Abstract
Through these epidemiological studies, which are based on statistical and observational calculations, without visual appeal, we tracked the incidence of public health problems. In this study, our research objective was to determine and evaluate the health patterns present in a population, along with identifying the factors that contribute to the risks or provide protection against specific diseases or conditions. The progression of cardiometabolic diseases is closely linked to various chronic conditions, such as diabetes, hypertension, dyslipidemia, and chronic kidney disease. This research study involved 578 patients, who were divided into six-year cohorts ranging from 2017 to 2022. The study examined parameters related to cardiometabolic diseases, including alcoholic hepatopathies, non-alcoholic hepatopathy, chronic kidney disease, hypertension, myocardial infarction, other forms of chronic coronary syndrome, peripheral vascular disease, microvascular diseases, macrovascular diseases, and hypercholesterolemia, while considering age and physical activity levels. The study concluded that individuals in the age group of 41–50 years exhibited the highest propensity for cardiometabolic damage. Additionally, the promotion of a healthy and active lifestyle is increasingly gaining traction among elderly patients.
1. Introduction
Although not visually appealing, epidemiological studies, which are based on statistical and observational analyses, are extremely valuable for monitoring large groups of individuals and identifying future trends. Regarding the study of cardiometabolic diseases, an epidemiological perspective, it highlighted their global importance as a public health problem [1]. Risk factors, including arterial hypertension (HTN), dyslipidemia, obesity, sedentary lifestyle, and unhealthy diet, exert a substantial influence on the development and progression of these diseases [2]. Comprehending and addressing these risk factors is crucial for the prevention and management of cardiometabolic diseases, as they hold significant potential for reducing the associated morbidity and mortality [3]. Therefore, epidemiological studies play a crucial role in identifying key aspects for each specific cohort.
The prevalence of cardiometabolic diseases [4,5] as revealed by epidemiological studies, has demonstrated a substantial increase in recent decades, particularly in middle- and high-income countries [5]. This rise can largely be attributed to lifestyle changes [6], unhealthy dietary habits [7], sedentary lifestyles, and obesity [8].
Significant risk factors for cardiometabolic diseases include HTN [9], dyslipidemia [10], smoking [11], diabetes, obesity, physical inactivity [12], and unhealthy diets [13]. Epidemiological studies have clearly established an association between these risk factors and the development of cardiometabolic diseases.
Cardiometabolic diseases have a considerable impact on public health by increasing both mortality and morbidity [14]. They contribute to cardiovascular complications such as myocardial infarction [15], stroke [16], and heart failure, as well as metabolic complications such as diabetic retinopathy and nephropathy [17].
Cardiovascular diseases, such as chronic coronary syndrome or congestive heart failure, as well as myocardial infarction, can be correlated with metabolic diseases [18,19]. In metabolic diseases such as type 2 diabetes or metabolic syndrome, heart disease often occurs as a comorbidity. The risk of these conditions increases with obesity, HTN, and visceral fat. A sedentary lifestyle also poses a risk factor. Dyslipidemia, particularly atherogenic dyslipidemia, increases the cardiovascular risk, exacerbates underlying metabolic diseases, and contributes to the development of new comorbidities [20,21].
Patients diagnosed with type 1 diabetes [22] and adolescent girls with polycystic ovary syndrome [23] have shown a heightened cardiometabolic risk, which has been associated with the presence of visceral obesity [24]. These metabolic conditions, directly associated with metabolic syndrome and type 2 diabetes, are closely linked to atherogenic dyslipidemia. The development of atherogenic dyslipidemia significantly diminishes both quality of life and life expectancy [25].
Cardiometabolic diseases have emerged as a global health issue, with a significant increase in prevalence and impact on the population in recent decades. Conditions such as coronary heart disease, stroke, diabetes, and metabolic syndrome demonstrate complex interconnections and mutual influences. The aim of this epidemiological study is to investigate the risk for atherosclerotic cardiovascular disease and evaluate their impact on public health. It also aims to determine whether the same approach is appropriate for both young and older people in reducing the risk of developing atherosclerosis. Starting from this premise, the ultimate goal is to develop a personalized approach to HTN, dyslipidemia, and metabolic diseases in young people, focusing on lifestyle modification and creating quality of life.
2. Materials and Methods
The study involved a total of 578 individuals with metabolic diseases, including metabolic syndrome and type 2 diabetes (DM2). They were divided into six groups based on the study year. The patients presented themselves at Arad County Emergency Clinical Hospital with hyperinsulinemia and hyperglycemia or diabetes, where cardiological, clinical, and paraclinical investigations were conducted. Each patient read the World Medical Association Declaration of Helsinki on Ethical Principles for Medical Research Involving Human Subjects [26]. Clinical and paraclinical analyses were performed to track the patients’ epidemiological progress, and the data were retrospectively processed. There were patients with both long-standing and new-onset hyperlipidemias. The clinical evaluation utilized the Tanita BC-545N body bioelectrical impedance analyzer available at the hospital to track fat mass and visceral fat. Changes in clinical and paraclinical parameters from the observation sheets were also monitored. The participants completed the evaluation forms at the beginning of the study period. Inclusion criteria required individuals to have at least three of the following medical conditions: obesity, visceral obesity, hypercholesterolemia, hypertriglyceridemia, HTN, myocardial infarction, congestive heart failure, chronic coronary syndrome, microvascular disease (microangiopathies showing chronic damage to small blood vessels), macrovascular disease (macroangiopathy means damage to large and medium arteries by the atherosclerosis process, including coronary artery disease, cerebrovascular disease and peripheral vascular disease), chronic kidney disease (any modification of the value outside the GFR values (glomerular filtration rate) 90 to 120 mL/min/1.73 m2), or alcoholic liver disease. Exclusion criteria included decompensated diabetes, renal stents, pacemakers, other cardiac or aortic medical devices, and autoimmune diseases. Due to the chronic nature of their conditions, patients were followed up for a minimum of 12 months (in mean 12.86 ± 0.12 months) with evaluations conducted every three months.
2.1. Statistical Analysis
Statistical analysis was performed using SPSS v20 and Microsoft Excel software packages. The initial step involved obtaining comprehensive descriptive statistics, which entailed calculating measures of central tendency and descriptive indicators and graphically presenting the most important findings. Using the Skewness and Kurtosis test (in the range of −3 to +3 for normally distributed data) we checked the normality of the data. A significance level of p < 0.05 was considered for all results. The Student’s t-test was utilized to calculate the mean values of parameters, frequency ranges, standard deviations, and perform statistical significance tests. The Spearman’s correlation coefficient was used to determine the independent indicator of units of measure for the two variables. A significance level of p < 0.05 indicated statistical significance for ANOVA, while a significance level of p < 0.01 denoted high-level statistical significance. Post hoc analysis (Bonferroni) was conducted as an additional subgroup analysis to analyze differences between groups.
2.2. Clinical Investigation
The clinical evaluation was conducted using the Tanita MC780MA body bio-electrical impedance analyzer from Germany, and the results were evaluated using medical software. BIA body analyzers are approved devices by the World Public Health Nutrition Association (WPHNA) that provide highly accurate measurements of body composition. The margin of error for these devices is 0.1 kg.
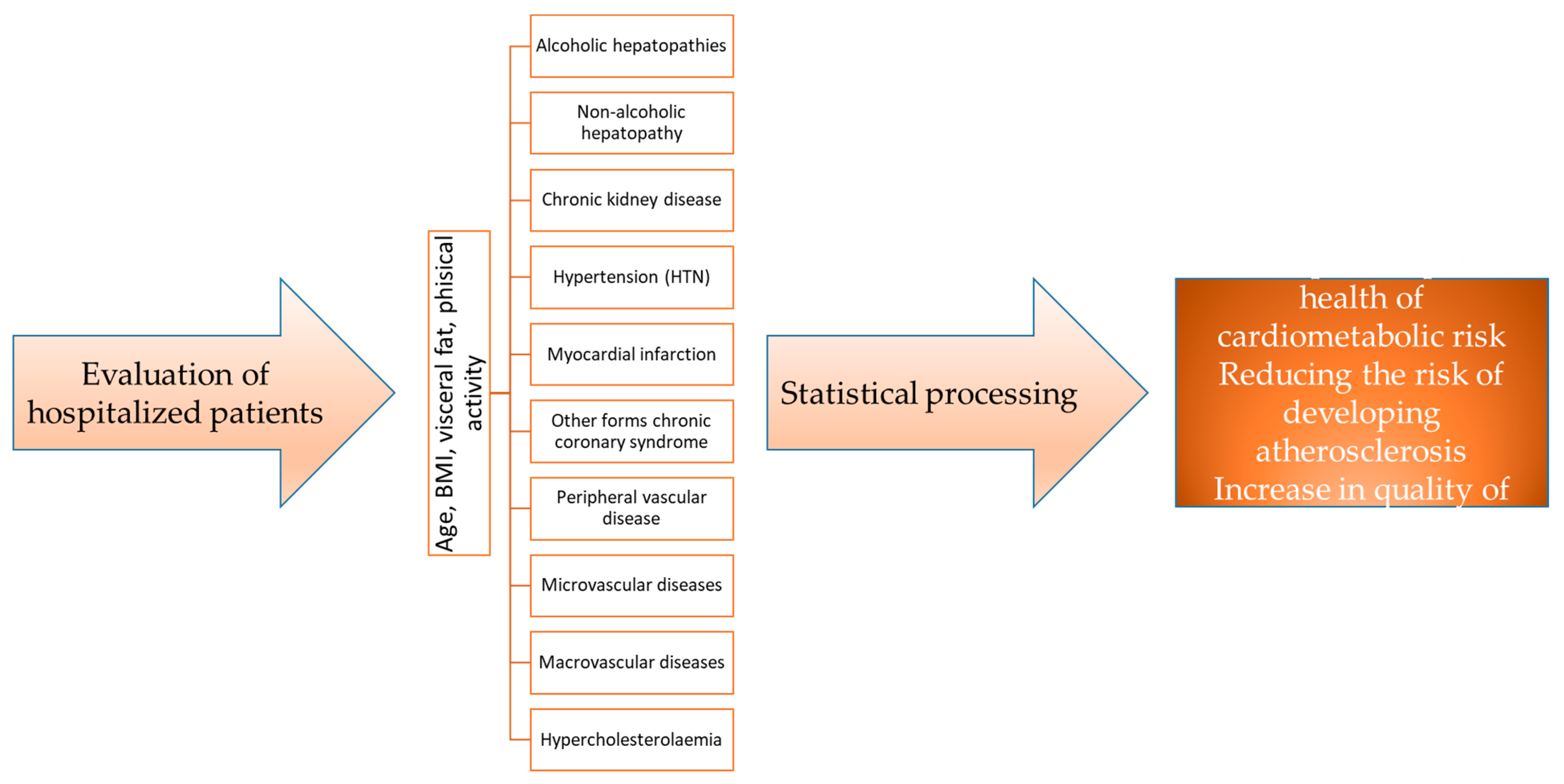
We monitored variations in four distinct groups based on sex, age, rural/urban environment, and clinical parameters such as BMI, visceral fat, hypercholesterolemia, hypertriglyceridemia, HTN, myocardial infarction, congestive heart failure, other forms of chronic coronary syndrome, peripheral vascular disease, macrovascular disease, microvascular disease, and chronic kidney disease, as well as alcoholic and non-alcoholic liver diseases, presented in the research flowchart (Figure 1).
