Association between Bone Quality and Physical Activity in Community-Dwelling Older Adults
Abstract
1. Introduction
2. Materials and Methods
2.1. Participant Characteristics
2.2. Bone Assessment
2.2.1. BMD
2.2.2. Bone Quality
2.3. Physical Activity (PA)
2.4. Measurement of Nutrient Intake
2.5. Statistics
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IOF Compendium of Osteoporosis, 2nd ed.; International Osteoporosis Foundation: Nyon, Switzerland, 2019; Available online: https://www.osteoporosis.foundation/educational-hub/files/iof-compendium-osteoporosis-2nd-edition (accessed on 25 January 2023).
- NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy. Osteoporosis Prevention, Diagnosis, and Therapy. JAMA 2001, 285, 785–795. [Google Scholar] [CrossRef] [PubMed]
- Doren, M. Effects of Specific Post-Menopausal Hormone Therapies on Bone Mineral Density in Post-Menopausal Women: A Meta-Analysis. Human Reprod. 2003, 18, 1737–1746. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Beverly, R.; Volkar, J. Risks and Benefits of Estrogen Plus Progestin in Healthy Postmenopausal Women: The Women’s Health Initiative. In 50 Studies Every Obstetrician-Gynecologist Should Know; Oxford University Press: Oxford, UK, 2021; pp. 262–266. ISBN 978-0-19-094708-8. [Google Scholar]
- Marques, E.A.; Elbejjani, M.; Gudnason, V.; Sigurdsson, G.; Lang, T.; Sigurdsson, S.; Aspelund, T.; Siggeirsdottir, K.; Launer, L.; Eiriksdottir, G.; et al. Cigarette Smoking and Hip Volumetric Bone Mineral Density and Cortical Volume Loss in Older Adults: The AGES-Reykjavik Study. Bone 2018, 108, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Papaioannou, A.; Kennedy, C.C.; Cranney, A.; Hawker, G.; Brown, J.P.; Kaiser, S.M.; Leslie, W.D.; O’Brien, C.J.M.; Sawka, A.M.; Khan, A.; et al. Risk Factors for Low BMD in Healthy Men Age 50 Years or Older: A Systematic Review. Osteoporos. Int. 2009, 20, 507–518. [Google Scholar] [CrossRef] [PubMed]
- Weaver, C. Nutrition and Bone Health. Oral. Dis. 2017, 23, 412–415. [Google Scholar] [CrossRef] [PubMed]
- Shea, B.; Wells, G.; Cranney, A.; Zytaruk, N.; Robinson, V.; Griffith, L.; Ortiz, Z.; Peterson, J.; Adachi, J.; Tugwell, P.; et al. VII. Meta-Analysis of Calcium Supplementation for the Prevention of Postmenopausal Osteoporosis. Endocr. Rev. 2002, 23, 552–559. [Google Scholar] [CrossRef] [PubMed]
- Godos, J.; Giampieri, F.; Chisari, E.; Micek, A.; Paladino, N.; Forbes-Hernández, T.Y.; Quiles, J.L.; Battino, M.; La Vignera, S.; Musumeci, G.; et al. Alcohol Consumption, Bone Mineral Density, and Risk of Osteoporotic Fractures: A Dose–Response Meta-Analysis. Int. J. Environ. Res. Public Health 2022, 19, 1515. [Google Scholar] [CrossRef] [PubMed]
- Berg, K.M.; Kunins, H.V.; Jackson, J.L.; Nahvi, S.; Chaudhry, A.; Harris, K.A.; Malik, R.; Arnsten, J.H. Association Between Alcohol Consumption and Both Osteoporotic Fracture and Bone Density. Am. J. Med. 2008, 121, 406–418. [Google Scholar] [CrossRef]
- Howe, T.E.; Shea, B.; Dawson, L.J.; Downie, F.; Murray, A.; Ross, C.; Harbour, R.T.; Caldwell, L.M.; Creed, G. Exercise for Preventing and Treating Osteoporosis in Postmenopausal Women. Cochrane Database Syst. Rev. 2011, CD000333. [Google Scholar] [CrossRef]
- Pinheiro, M.B.; Oliveira, J.; Bauman, A.; Fairhall, N.; Kwok, W.; Sherrington, C. Evidence on Physical Activity and Osteoporosis Prevention for People Aged 65+ Years: A Systematic Review to Inform the WHO Guidelines on Physical Activity and Sedentary Behaviour. Int. J. Behav. Nutr. Phys. Act. 2020, 17, 150. [Google Scholar] [CrossRef]
- Osteoporosis Prevention Diagnosis and Management in Postmenopausal Women and Men over 50 Years of Age. Available online: https://www.racgp.org.au/clinical-resources/clinical-guidelines/key-racgp-guidelines/view-all-racgp-guidelines/osteoporosis (accessed on 25 January 2024).
