptosis suppression with neuroprotection against pharmacologically induced Parkinson’s disease. J. Exerc. Nutr. Biochem. 2018, 22, 1–8. [Google Scholar] [CrossRef]
- Shin, M.S.; Kim, T.W.; Lee, J.M.; Ji, E.S.; Lim, B.V. Treadmill exercise alleviates nigrostriatal dopaminergic loss of neurons and fibers in rotenone-induced Parkinson rats. J. Exerc. Rehabil. 2017, 13, 30–35. [Google Scholar] [CrossRef]
- Son, H.G.; Choi, E.-O. The Effects of Mindfulness Meditation-Based Complex Exercise Program on Motor and Nonmotor Symptoms and Quality of Life in Patients with Parkinson’s Disease. Asian Nurs. Res. 2018, 12, 145–153. [Google Scholar] [CrossRef]
- AHRQ. The Mission, Priority Areas of Focus, Customers, and Background of the Agency for Healthcare Research and Quality (AHRQ). 2021. Available online: https://www.ahrq.gov/cpi/about/index.html (accessed on 2 June 2024).
- Jadad, A.R.; Moore, R.A.; Carroll, D.; Jenkinson, C.; Reynolds, D.J.; Gavaghan, D.J.; McQuay, H.J. Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Control. Clin. Trials 1996, 17, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Landers, M.R.; Navalta, J.W.; Murtishaw, A.S.; Kinney, J.W.; Pirio Richardson, S. A high-intensity exercise boot camp for persons with Parkinson disease: A phase II, pragmatic, randomized clinical trial of feasibility, safety, signal of efficacy, and disease mechanisms. Journal of neurologic physical therapy. J. Neurol. Phys. Ther. 2019, 43, 12–25. [Google Scholar] [CrossRef] [PubMed]
- Cheung, C.; Bhimani, R.; Wyman, J.F.; Konczak, J.; Zhang, L.; Mishra, U.; Terluk, M.; Kartha, R.V.; Tuite, P. Effects of yoga on oxidative stress, motor function, and non-motor symptoms in Parkinson’s disease: A pilot randomized controlled trial. Pilot. Feasibility Stud. 2018, 4, 162. [Google Scholar] [CrossRef] [PubMed]
- Ni, M.; Mooney, K.; Signorile, J.F. Controlled pilot study of the effects of power yoga in Parkinson’s disease. Complement. Ther. Med. 2016, 25, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Ni, M.; Signorile, J.F.; Balachandran, A.; Potiaumpai, M. Power training induced change in bradykinesia and muscle power in Parkinson’s disease. Park. Rel. Disord. 2015, 23, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Schenkman, M.; Hall, D.A.; Barón, A.E.; Schwartz, R.S.; Mettler, P.; Kohrt, W.M. Exercise for people in early- or mid-stage Parkinson disease: A 16-month randomized controlled trial. Phys. Ther. 2012, 92, 1395–1410. [Google Scholar] [CrossRef] [PubMed]
- Haas, A.N.; Delabary, M.D.S.; Passos-Monteiro, E.; Wolffenbuttel, M.; Donida, R.G.; Casal, M.Z.; Zanardi, A.P.J.; Rodrigues, L.P.; Martinez, F.G.; Peyré-Tartaruga, L.A. The effects of Brazilian dance, deep-water exercise and nordic walking, pre- and post-12 weeks, on functional-motor and non-motor symptoms in trained PwPD. Arch. Gerontol. Geriatr. 2024, 118, 105285. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Chien, H.F.; Francato, D.C.V.; Barbosa, A.F.; Souza, C.O.; Voos, M.C.; Greve, J.M.D.; Barbosa, E.R. Effects of resistance training on postural control in Parkinson’s disease: A randomized controlled trial. Arq. Neuropsiquiatr. 2021, 79, 511–520. [Google Scholar] [CrossRef]