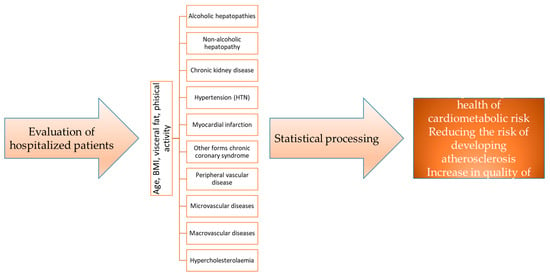
Figure 1.
Research flowchart.
3. Results
3.1. Demographic Description
A total of 578 patients were included in the research study, divided over six years. The average age of the participants ranged from 44.50 ± 12.37 to 50.71 ± 17.67, as shown in Table 1. The distribution of patients based on sex is as follows: 239 men (41.3%) and 339 women (58.7%). There were no significant differences observed in the distribution of patients by sex over the six-year period. The breakdown of patients by sex for each year is as follows: in 2017, there were 65 (14.1%) men and 96 (20.8%) women; in 2018, there were 31 (6.7%) men and 47 (10.2%) women; in 2019, there were 43 (9.3%) men and 66 (14.3%) women; and in the final year of the study (2020), there were 52 (11.3%) men and 62 (13.4%) women. Overall, the proportion of women in the cohort was 17.4% higher than that of men, with significant differences observed in each year of the study.

Table 1.
Demographic Description of the Cohort by Year of Study.
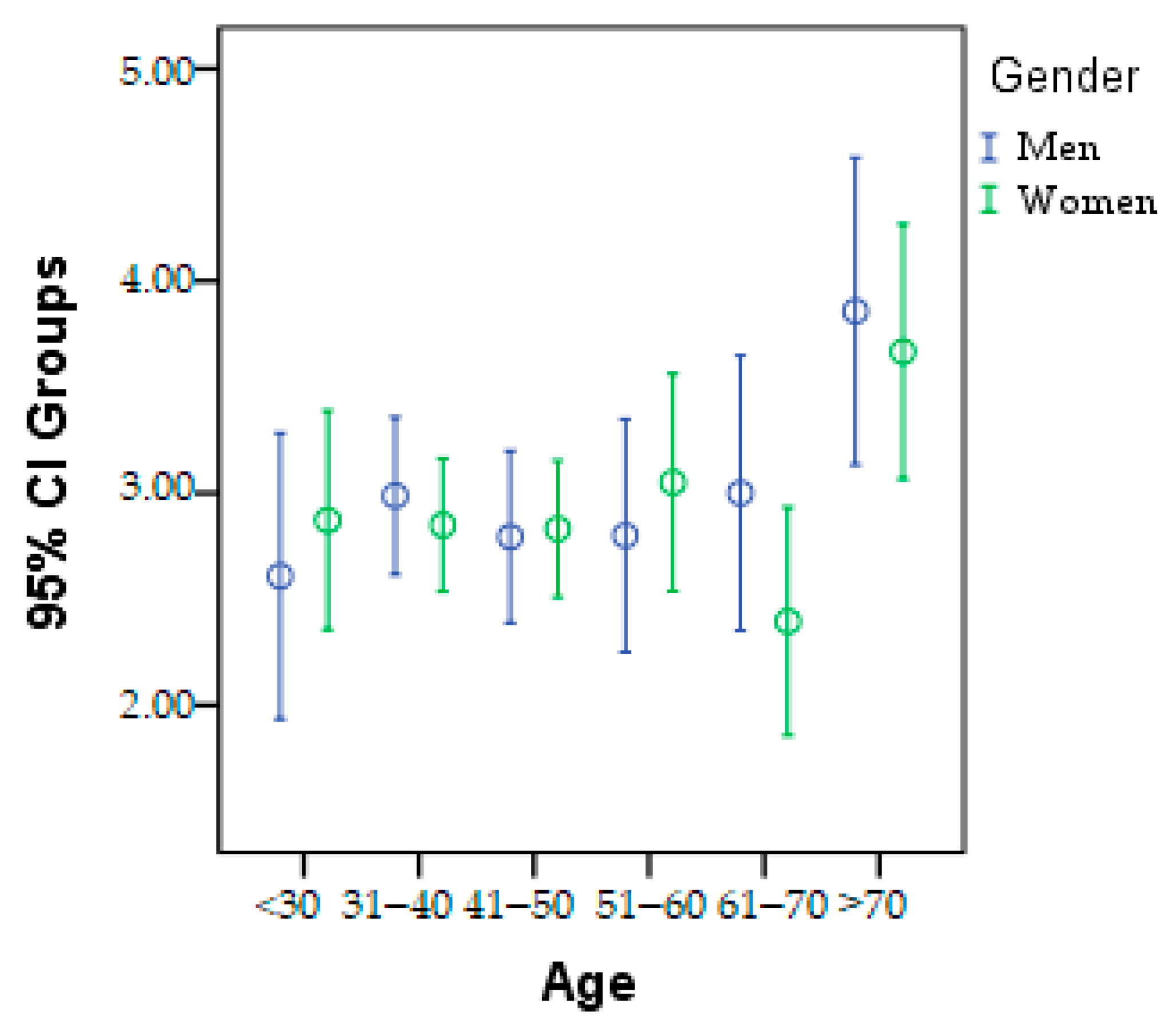
The patients were divided into six age categories to track epidemiological changes in the research parameters. This division is presented in Figure 2, categorized by gender. It can be observed that in the <30 years, 41–50 years, and 51–60 years age groups, there is a higher prevalence of women with metabolic problems. On the other hand, in the 31–40 years, 61–70 years, and >70 years age groups, men have a higher incidence.
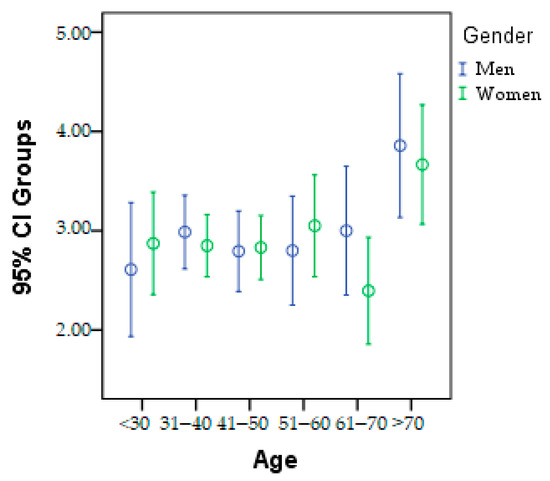
Figure 2.
Graphic presentation of the age category according to gender.
Among the research parameters, the following findings were observed:
Elevated cholesterol levels were observed in 375 individuals, with the highest prevalence of hypercholesterolemia in 2020 (17.53%).
High triglyceride levels were recorded in 341 individuals in 2022, with the highest frequency at 20.27%.
HTN was present in 449 patients, with the lowest incidence in 2018 (15.21%).
Myocardial infarction was observed in 29 individuals, with the highest incidence of 24.82% in 2021.
Congestive heart failure was diagnosed in 147 out of 578 patients, with an incidence of 22.69% in 2019.
Other forms of chronic coronary syndrome were reported in a total of 239 individuals, with the highest incidence of 31.38% in 2021.
Peripheral vascular disease was identified in 164 patients, with the highest prevalence of 42.01% in 2021. Microvascular disease was present in 144 patients, while macrovascular disease was reported in only 74 patients.