- Chiba, K.; Suetoshi, R.; Cretin, D.; Arai, T.; Kawajiri, T.; Okayama, A.; Tsuji, S.; Okazaki, N.; Osaki, M.; Yoh, K. Development of a QUS Device to Evaluate Deterioration of Cortical Bone: Verification by HR-PQCT and Measurements in Healthy Individuals and Dialysis Patients. J. Clin. Densitom. 2021, 24, 94–105. [Google Scholar] [CrossRef] [PubMed]
- Otani, T.; Fukunaga, M.; Yho, K.; Miki, T.; Yamazaki, K.; Kishimoto, H.; Matsukawa, M.; Endoh, N.; Hachiya, H.; Kanai, H.; et al. Attempt at Standardization of Bone Quantitative Ultrasound in Japan. J. Med. Ultrason. 2018, 45, 3–13. [Google Scholar] [CrossRef] [PubMed]
- 2015 Guidelines for Prevention and Treatment of Osteoporosis. Available online: http://www.josteo.com/ja/guideline/index.html (accessed on 1 February 2023).
- Pate, R.R.; O’Neill, J.R.; Lobelo, F. The Evolving Definition of “Sedentary”. Exerc. Sport. Sci. Rev. 2008, 36, 173–178. [Google Scholar] [CrossRef]
- Trost, S.G.; Pate, R.R.; Freedson, P.S.; Sallis, J.F.; Taylor, W.C. Using objective physical activity measures with youth: How many days of monitoring are needed? Med. Sci. Sports Exerc. 2000, 32, 426. [Google Scholar] [CrossRef] [PubMed]
- Nagai, K.; Miyamato, T.; Okamae, A.; Tamaki, A.; Fujioka, H.; Wada, Y.; Uchiyama, Y.; Shinmura, K.; Domen, K. Physical Activity Combined with Resistance Training Reduces Symptoms of Frailty in Older Adults: A Randomized Controlled Trial. Arch. Gerontol. Geriatr. 2018, 76, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, S.; Murakami, K.; Sasaki, S.; Okubo, H.; Hirota, N.; Notsu, A.; Fukui, M.; Date, C. Comparison of Relative Validity of Food Group Intakes Estimated by Comprehensive and Brief-Type Self-Administered Diet History Questionnaires against 16 d Dietary Records in Japanese Adults. Public Health Nutr. 2011, 14, 1200–1211. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, S.; Honda, S.; Murakami, K.; Sasaki, S.; Okubo, H.; Hirota, N.; Notsu, A.; Fukui, M.; Date, C. Both Comprehensive and Brief Self-Administered Diet History Questionnaires Satisfactorily Rank Nutrient Intakes in Japanese Adults. J. Epidemiol. 2012, 22, 151–159. [Google Scholar] [CrossRef] [PubMed]