- Tollár, J.; Nagy, F.; Kovács, N.; Hortobágyi, T. A High-Intensity Multicomponent Agility Intervention Improves Parkinson Patients’ Clinical and Motor Symptoms. Arc. Phys. Med. Rehabil. 2018, 99, 2478–2484. [Google Scholar] [CrossRef]
- Volpe, D.; Signorini, M.; Marchetto, A.; Lynch, T.; Morris, M.E. A comparison of Irish set dancing and exercises for people with Parkinson’s disease: A phase II feasibility study. BMC Geriatr. 2013, 13, 54. [Google Scholar] [CrossRef]
- Collett, J.; Franssen, M.; Meaney, A.; Wade, D.; Izadi, H.; Tims, M.; Winward, C.; Bogdanovic, M.; Farmer, A.; Dawes, H. Phase II randomised controlled trial of a 6-month self-managed community exercise programme for people with Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 2017, 88, 204–211. [Google Scholar] [CrossRef]
- Wagner, L.; Hauptmann, B.; Hoffmann, A.K.; Jochems, N.; Schmeier, B.; Schrader, A.; Kohlmann, T.; Deck, R. Evaluation of an individualized, tablet-based physiotherapy training programme for patients with Parkinson’s disease: The ParkProTrain study, a quasi-randomised controlled trial. BMC Neurol. 2022, 22, 176. [Google Scholar] [CrossRef] [PubMed]
- van der Kolk, N.M.; Vries, N.M.; Kessels, R.P.; Joosten, H.; Zwinderman, A.H.; Post, B.; Bloem, B.R. Effectiveness of home-based and remotely supervised aerobic exercise in Parkinson’s disease: A double-blind, randomised controlled trial. Lancet 2019, 18, P998–P1008. [Google Scholar] [CrossRef]
- Kwok, J.Y.Y.; Choi, E.P.H.; Wong, J.Y.H.; Lok, K.Y.W.; Ho, M.-H.; Fong, D.Y.T.; Kwan, J.C.Y.; Pang, S.Y.Y.; Auyeung, M. A randomized clinical trial of mindfulness meditation versus exercise in Parkinson’s disease during social unrest. npj Park. Dis. 2023, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kwok, J.Y.Y.; Kwan, J.C.Y.; Auyeung, M.; Mok, V.C.T.; Lau, C.K.Y.; Choi, K.C.; Chan, H.Y.L. Effects of Mindfulness Yoga vs Stretching and Resistance Training Exercises on Anxiety and Depression for People With Parkinson Disease: A Randomized Clinical Trial. JAMA Neurol. 2019, 76, 755–763. [Google Scholar] [CrossRef] [PubMed]
- Schootemeijer, S.; van der Kolk, N.M.; Ellis, T.; Mirelman, A.; Nieuwboer, A.; Nieuwhof, F.; Schwarzschild, M.A.; de Vries, N.M.; Bloem, B.R. Barriers and Motivators to Engage in Exercise for Persons with Parkinson’s Disease. J. Park. Dis. 2020, 10, 1293–1299. [Google Scholar] [CrossRef]
- International Health Conference. Constitution of the World Health Organization. 1946. Bull. World Health Organ. 2002, 80, 983–984. [Google Scholar]
- Biddiscombe, K.J.; Ong, B.; Kalinowski, P.; Pike, K.E. Physical activity and cognition in young-onset Parkinson’s disease. Acta Neurol. Scand. 2020, 142, 151–160. [Google Scholar] [CrossRef]
- King, L.A.; Wilhelm, J.; Chen, Y.; Blehm, R.; Nutt, J.; Chen, Z.; Serdar, A.; Horak, F.B. Effects of Group, Individual, and Home Exercise in Persons with Parkinson Disease: A Randomized Clinical Trial. J. Neurol. Phys. Ther. 2015, 39, 204–212. [Google Scholar] [CrossRef]
- Gomez-Pinilla, F.; Zhuang, Y.; Feng, J.; Ying, Z.; Fan, G. Exercise impacts brain-derived neurotrophic factor plasticity by engaging mechanisms of epigenetic regulation. Eur. J. Neurosci. 2011, 33, 383–390. [Google Scholar] [CrossRef]