Chronic kidney disease was observed in 361 individuals, accounting for 24.19% of the total, with the highest incidence in 2020.
Alcoholic liver diseases were present in 77.9% of patients, with the highest prevalence in 2022 (20.02%). Non-alcoholic liver diseases accounted for 46.7% of the total, with the highest incidence of 27.05% in 2022.
Statistical analysis using the Chi-square technique revealed significant differences (Table 2) within each age category as follows:

Table 2.
Chi-square statistical analysis of the research parameters’ progression in each age group.
In individuals under the age of 30, non-alcoholic liver diseases (46.8%) and other forms of chronic coronary syndrome (32.3%) showed different trends over the 6-year study period.
Among individuals aged 31–40 years, there were no significant differences in myocardial infarction (9.4%) and the incidence of hypercholesterolemia (71.3%) over the 6-year study period. However, other parameters showed statistically significant differences (p < 0.05).
In the 41–50 age group, the incidence of non-alcoholic liver diseases (52.6%) differed significantly, and no significant differences in HTN (75.7%), myocardial infarction (1.3%), macrovascular disease (23%), and hypercholesterolemia (73%) were found over the 6-year study period.
Among individuals aged 51–60 years, significant differences were observed in the incidence of myocardial infarction (4.3%), and peripheral vascular disease (37.1%), and no significant differences in alcoholic liver diseases (75.7%) or in HTN (87.1%), over the 6-year study period.
In the 61–70 age group, there were no significant differences in the incidence of heart diseases, HTN (81.5%), myocardial infarction (6.2%), or alcoholic liver diseases (76.9%) across each year of the study.
For individuals over 70 years of age, liver diseases exhibited statistically significant variations depending on the year of study (p < 0.05), as did HTN (87.5%).
Congestive heart failure and triglyceride levels did not show statistically significant changes over the 6-year study period across the different age categories. Non-alcoholic liver disease and chronic coronary syndrome exhibited significant differences within each age category across all study years.
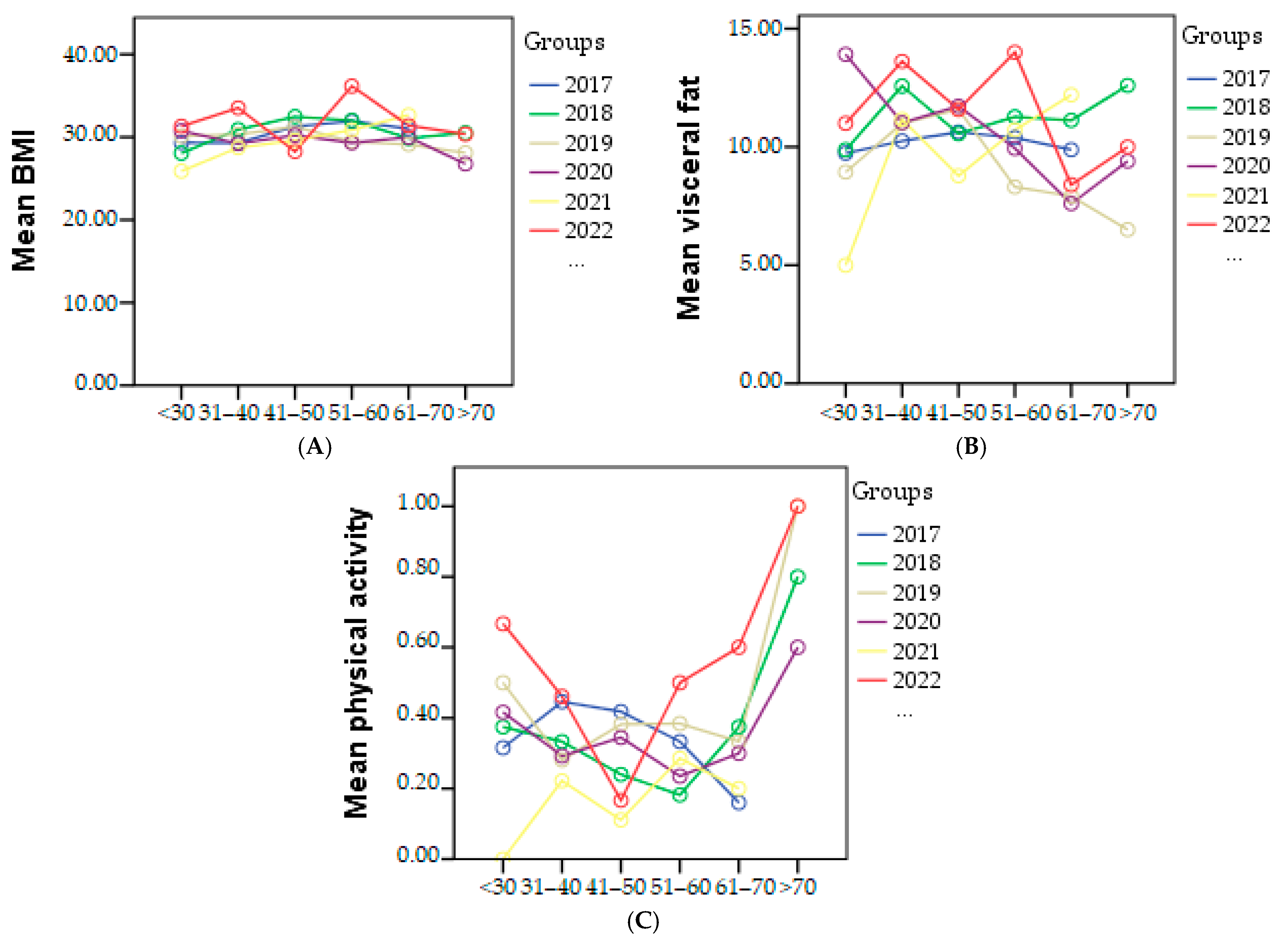
BMI reached its highest value in 2022, with a value of 31.32, indicating grade 1 obesity. The lowest BMI was observed in 2020, with a value of 29.38, which falls within the overweight category.
Visceral fat measurements showed an increase from 10.01 in 2020 to 11.54 in 2022.
Regarding physical activity, in 2021, the lowest percentage of individuals engaging in sports was 8.91% out of a total of 217 people. The highest percentage was recorded in 2022, with 24.85% of individuals participating in sports. The graphical representation of BMI, visceral fat, and physical activity in each age category based on the year of study is presented in Figure 3.
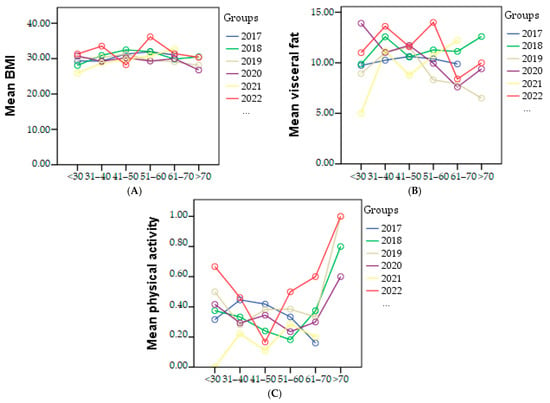
Figure 3.
Graphical representation of mean of the BMI (A), visceral fat (B), and physical activity (C) in each age category based on the year of study.
The study examined the progression of cardiometabolic diseases in relation to physical activity. The cohort was monitored, and it was observed that only 217 individuals (37.54%) engaged in some form of physical exercise, while 361 individuals remained sedentary. Sedentary lifestyle was considered a risk factor for cardiometabolic diseases and formed the basis of the evaluation. Table 3 presents the significant differences between physical activity and the research parameters.

Table 3.
Progression of cardiometabolic parameters based on physical activity.
The assessment of physical activity in the study was qualitative rather than quantitative. Therefore, any physical activity performed by the participants that exceeded a minimum of 30 min at least once a week was assigned a value of “1”. The absence of physical activity was denoted by a value of “0”.