- The 5th Revision of Standard Food Composition Table of Japanese Foods. Available online: https://www.mext.go.jp/b_menu/shingi/gijyutu/gijyutu3/houkoku/1298713.htm (accessed on 31 January 2023). (In Japanese)
- Polidoulis, I.; Beyene, J.; Cheung, A.M. The Effect of Exercise on PQCT Parameters of Bone Structure and Strength in Postmenopausal Women—A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Osteoporos. Int. 2012, 23, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Bolam, K.A.; van Uffelen, J.G.Z.; Taaffe, D.R. The Effect of Physical Exercise on Bone Density in Middle-Aged and Older Men: A Systematic Review. Osteoporos. Int. 2013, 24, 2749–2762. [Google Scholar] [CrossRef]
- Zhao, R.; Zhao, M.; Xu, Z. The Effects of Differing Resistance Training Modes on the Preservation of Bone Mineral Density in Postmenopausal Women: A Meta-Analysis. Osteoporos. Int. 2015, 26, 1605–1618. [Google Scholar] [CrossRef]
- Kistler-Fischbacher, M.; Weeks, B.K.; Beck, B.R. The Effect of Exercise Intensity on Bone in Postmenopausal Women (Part 2): A Meta-Analysis. Bone 2021, 143, 115697. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, T.; Hiraya, K.; Denda, T.; Yamamoto, S. A Comparison of Different Exercise Intensities for Improving Bone Mineral Density in Postmenopausal Women with Osteoporosis: A Systematic Review and Meta-Analysis. Bone Rep. 2022, 17, 101631. [Google Scholar] [CrossRef] [PubMed]
- Elhakeem, A.; Heron, J.; Tobias, J.H.; Lawlor, D.A. Physical Activity Throughout Adolescence and Peak Hip Strength in Young Adults. JAMA Netw. Open 2020, 3, e2013463. [Google Scholar] [CrossRef] [PubMed]
- Bielemann, R.M.; Domingues, M.R.; Horta, B.L.; Gigante, D.P. Physical Activity from Adolescence to Young Adulthood and Bone Mineral Density in Young Adults from the 1982 Pelotas (Brazil) Birth Cohort. Prev. Med. 2014, 62, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Bielemann, R.M.; Domingues, M.R.; Horta, B.L.; Menezes, A.M.B.; Gonçalves, H.; Assunção, M.C.F.; Hallal, P.C. Physical Activity throughout Adolescence and Bone Mineral Density in Early Adulthood: The 1993 Pelotas (Brazil) Birth Cohort Study. Osteoporos. Int. 2014, 25, 2007–2015. [Google Scholar] [CrossRef][Green Version]