- Mandolesi, L.; Polverino, A.; Montuori, S.; Foti, F.; Ferraioli, G.; Sorrentino, P.; Sorrentino, G. Effects of Physical Exercise on Cognitive Functioning and Wellbeing: Biological and Psychological Benefits. Front. Psychol. 2018, 9, 509. [Google Scholar] [CrossRef]
- Sleiman, S.F.; Henry, J.; Al-Haddad, R.; El Hayek, L.; Abou Haidar, E.; Stringer, T.; Ulja, D.; Karuppagounder, S.S.; Holson, E.B.; Ratan, R.R.; et al. Exercise promotes the expression of brain derived neurotrophic factor (BDNF) through the action of the ketone body beta-hydroxybutyrate. eLife 2016, 5, e15092. [Google Scholar] [CrossRef]
- Barber, J.L.; Zellars, K.N.; Barringhaus, K.G.; Bouchard, C.; Spinale, F.G.; Sarzynski, M.A. The Effects of Regular Exercise on Circulating Cardiovascular-related MicroRNAs. Sci. Rep. 2019, 9, 7527. [Google Scholar] [CrossRef]
- Fernandes, J.; Arida, R.M.; Gomez-Pinilla, F. Physical exercise as an epigenetic modulator of brain plasticity and cognition. Neurosci. Biobehav. Rev. 2017, 80, 443–456. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, F.C.; Rode, M.P.; Vietta, G.G.; Iop, R.D.R.; Creczynski-Pasa, T.B.; Martin, A.S.; Da Silva, R. Expression levels of specific microRNAs are increased after exercise and are associated with cognitive improvement in Parkinson’s disease. Mol. Med. Rep. 2021, 24, 1–10. [Google Scholar] [CrossRef]
- Chaire, A.; Becke, A.; Duzel, E. Effects of Physical Exercise on Working Memory and Attention-Related Neural Oscillations. Front. Neurosci. 2020, 14, 239. [Google Scholar] [CrossRef]
- Pruzin, J.J.; Klein, H.; Rabin, J.S.; Schultz, A.P.; Kirn, D.R.; Yang, H.S.; Buckley, R.F.; Scott, M.R.; Properzi, M.; Rentz, D.M.; et al. Physical activity is associated with increased resting-state functional connectivity in networks predictive of cognitive decline in clinically unimpaired older adults. Alzheimer’s Dement. Diagn. Assess. Dis. Monit. 2022, 14, e12319. [Google Scholar] [CrossRef] [PubMed]
- van Wijk, B.C.; Beek, P.J.; Daffertshofer, A. Neural synchrony within the motor system: What have we learned so far? Front. Hum. Neurosci. 2012, 6, 252. [Google Scholar] [CrossRef]
- He, X.F.; Liu, D.X.; Zhang, Q.; Liang, F.Y.; Dai, G.Y.; Zeng, J.S.; Pei, Z.; Xu, G.Q.; Lan, Y. Voluntary Exercise Promotes Glymphatic Clearance of Amyloid Beta and Reduces the Activation of Astrocytes and Microglia in Aged Mice. Front. Mol. Neurosci. 2017, 10, 144. [Google Scholar] [CrossRef] [PubMed]
- Kylkilahti, T.M.; Berends, E.; Ramos, M.; Shanbhag, N.C.; Toger, J.; Markenroth Bloch, K.; Lundgaard, I. Achieving brain clearance and preventing neurodegenerative diseases-A glymphatic perspective. J. Cereb. Blood Flow Metab. 2021, 41, 2137–2149. [Google Scholar] [CrossRef]
- Scott-Massey, A.; Boag, M.K.; Magnier, A.; Bispo, D.; Khoo, T.K.; Pountney, D.L. Glymphatic System Dysfunction and Sleep Disturbance May Contribute to the Pathogenesis and Progression of Parkinson’s Disease. Int. J. Mol. Sci. 2022, 23, 12928. [Google Scholar] [CrossRef] [PubMed]
- Si, X.; Guo, T.; Wang, Z.; Fang, Y.; Gu, L.; Cao, L.; Yang, W.; Gao, T.; Song, Z.; Tian, J.; et al. Neuroimaging evidence of glymphatic system dysfunction in possible REM sleep behavior disorder and Parkinson’s disease. npj Parkinson’s Dis. 2022, 8, 1–9. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).