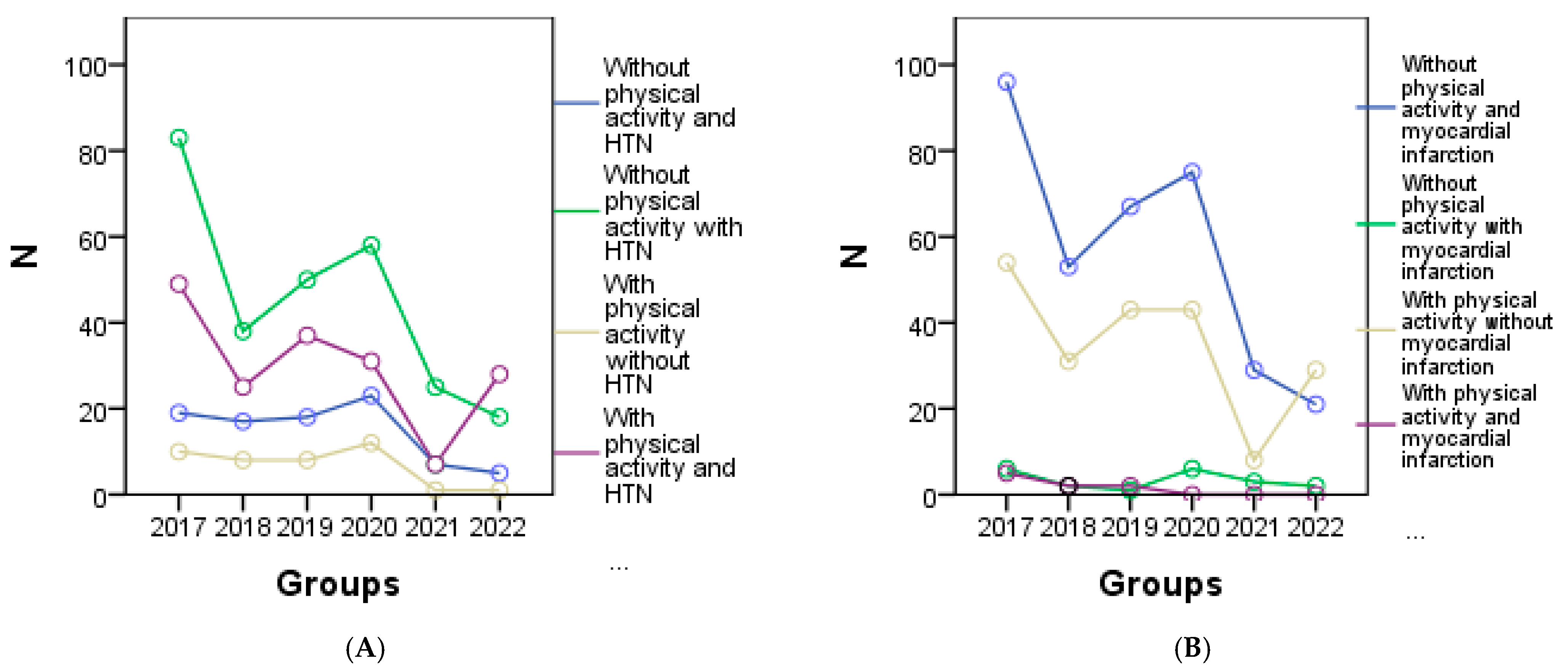
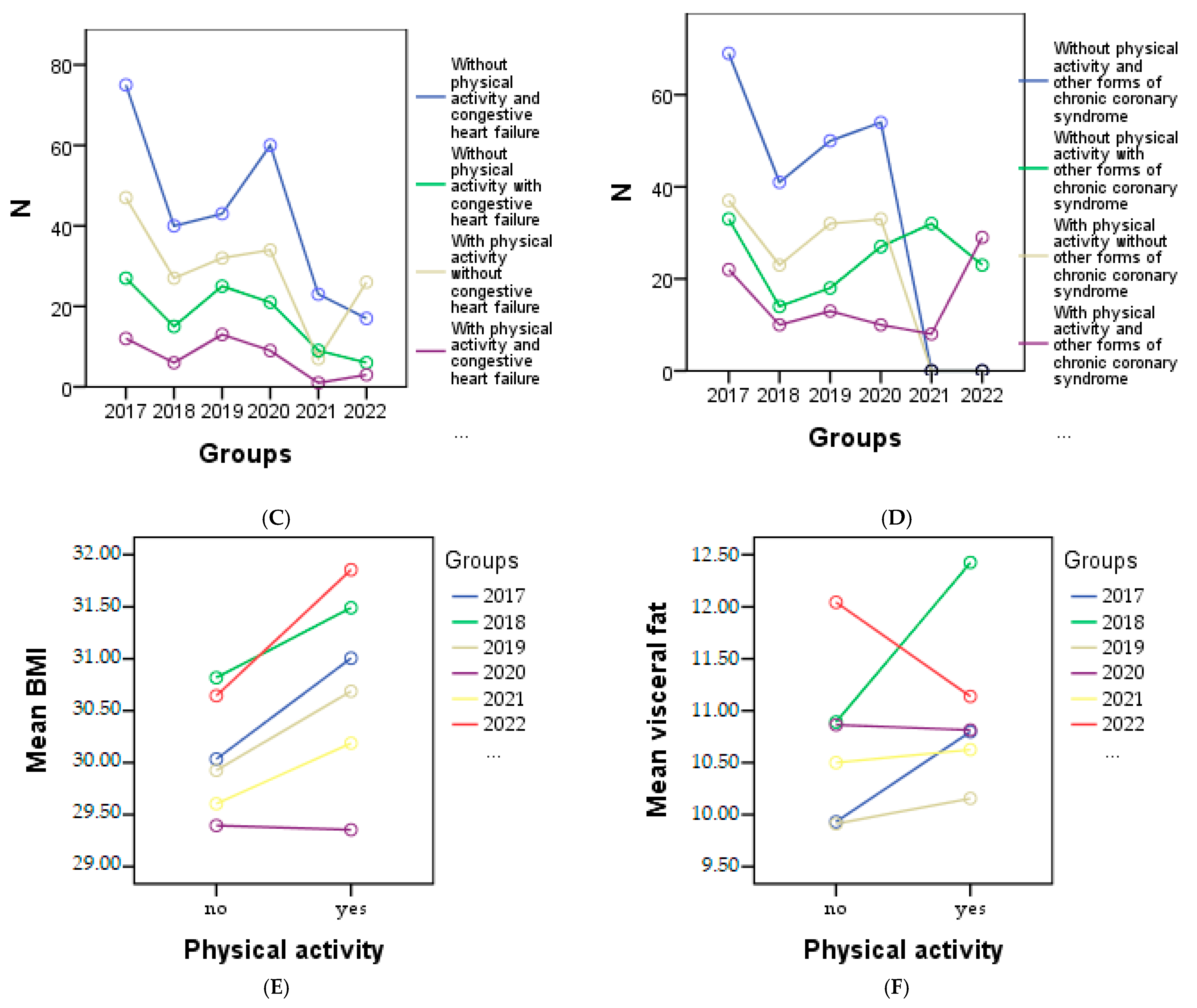
From the graphical representation in Figure 4, the cardiometabolic parameters can be observed for each year of the study according to physical activity. It is evident that a decrease in physical activity was observed among patients with HTN in 2020 (Figure 4A), patients with myocardial infarction in 2020 (Figure 4B), 2021, and 2022, and among all patients with congestive heart failure (Figure 4C). Regarding patients with other forms of chronic coronary syndrome, an improvement in physical activity was noted in 2017, 2018, and 2019 (Figure 4D), while in other cases a decrease or complete lack of physical activity was observed. Additionally, Figure 4E shows a correlation between increasing BMI and physical activity, indicating that higher BMI values are associated with higher physical activity levels. However, a significant decrease in visceral fat was only observed in 2022 (Figure 4F).
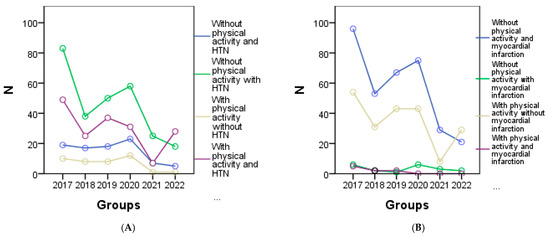
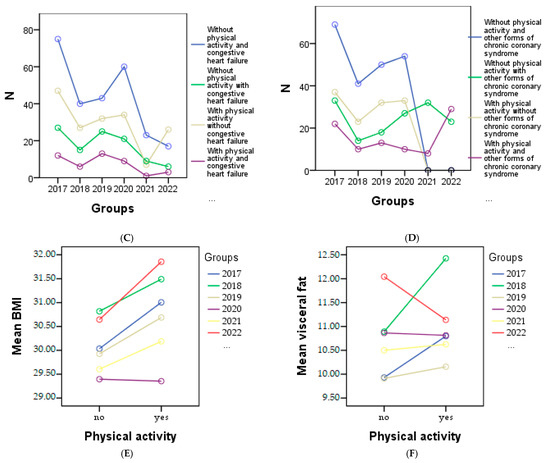
Figure 4.
Graphical representation of the evolution of cardiometabolic parameters according to physical activity in each year of the study is as follows: (A) depicts individuals with or without HTN, and myocardial infarction (B), (C) shows individuals with congestive heart failure, and the (D) representing other forms of chronic coronary syndrome. (E) represents the evolution of BMI, and (F) the evolution of visceral fat for individuals with or without physical activity.
Based on the non-parametric statistical evaluation using the Chi-square technique, a statistically significant difference was observed among patients under the age of 30 in terms of BMI (mean 29.78 ± 4.36). Furthermore, significant differences were found in patients aged between 31 and 40 years with congestive heart failure between the groups (26.5% of people) with physical activity (34.8% of people) and those without physical activity.
In patients aged over 60 (both between 61 and 70 years and over 70 years), with myocardial infarction (4 people between 61 and 70 years old, and 3 people over 70 years old) and congestive heart failure (16.9% between 61 and 70 years old, and 6.2% in people over 70 years old), significant differences were identified between the groups with physical activity (37.5%) and those without physical activity (62.5%). These findings are presented in detail in Table 4, which provides the Chi-square statistical description of the evolution of research parameters according to physical activity.

Table 4.
Chi-square statistical description of the evolution of research parameters according to physical activity.
3.2. Correlations
Spearman’s correlation was used to analyze the relationships between all research parameters, presented in Table 5. A statistically significant, inversely proportional relationship was observed between congestive heart failure and age, as well as between age and visceral fat. This is evident from the negative value of the Spearman’s coefficient and the significance value of p < 0.05. The findings indicate that congestive heart failure tends to increase as age decreases, suggesting a higher risk for young patients. Similarly, a negative correlation was observed between age and visceral fat, indicating that higher levels of visceral fat were found in younger age groups. Additionally, in the current study, a positive correlation was found between physical activity and age, indicating that as individuals age, there is a greater emphasis on incorporating physical activity into a healthy lifestyle.