- Muthuri, S.G.; Ward, K.A.; Kuh, D.; Elhakeem, A.; Adams, J.E.; Cooper, R. Physical Activity Across Adulthood and Bone Health in Later Life: The 1946 British Birth Cohort. J. Bone Miner. Res. 2019, 34, 252–261. [Google Scholar] [CrossRef]
- Cauley, J.A. Estrogen and Bone Health in Men and Women. Steroids 2015, 99, 11–15. [Google Scholar] [CrossRef]
| Variables 1 | Overall (n = 452) | Women (n = 278) | Men (n = 174) | p-Value |
|---|---|---|---|---|
| Age, years, mean (SD) | 72.2 (5.8) | 71.8 (5.5) | 73.0 (6.1) | 0.030 |
| BMI, kg/m2, mean (SD) | 22.6 (2.9) | 22.2 (2.9) | 23.0 (2.8) | 0.004 |
| Diabetes, n (%) | 64 (14.1) | 30 (10.8) | 34 (19.5) | 0.009 |
| Kidney disease, n (%) | 13 (2.9) | 5 (1.8) | 8 (4.6) | 0.117 |
| Number of drugs taken, mean (SD) | 1.6 (1.9) | 1.4 (1.6) | 1.9 (2.2) | 0.008 |
| tib cSOS, m/s, mean (SD) | 3859.8 (86.8) | 3833.1 (85.9) | 3902.3 (69.8) | <0.001 |
| SOS, m/s, mean (SD) | 1494.4 (23.3) | 1490.0 (21.2) | 1501.3 (24.9) | <0.001 |
| Nutritional intake | ||||
| Calcium (Ca), mg/day, mean (SD) | 790.4 (330.7) | 809.5 (299.3) | 759.8 (374.4) | 0.140 |
| Vitamin D, μg/day, mean (SD) | 23.8 (16.5) | 24.8 (16.3) | 22.2 (16.9) | 0.106 |
| Vitamin K, μg/day, mean (SD) | 412.6 (216.9) | 420.7 (202.6) | 399.7 (238.1) | 0.319 |
| Physical activity | ||||
| MVPA, min/day, mean (SD) | 42.0 (31.3) | 42.0 (29.5) | 42.1 (34.0) | 0.960 |
| LPA, min/day, mean (SD) | 483.6 (139.5) | 486.2 (139.3) | 479.4 (140.1) | 0.614 |
| Variables | Overall (n = 449) | Women (n = 277) | Men (n = 172) | p-Value |
| Gait speed, m/s, mean (SD) | 1.5 (0.2) | 1.5 (0.2) | 1.5 (0.2) | 0.372 |
| Grip strength, kg, mean (SD) | 28.7 (7.3) | 24.6 (4.5) | 35.3 (6.0) | <0.001 |
| (A) Women | ||||
| PA | ||||
| Age | BMI | MVPA | LPA | |
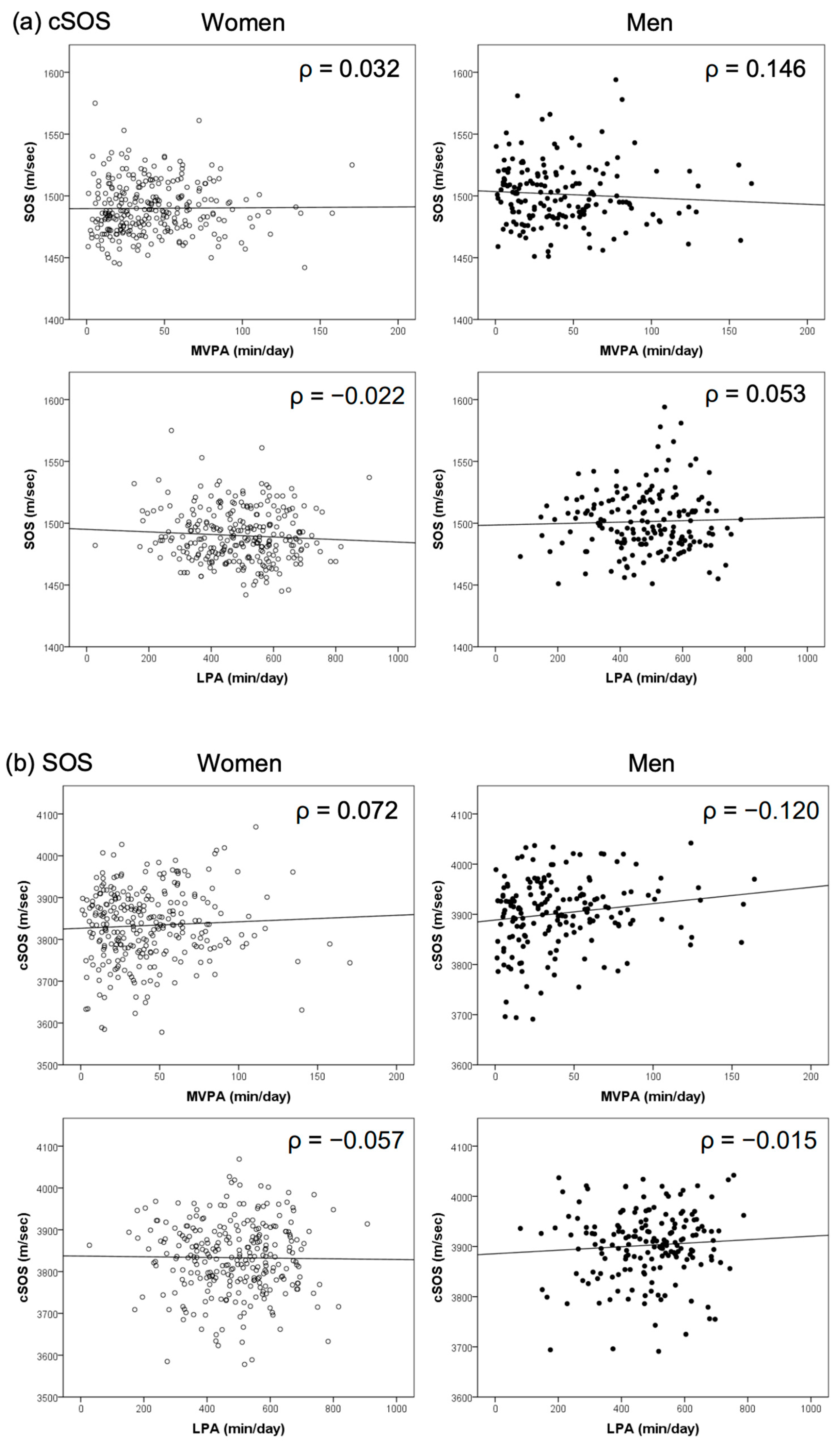
| Cortical QUS (mid-tibia) cSOS | −0.189 ** | 0.063 | 0.032 | −0.022 |
| Calcaneus QUS SOS | −0.151 * | 0.088 | 0.072 | −0.057 |
| (B) Men | ||||
| Age | BMI | MVPA | LPA | |
| Cortical QUS (mid-tibia) cSOS | −0.111 | −0.023 | 0.146 | 0.053 |
| Calcaneus QUS SOS | −0.147 | 0.224 ** | −0.120 | −0.015 |
| Models | Subjects | B | β-Coefficient | SE | p-Value | R2 |
|---|---|---|---|---|---|---|
| cSOS | ||||||
| MVPA | ||||||