Table 5.
The Spearman’s correlation results, including the research parameters and their relationship with age categories, along with the corresponding statistical significance.
In the age group of 31–40, the incidence of congestive heart failure was found to be the highest. This observation can be attributed to the elevated levels of visceral fat, which is a significant cardiometabolic risk factor. Furthermore, individuals in this age group had a high BMI within the overweight category, with an average of 29.90 ± 4.26.
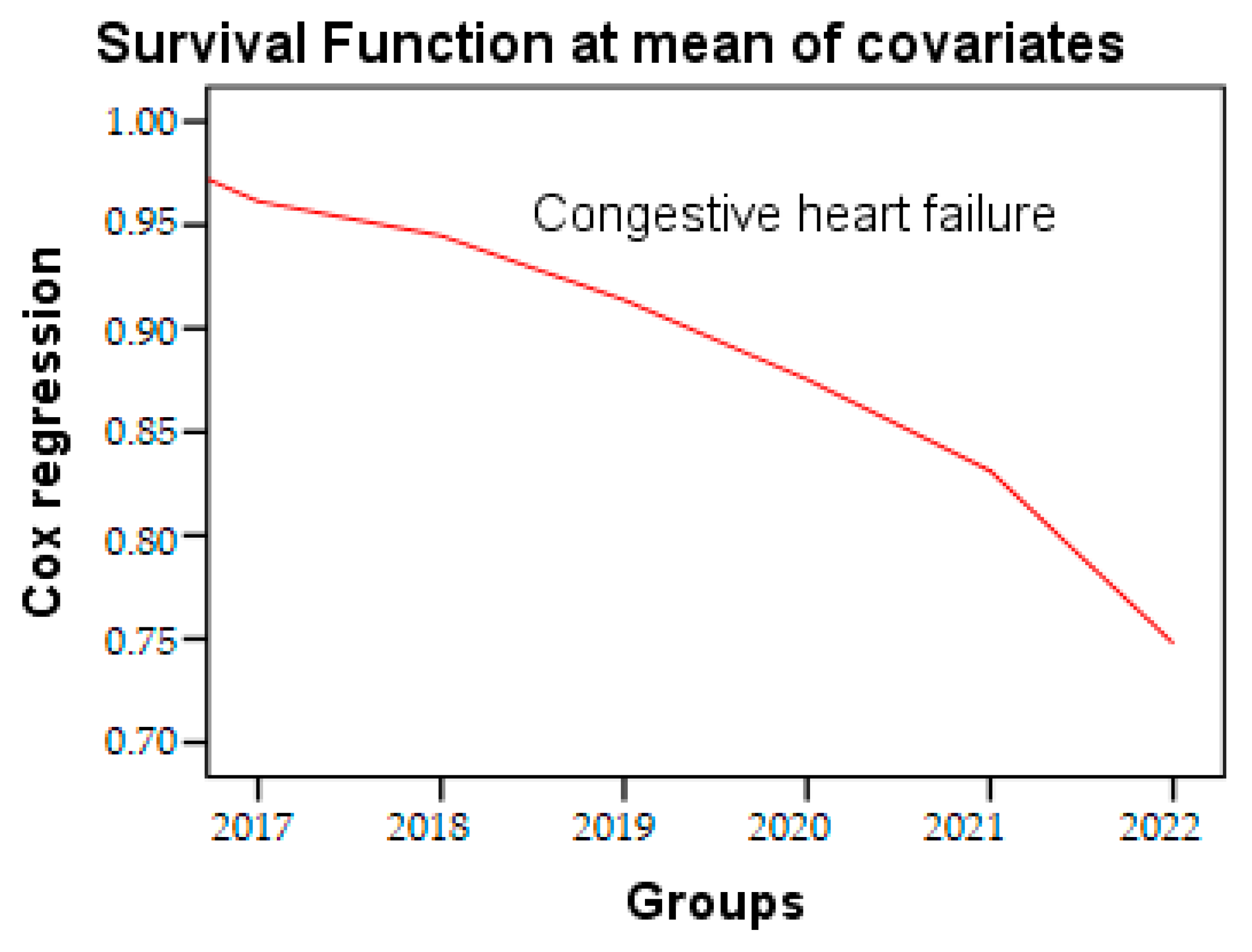
In the Cox regression analysis, a decrease of 1.7% in age was associated with a 6% increase in the risk rate of congestive heart failure (ExpB: 1.77, 95% CI: 1.27–2.47, p = 0.001). This increase was observed independently of several potential confounders, such as age, sex, hypertension (HTN), and dyslipidemias. The time to event parameter used in our test was one year. Survival analysis, analyzed by Cox regression, is a powerful analytical approach commonly employed in medical research to evaluate the risk of congestive heart failure over the course of the study period (Figure 5), presented in Table 6.
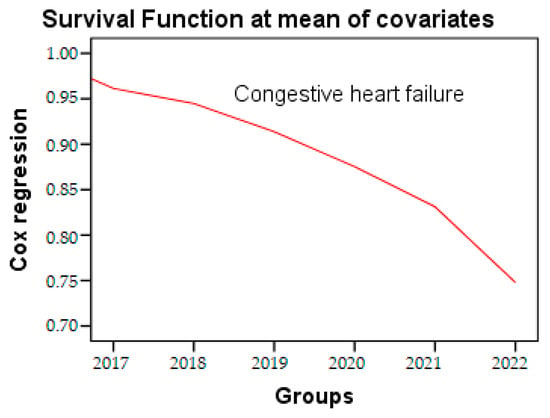
Figure 5.
Cox regression of congestive heart failure during the research period.

Table 6.
Cox regression of parameters during the research period.
4. Discussion
Obesity is widely recognized as a significant health risk [27,28]. Numerous studies have explored the relationship between weight status and metabolic diseases and their therapeutical approaches [29,30]. Several studies have highlighted the direct link between obesity and metabolic syndrome [19,31,32], type 2 diabetes (T2DM) [8,33], with dysbiosis [34,35], and stress [36,37]. In our current study, we observed a higher prevalence of overweight and obesity among the patients. Metabolic syndrome involves an imbalance in carbohydrate metabolism [38], which is closely associated with increased visceral fat accumulation [39]. Previous research conducted in 2010 [24], highlighted the significant cardiovascular risk associated with increased visceral fat, while a study in 2018 [40] demonstrated a similar relationship with fat mass [41]. Since visceral fat is a major risk factor in metabolic diseases, this correlation helps explain the progression of cardiometabolic disorders. The assessment of visceral fat using a BIA analyzer has been compared with computed tomography (CT) assessment in several studies, and both methods have shown relevance in obtaining accurate results [42]. In our study, we observed a high level of visceral fat, reaching a value of 10, which is associated with an increased risk not only of cardiovascular diseases [10], but also of joint disorders [43], obstructive sleep apnea [44], and kidney diseases [45].
Atherogenic dyslipidemia has been extensively studied in Europe [5], China [46], and India. Our findings deviate significantly from the European studies, as we observed a dyslipidemia prevalence of 75% among the subjects from Arad, whereas Europe reported a prevalence of 55%. Hypercholesterolemia and hypertriglyceridemia, as components of dyslipidemia, are frequently evaluated together in most studies. In terms of gender, our study found a higher incidence of dyslipidemia in women compared to men, which is consistent with the findings reported in the literature. However, the observed differences did not reach statistical significance. Over the course of our study, we observed an increasing trend in the prevalence of dyslipidemia, particularly marked in hypercholesterolemia and hypertriglyceridemia. An epidemiological study from Malaysia [47], published in 2022, reported an incidence of 23.55% for hypercholesterolemia and 27.08% for hypertriglyceridemia, which are higher than our highest values of 17.53% for hypercholesterolemia and 20.27% for hypertriglyceridemia. This result can be explained by the fact that developing countries place greater emphasis on reducing health risks rather than treating complications, and this perspective is also reflected in the importance of conducting such studies in Romania in order to intervene and mitigate the risks.
Daily fat intake plays a crucial role in modifying the lipid profile [48]. Plasma saturated fat content is associated with dietary saturated fat, although endogenous sources can also contribute to its presence [49]. Dietary intake represents the primary source of Omega-6 and Omega-3 fatty acids [50].
Non-alcoholic liver diseases, which are detailed in numerous published papers [51,52], along with metabolic syndrome, affect approximately one third of the American population [53]. For patients over 60 years old, it is generally recommended to engage in adapted physical activity to reduce the risk of obesity, diabetes, and cardiovascular diseases. Lifestyle modifications, including exercise and diet, remain the mainstay of prevention [13,53,54,55], Therefore, it is crucial to closely monitor and recognize the imminent risk. Our study revealed a higher prevalence of non-alcoholic liver diseases, reaching 42% among the population in Arad.
In 2022, Talle conducted a study to track the prevalence of acute heart diseases [56]. The study recorded an incidence of 22.6% for acute myocardial infarction in Greece and 25% in Italy. These values are within the range of our data, with our recorded incidence of 24.82% being the highest in 2021. Additionally, the study also found a prevalence of 31.38% for chronic coronary syndrome.
The predisposition of the population to accumulate excess fat raises several concerns due to the increased incidence and prevalence of metabolic syndrome and T2DM. The alarming rise in the risk of developing peripheral vascular disease, whether microvascular or macrovascular, in individuals with these syndromes has necessitated closer monitoring. In addition to epidemiological assessments, we have also integrated interview directives [55]. The incidence of peripheral vascular disease increases with higher BMI, fat mass, and visceral fat accumulation [57]. Swenty’s study in 2020 focused on identifying the specific types of vascular diseases to establish the most effective strategies. A 2023 study tracked the prevalence of peripheral vascular disease among different age groups and found a significant increase with advancing age [58]. Over the course of the 4-year study, peripheral vascular disease was observed in 28% of the patients, indicating a significant rise in cardiovascular and circulatory risks. Furthermore, the incidence of peripheral vascular disease in 2021, at 42.01%, was higher than the average for the age group studied, suggesting a higher occurrence of the condition at younger ages. This finding highlights the importance of early detection and intervention in managing peripheral vascular disease and mitigating its associated risks.
Progressive renal damage has been observed in patients with dyslipidemia in a study conducted in 2017 [59]. Chronic kidney disease, a component of metabolic syndrome [60,61], is considered a consequence of the proinflammatory process [62]. It has also been correlated with coronary insufficiency [63], atherosclerosis [64], and cardiovascular mortality [65]. Some studies have identified chronic kidney disease as a risk factor primarily affecting men [66], while others have shown risks for both men and women [67]. In our study, we did not observe statistically significant differences between the sexes, although there were more women registered. Chronic kidney disease is strongly correlated with atherogenic dyslipidemia [68], and aligns with the pathophysiology of metabolic syndrome, posing a significant cardiovascular risk [69].
In 2022, Riazi published a study on non-alcoholic fatty liver disease (NAFLD) in 17 countries [70]. The study concluded a worldwide prevalence of 32.4% for NAFLD. In our own study, we observed a higher incidence of 27.05% in 2022, which was the highest recorded. The total prevalence in our study was 46.7%. This can be explained by the fact that the population included in our study may have already had existing risk factors for NAFLD, leading to a higher incidence compared to the global average. These findings emphasize the importance of understanding and addressing the risk factors associated with NAFLD to effectively manage and prevent the disease.
Zheng et al. observed that higher levels of metabolic syndrome (MS) were associated with a hypertensive pattern [71]. In a study conducted in 2015, an increase in the prevalence of HTN and metabolic syndrome was observed, with a revealed correlation between the two conditions in regression analysis. Our study also noted a higher prevalence of obesity, as reflected by high BMI, among the patients, with 75% of them presenting HTN, 76% with alcoholic liver disease, and 62% with chronic kidney disease.
One limitation may stem from the observational nature of the study, where researchers solely observed and analyzed the data without intervening or controlling for variables. Another limitation could arise from confounding factors, referring to unaccounted factors that may alter the established correlations. Previous measurement or recording errors can also be considered as limitations since we lack access to verification or correction. Lastly, a significant limitation may pertain to external validity, meaning the study’s findings may not be applicable to diverse areas and regions.
5. Conclusions
Based on the epidemiological study conducted on cardiometabolic risk, several conclusions can be drawn. Firstly, in 2022, the highest levels of visceral fat and BMI were recorded, indicating an increased risk of metabolic disorders. Additionally, there was a notable incidence of hypertriglyceridemia among the participants.
The practice of sports showed variations across different age categories. In 2022, older individuals engaged in sports activities to a greater extent. However, there was a significant discrepancy among age groups, particularly with the 41–50-years-old category showing the lowest participation in sports. This finding can be attributed to the high levels of stress experienced by individuals in this age group, leading to limited time for physical activity. Moreover, this age category exhibited a higher incidence of vascular and kidney diseases, further contributing to the cardiometabolic risk. It can be concluded that individuals between 41 and 50 years old face significant challenges in engaging in physical exercise, thereby increasing their susceptibility to cardiometabolic disorders and heightened mortality risk, especially considering the presence of oxidative stress. This study highlights the importance of promoting a healthy and active lifestyle, particularly among the elderly population, where a growing trend towards adopting such practices is observed.
Author Contributions
Conceptualization, L.M. and T.C.G.; methodology, T.C.G.; software, T.C.G.; validation, T.C.G. and L.M.; formal analysis, T.C.G.; investigation, L.M.; resources, L.M.; data curation, L.M.; writing—original draft preparation, L.M.; writing—review and editing, T.C.G.; visualization, T.C.G.; supervision, T.C.G.; project administration, T.C.G.; funding acquisition, T.C.G. All authors have read and agreed to the published version of the manuscript.
Funding
The APC was funded by University of Oradea, Oradea, Romania. The funding number is 410068.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board (or Ethics Committee) of the University of Oradea (protocol code CEFMF/1 from 31 January 2023 and date of approval).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Written informed consent has been obtained from the patient(s) to publish this paper.
Data Availability Statement
All the data processed in this article are part of the research for a doctoral thesis, being archived in the aesthetic medical office, where the interventions were performed.
Acknowledgments
The authors would like to thank to the University of Oradea, for supporting the payment of the invoice, through an internal project.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Duchatelle, V.; Delhasse, S.; Karpuz, B.; Tapponnier, M.; Alexe, R.; Sierro, C.; Girod, G. Is global warming responsible for an increase in the incidence of stress cardiomyopathy (takostubo cardiomyopathy)? Arch. Cardiovasc. Dis. Suppl. 2018, 10, 33–34. [Google Scholar] [CrossRef]
- Artham, S.M.; Lavie, C.J.; Schutter, A.D.; Ventura, H.O.; Milani, R.V. Obesity, Age, and Cardiac Risk. Curr. Cardiovasc. Risk Rep. 2011, 5, 128–137. [Google Scholar] [CrossRef]
- Abu-Assi, E.; López-López, A.; González-Salvado, V.; Redondo-Diéguez, A.; Peña-Gil, C.; Bouzas-Cruz, N.; Raposeiras-Roubín, S.; Abumuaileq, R.R.-Y.; García-Acuña, J.M.; González-Juanatey, J.R. The Risk of Cardiovascular Events After an Acute Coronary Event Remains High, Especially During the First Year, Despite Revascularization. Rev. Esp. Cardiol. Engl. Ed. 2016, 69, 11–18. [Google Scholar] [CrossRef]