| Crude model | Women | 0.17 | 0.058 | 0.179 | 0.344 | 0.003 |
| Men | 0.31 | 0.151 | 0.16 | 0.054 | 0.026 | |
| Adjusted model | Women | 0.195 | 0.067 | 0.176 | 0.267 | 0.091 |
| Men | 0.247 | 0.133 | 0.161 | 0.091 | 0.091 | |
| LPA | ||||||
| Crude model | Women | −0.016 | −0.025 | 0.038 | 0.681 | 0.003 |
| Men | 0.016 | 0.032 | 0.039 | 0.677 | 0.026 | |
| Adjusted model | Women | −0.023 | −0.038 | 0.038 | 0.536 | 0.091 |
| Men | −0.01 | −0.02 | 0.04 | 0.807 | 0.091 | |
| SOS | ||||||
| MVPA | ||||||
| Crude model | Women | 0.017 | 0.024 | 0.044 | 0.692 | 0.005 |
| Men | −0.061 | −0.084 | 0.057 | 0.288 | 0.008 | |
| Adjusted model | Women | 0.024 | 0.033 | 0.044 | 0.592 | 0.062 |
| Men | −0.07 | −0.096 | 0.056 | 0.215 | 0.122 | |
| LPA | ||||||
| Crude model | Women | −0.011 | −0.073 | 0.009 | 0.237 | 0.005 |
| Men | 0.01 | 0.054 | 0.014 | 0.492 | 0.008 | |
| Adjusted model | Women | −0.011 | −0.074 | 0.009 | 0.233 | 0.062 |
| Men | 0.011 | 0.063 | 0.014 | 0.426 | 0.122 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akai, K.; Nagai, K.; Tsuji, S.; Hirose, K.; Maruyama, D.; Matsuzawa, R.; Tamaki, K.; Kusunoki, H.; Wada, Y.; Shinmura, K. Association between Bone Quality and Physical Activity in Community-Dwelling Older Adults. Geriatrics 2024, 9, 62. https://doi.org/10.3390/geriatrics9030062
Akai K, Nagai K, Tsuji S, Hirose K, Maruyama D, Matsuzawa R, Tamaki K, Kusunoki H, Wada Y, Shinmura K. Association between Bone Quality and Physical Activity in Community-Dwelling Older Adults. Geriatrics. 2024; 9(3):62. https://doi.org/10.3390/geriatrics9030062
Chicago/Turabian StyleAkai, Koki, Koutatsu Nagai, Shotaro Tsuji, Katsuyoshi Hirose, Daisuke Maruyama, Ryota Matsuzawa, Kayoko Tamaki, Hiroshi Kusunoki, Yosuke Wada, and Ken Shinmura. 2024. "Association between Bone Quality and Physical Activity in Community-Dwelling Older Adults" Geriatrics 9, no. 3: 62. https://doi.org/10.3390/geriatrics9030062
APA StyleAkai, K., Nagai, K., Tsuji, S., Hirose, K., Maruyama, D., Matsuzawa, R., Tamaki, K., Kusunoki, H., Wada, Y., & Shinmura, K. (2024). Association between Bone Quality and Physical Activity in Community-Dwelling Older Adults. Geriatrics, 9(3), 62. https://doi.org/10.3390/geriatrics9030062