- Balakumar, P.; Maung-U, K.; Jagadeesh, G. Prevalence and prevention of cardiovascular disease and diabetes mellitus. Pharmacol. Res. 2016, 113, 600–609. [Google Scholar] [CrossRef]
- Halcox, J.P.; Banegas, J.R.; Roy, C.; Dallongeville, J.; De Backer, G.; Guallar, E.; Perk, J.; Hajage, D.; Henriksson, K.M.; Borghi, C. Prevalence and treatment of atherogenic dyslipidemia in the primary prevention of cardiovascular disease in Europe: EURIKA, a cross-sectional observational study. BMC Cardiovasc. Disord. 2017, 17, 160. [Google Scholar] [CrossRef]
- de Toro-Martín, J.; Arsenault, B.J.; Després, J.P.; Vohl, M.C. Precision Nutrition: A Review of Personalized Nutritional Approaches for the Prevention and Management of Metabolic Syndrome. Nutrients 2017, 9, 913. [Google Scholar] [CrossRef] [PubMed]
- Arora, A.; Behl, T.; Sehgal, A.; Singh, S.; Sharma, N.; Bhatia, S.; Sobarzo-Sanchez, E.; Bungau, S. Unravelling the involvement of gut microbiota in type 2 diabetes mellitus. Life Sci. 2021, 273, 119311. [Google Scholar] [CrossRef] [PubMed]
- Welin, L.; Adlerberth, A.; Caidahl, K.; Eriksson, H.; Hansson, P.O.; Johansson, S.; Rosengren, A.; Svärdsudd, K.; Welin, C.; Wilhelmsen, L. Prevalence of cardiovascular risk factors and the metabolic syndrome in middle-aged men and women in Gothenburg, Sweden. BMC Public Health 2008, 8, 403. [Google Scholar] [CrossRef] [PubMed]
- Bozkurt, B.; Aguilar, D.; Deswal, A.; Dunbar, S.B.; Francis, G.S.; Horwich, T.; Jessup, M.; Kosiborod, M.; Pritchett, A.M.; Ramasubbu, K.; et al. Contributory Risk and Management of Comorbidities of Hypertension, Obesity, Diabetes Mellitus, Hyperlipidemia, and Metabolic Syndrome in Chronic Heart Failure: A Scientific Statement From the American Heart Association. Circulation 2016, 134, e535–e578. [Google Scholar] [CrossRef]
- Plana, N.; Ibarretxe, D.; Cabré, A.; Ruiz, E.; Masana, L. Prevalence of atherogenic dyslipidemia in primary care patients at moderate-very high risk of cardiovascular disease. Cardiovascular risk perception. Clin. Investig. Arterioscler. 2014, 26, 274–284. [Google Scholar] [CrossRef]
- Piepoli, M.F.; Hoes, A.W.; Agewall, S.; Albus, C.; Brotons, C.; Catapano, A.L.; Cooney, M.T.; Corrà, U.; Cosyns, B.; Deaton, C.; et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts) Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur. Heart J. 2016, 37, 2315–2381. [Google Scholar] [CrossRef] [PubMed]
- Myers, J.; Kokkinos, P.; Nyelin, E. Physical Activity, Cardiorespiratory Fitness, and the Metabolic Syndrome. Nutrients 2019, 11, 1652. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Martínez, P.; Mikhailidis, D.P.; Athyros, V.G.; Bullo, M.; Couture, P.; Covas, M.I.; de Koning, L.; Delgado-Lista, J.; Díaz-López, A.; Drevon, C.A.; et al. Lifestyle recommendations for the prevention and management of metabolic syndrome: An international panel recommendation. Nutr. Rev. 2017, 75, 307–326. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Zhang, Y.; Li, M.; Wu, J.H.; Mai, L.; Li, J.; Yang, Y.; Hu, Y.; Huang, Y. Association between prediabetes and risk of all cause mortality and cardiovascular disease: Updated meta-analysis. BMJ 2020, 370, m2297. [Google Scholar] [CrossRef]
- Chang, H.-J.; Lin, F.Y.; Lee, S.-E.; Andreini, D.; Bax, J.; Cademartiri, F.; Chinnaiyan, K.; Chow, B.J.; Conte, E.; Cury, R.C.; et al. Coronary atherosclerotic precursors of acute coronary syndromes. J. Am. Coll. Cardiol. 2018, 71, 2511–2522. [Google Scholar] [CrossRef]
- Shah, S.J.; Lam, C.S.P.; Svedlund, S.; Saraste, A.; Hage, C.; Tan, R.S.; Beussink-Nelson, L.; Faxén, U.L.; Fermer, M.L.; Broberg, M.A.; et al. Prevalence and correlates of coronary microvascular dysfunction in heart failure with preserved ejection fraction: PROMIS-HFpEF. Eur. Heart J. 2018, 39, 3439–3450. [Google Scholar] [CrossRef]
- Brenner, B.M.; Cooper, M.E.; de Zeeuw, D.; Keane, W.F.; Mitch, W.E.; Parving, H.H.; Remuzzi, G.; Snapinn, S.M.; Zhang, Z.; Shahinfar, S. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N. Engl. J. Med. 2001, 345, 861–869. [Google Scholar] [CrossRef]
- Galassi, A.; Reynolds, K.; He, J. Metabolic syndrome and risk of cardiovascular disease: A meta-analysis. Am. J. Med. 2006, 119, 812–819. [Google Scholar] [CrossRef]
- Ghitea, T.; Vlad, S.; Birle, D.; Tit, D.; Lazar, L.; Nistor-Cseppento, C.; Behl, T.; Bungau, S. The influence of diet therapeutic intervention on the sarcopenic index of patients with metabolic syndrome. Acta Endocrinol. 2020, 16, 470. [Google Scholar] [CrossRef]
- Dey, R.; Rajappa, M.; Parameswaran, S.; Revathy, G. Hypomagnesemia and atherogenic dyslipidemia in chronic kidney disease: Surrogate markers for increased cardiovascular risk. Clin. Exp. Nephrol. 2015, 19, 1054–1061. [Google Scholar] [CrossRef]
- Dongiovanni, P.; Meroni, M.; Baselli, G.; Mancina, R.M.; Ruscica, M.; Longo, M.; Rametta, R.; Cespiati, A.; Pelusi, S.; Ferri, N.; et al. PCSK7 gene variation bridges atherogenic dyslipidemia with hepatic inflammation in NAFLD patients. J. Lipid Res. 2019, 60, 1144–1153. [Google Scholar] [CrossRef] [PubMed]
- Niechciał, E.; Marcovecchio, M.L. Treatment of cardiometabolic risk factors in patients with type 1 diabetes. Curr. Opin. Pediatr. 2020, 32, 589–594. [Google Scholar] [CrossRef]
- Vatopoulou, A.; Dionelli, M.E.; Papanikolaou, A.; Grover, S. Cardiometabolic Risk Factors in Adolescents with Polycystic Ovary Syndrome. Curr. Pharm. Des. 2021, 27, 3808–3811. [Google Scholar] [CrossRef] [PubMed]
- Amato, M.C.; Giordano, C.; Galia, M.; Criscimanna, A.; Vitabile, S.; Midiri, M.; Galluzzo, A.; AlkaMeSy Study Group. Visceral Adiposity Index: A reliable indicator of visceral fat function associated with cardiometabolic risk. Diabetes Care 2010, 33, 920–922. [Google Scholar] [CrossRef]
- Henzen, C. Risk factors for arteriosclerosis. Praxis 2001, 90, 91–95. [Google Scholar] [PubMed]
- World Medical Association. World Medical Association Declaration of Helsinki. Ethical principles for medical research involving human subjects. Bull. World Health Organ. 2001, 79, 373. [Google Scholar]
- Popa, A.R.; Fratila, O.; Rus, M.; Corb Aron, R.A.; Vesa, C.M.; Pantis, C.; Diaconu, C.C.; Bratu, O.; Bungau, S.; Nemeth, S. Risk factors for adiposity in the urban population and influence on the prevalence of overweight and obesity. Exp. Ther. Med. 2020, 20, 129–133. [Google Scholar] [CrossRef]
- Timmis, A.; Townsend, N.; Gale, C.P.; Torbica, A.; Lettino, M.; Petersen, S.E.; Mossialos, E.A.; Maggioni, A.P.; Kazakiewicz, D.; May, H.T.; et al. European Society of Cardiology: Cardiovascular Disease Statistics 2019. Eur. Heart J. 2020, 41, 12–85. [Google Scholar] [CrossRef]
- Iorga, R.A.; Bacalbasa, N.; Carsote, M.; Bratu, O.G.; Stanescu, A.M.A.; Bungau, S.; Pantis, C.; Diaconu, C.C. Metabolic and cardiovascular benefits of GLP-1 agonists, besides the hypoglycemic effect. Exp. Ther. Med. 2020, 20, 2396–2400. [Google Scholar] [CrossRef]
- Vesa, C.M.; Bungau, S.G. Novel Molecules in Diabetes Mellitus, Dyslipidemia and Cardiovascular Disease. Int. J. Mol. Sci. 2023, 24, 4029. [Google Scholar] [CrossRef]
- Barness, L.A.; Opitz, J.M.; Gilbert-Barness, E. Obesity: Genetic, molecular, and environmental aspects. Am. J. Med. Genet. Part A 2007, 143A, 3016–3034. [Google Scholar] [CrossRef]
- Ghitea, T.C. Correlation of Periodontal Bacteria with Chronic Inflammation Present in Patients with Metabolic Syndrome. Biomedicines 2021, 9, 1709. [Google Scholar] [CrossRef] [PubMed]
- Diabetes and Obesity. Available online: https://www.diabetes.co.uk/diabetes-and-obesity.html (accessed on 2 March 2021).
- Sircana, A.; Framarin, L.; Leone, N.; Berrutti, M.; Castellino, F.; Parente, R.; De Michieli, F.; Paschetta, E.; Musso, G. Altered Gut Microbiota in Type 2 Diabetes: Just a Coincidence? Curr. Diabetes Rep. 2018, 18, 98. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; Jung, S.R.; Lee, S.Y.; Lee, N.K.; Paik, H.D.; Lim, S.I. Lactobacillus plantarum Strain Ln4 Attenuates Diet-Induced Obesity, Insulin Resistance, and Changes in Hepatic mRNA Levels Associated with Glucose and Lipid Metabolism. Nutrients 2018, 10, 643. [Google Scholar] [CrossRef]
- Reiterer, M.; Rajan, M.; Gómez-Banoy, N.; Lau, J.D.; Gomez-Escobar, L.G.; Gilani, A.; Alvarez-Mulett, S.; Sholle, E.T.; Chandar, V.; Bram, Y.; et al. Hyperglycemia in Acute COVID-19 is Characterized by Adipose Tissue Dysfunction and Insulin Resistance. medRxiv 2021, 33, 2174–2188. [Google Scholar] [CrossRef]
- Kyrou, I.; Randeva, H.S.; Tsigos, C. Stress, insulin resistance, and type 2 diabetes. In Stress: Neuroendocrinology and Neurobiology, Handbook of Stress; Academic Press: Samford Valley, Australia, 2020; pp. 351–358. [Google Scholar] [CrossRef]
- Dashty, M. A quick look at biochemistry: Carbohydrate metabolism. Clin. Biochem. 2013, 46, 1339–1352. [Google Scholar] [CrossRef]
- Yang, Y.; Ding, L.; Zou, X.; Shen, Y.; Hu, D.; Hu, X.; Li, Z.; Kamel, I.R. Visceral Adiposity and High Intramuscular Fat Deposition Independently Predict Critical Illness in Patients with SARS-CoV-2. Obesity 2020, 28, 2040–2048. [Google Scholar] [CrossRef]
- Després, J.-P.; Lemieux, I.; Bergeron, J.; Pibarot, P.; Mathieu, P.; Larose, E.; Rodés-Cabau, J.; Bertrand, O.F.; Poirier, P. Abdominal Obesity and the Metabolic Syndrome: Contribution to Global Cardiometabolic Risk. Arter. Thromb. Vasc. Biol. 2008, 28, 1039–1049. [Google Scholar] [CrossRef]
- Shuster, A.; Patlas, M.; Pinthus, J.H.; Mourtzakis, M. The clinical importance of visceral adiposity: A critical review of methods for visceral adipose tissue analysis. Br. J. Radiol. 2012, 85, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Omura-Ohata, Y.; Son, C.; Makino, H.; Koezuka, R.; Tochiya, M.; Tamanaha, T.; Kishimoto, I.; Hosoda, K. Efficacy of visceral fat estimation by dual bioelectrical impedance analysis in detecting cardiovascular risk factors in patients with type 2 diabetes. Cardiovasc. Diabetol. 2019, 18, 137. [Google Scholar] [CrossRef]
- Francisco, V.; Ruiz-Fernández, C.; Pino, J.; Mera, A.; Gonzalez-Gay, M.A.; Gómez, R.; Lago, F.; Mobasheri, A.; Gualillo, O. Adipokines: Linking metabolic syndrome, the immune system, and arthritic diseases. Biochem. Pharmacol. 2019, 165, 196–206. [Google Scholar] [CrossRef]
- Ghitea, T.C.; Aleya, L.; Tit, D.M.; Behl, T.; Stoicescu, M.; Sava, C.; Iovan, C.; El-Kharoubi, A.; Uivarosan, D.; Pallag, A.; et al. Influence of diet and sport on the risk of sleep apnea in patients with metabolic syndrome associated with hypothyroidism—A 4-year survey. Environ. Sci. Pollut. Res. 2021, 29, 23158–23168. [Google Scholar] [CrossRef]
- Akarken, I.; Tarhan, H.; Ekin, R.G.; Çakmak, Ö.; Koç, G.; Ilbey, Y.Ö.; Zorlu, F. Visceral obesity: A new risk factor for stone disease. Can. Urol. Assoc. J. 2015, 9, E795–E799. [Google Scholar] [CrossRef]
- Pan, L.; Yang, Z.; Wu, Y.; Yin, R.X.; Liao, Y.; Wang, J.; Gao, B.; Zhang, L. The prevalence, awareness, treatment and control of dyslipidemia among adults in China. Atherosclerosis 2016, 248, 2–9. [Google Scholar] [CrossRef]
- Baharudin, N.; Mohamed-Yassin, M.S.; Daher, A.M.; Ramli, A.S.; Khan, N.M.N.; Abdul-Razak, S. Prevalence and factors associated with lipid-lowering medications use for primary and secondary prevention of cardiovascular diseases among Malaysians: The REDISCOVER study. BMC Public Health 2022, 22, 228. [Google Scholar] [CrossRef]
- Udenigwe, C.C.; Rouvinen-Watt, K. The role of food peptides in lipid metabolism during dyslipidemia and associated health conditions. Int. J. Mol. Sci. 2015, 16, 9303–9313. [Google Scholar] [CrossRef]
- Skoczyńska, M.; Świerkot, J. The role of diet in rheumatoid arthritis. Reumatologia 2018, 56, 259–267. [Google Scholar] [CrossRef]
- Rocha, D.; Caldas, A.; Oliveira, L.; Bressan, J.; Hermsdorff, H. Saturated fatty acids trigger TLR4-mediated inflammatory response. Atherosclerosis 2016, 244, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Bungau, S.; Behl, T.; Tit, D.M.; Banica, F.; Bratu, O.G.; Diaconu, C.C.; Nistor-Cseppento, C.D.; Bustea, C.; Aron, R.A.C.; Vesa, C.M. Interactions between leptin and insulin resistance in patients with prediabetes, with and without NAFLD. Exp. Ther. Med. 2020, 20, 197. [Google Scholar] [CrossRef] [PubMed]
- Vesa, C.M.; Behl, T.; Nemeth, S.; Bratu, O.G.; Diaconu, C.C.; Moleriu, R.D.; Negrut, N.; Zaha, D.C.; Bustea, C.; Radu, F.I.; et al. Prediction of NAFLD occurrence in prediabetes patients. Exp. Ther. Med. 2020, 20, 190. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Touros, A.; Kim, W.R. Nonalcoholic Fatty Liver Disease and Metabolic Syndrome. Clin. Liver Dis. 2018, 22, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Mudaliar, S.; Henry, R.R.; Sanyal, A.J.; Morrow, L.; Marschall, H.U.; Kipnes, M.; Adorini, L.; Sciacca, C.I.; Clopton, P.; Castelloe, E.; et al. Efficacy and safety of the farnesoid X receptor agonist obeticholic acid in patients with type 2 diabetes and nonalcoholic fatty liver disease. Gastroenterology 2013, 145, 574–582.e1. [Google Scholar] [CrossRef] [PubMed]
- Frisbee, J.C.; Goodwill, A.G.; Frisbee, S.J.; Butcher, J.T.; Wu, F.; Chantler, P.D. Microvascular perfusion heterogeneity contributes to peripheral vascular disease in metabolic syndrome. J. Physiol. 2016, 594, 2233–2243. [Google Scholar] [CrossRef] [PubMed]
- Talle, M.A.; Ngarande, E.; Doubell, A.F.; Herbst, P.G. Cardiac Complications of Hypertensive Emergency: Classification, Diagnosis and Management Challenges. J. Cardiovasc. Dev. Dis. 2022, 9, 276. [Google Scholar] [CrossRef]
- Swenty, C.F.; Hall, M. Peripheral Vascular Disease. Home Healthc. Now 2020, 38, 294–301. [Google Scholar] [CrossRef]
- Horváth, L.; Németh, N.; Fehér, G.; Kívés, Z.; Endrei, D.; Boncz, I. Epidemiology of Peripheral Artery Disease: Narrative Review. Life 2022, 12, 1041. [Google Scholar] [CrossRef]
- Hager, M.R.; Narla, A.D.; Tannock, L.R. Dyslipidemia in patients with chronic kidney disease. Rev. Endocr. Metab. Disord. 2017, 18, 29–40. [Google Scholar] [CrossRef]
- Moisi, M.I.; Rus, M.; Bungau, S.; Zaha, D.C.; Uivarosan, D.; Fratila, O.; Tit, D.M.; Endres, L.; Nistor-Cseppento, D.C.; Popescu, M.I. Acute Coronary Syndromes in Chronic Kidney Disease: Clinical and Therapeutic Characteristics. Medicina 2020, 56, 118. [Google Scholar] [CrossRef]
- Moisi, M.I.; Bungau, S.G.; Vesa, C.M.; Diaconu, C.C.; Behl, T.; Stoicescu, M.; Toma, M.M.; Bustea, C.; Sava, C.; Popescu, M.I. Framing Cause-Effect Relationship of Acute Coronary Syndrome in Patients with Chronic Kidney Disease. Diagnostics 2021, 11, 1518. [Google Scholar] [CrossRef]
- Abdollahi-Roodsaz, S.; Abramson, S.B.; Scher, J.U. The metabolic role of the gut microbiota in health and rheumatic disease: Mechanisms and interventions. Nat. Rev. Rheumatol. 2016, 12, 446–455. [Google Scholar] [CrossRef]
- Budoff, M.J.; Rader, D.J.; Reilly, M.P.; Mohler, E.R., 3rd; Lash, J.; Yang, W.; Rosen, L.; Glenn, M.; Teal, V.; Feldman, H.I. Relationship of estimated GFR and coronary artery calcification in the CRIC (Chronic Renal Insufficiency Cohort) Study. Am. J. Kidney Dis. 2011, 58, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.-Y.; Chiu, J.-J. Atherosclerosis and flow: Roles of epigenetic modulation in vascular endothelium. J. Biomed. Sci. 2019, 26, 56. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.H.; Zhong, W.F.; Lv, Y.B.; Kraus, V.B.; Gao, X.; Chen, P.L.; Huang, Q.M.; Ni, J.D.; Shi, X.M.; Mao, C.; et al. Associations of plasma high-sensitivity C-reactive protein concentrations with all-cause and cause-specific mortality among middle-aged and elderly individuals. Immun. Ageing 2019, 16, 28. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.A.; Gaffo, A. Gout epidemiology and comorbidities. Semin. Arthritis Rheum. 2020, 50, S11–S16. [Google Scholar] [CrossRef] [PubMed]
- Xie, K.; Bao, L.; Jiang, X.; Ye, Z.; Bing, J.; Dong, Y.; Gao, D.; Ji, X.; Jiang, T.; Li, J.; et al. The association of metabolic syndrome components and chronic kidney disease in patients with hypertension. Lipids Health Dis. 2019, 18, 229. [Google Scholar] [CrossRef] [PubMed]
- Ferro, C.J.; Mark, P.B.; Kanbay, M.; Sarafidis, P.; Heine, G.H.; Rossignol, P.; Massy, Z.A.; Mallamaci, F.; Valdivielso, J.M.; Malyszko, J.; et al. Lipid management in patients with chronic kidney disease. Nat. Rev. Nephrol. 2018, 14, 727–749. [Google Scholar] [CrossRef]
- McCracken, E.; Monaghan, M.; Sreenivasan, S. Pathophysiology of the metabolic syndrome. Clin. Dermatol. 2018, 36, 14–20. [Google Scholar] [CrossRef]
- Riazi, K.; Azhari, H.; Charette, J.H.; Underwood, F.E.; King, J.A.; Afshar, E.E.; Swain, M.G.; Congly, S.E.; Kaplan, G.G.; Shaheen, A.A. The prevalence and incidence of NAFLD worldwide: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2022, 7, 851–861. [Google Scholar] [CrossRef]
- Zheng, Y.; Yu, B.; Alexander, D.; Mosley, T.H.; Heiss, G.; Nettleton, J.A.; Boerwinkle, E. Metabolomics and incident hypertension among blacks: The atherosclerosis risk in communities study. Hypertension 2013, 62, 398–403. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).