Abstract
Delirium, an acute neuropsychiatric disorder characterized by a disturbance of attention and awareness, is often superimposed on dementia with its progressive cognitive decline. Despite the high frequency and clinical relevance of this condition, often called delirium-superimposed dementia (DSD), little is known about possible triggers. In this study using the GePsy-B databank, we investigated the impact of the underlying brain disorder and multimorbidity (MM) on DSD. MM was measured by CIRS and the number of ICD-10 diagnoses. Dementia was diagnosed by CDR, and delirium by DSM IV TR criteria. A total of 218 patients were diagnosed with DSD and these were compared to 105 patients with only dementia, 46 with only delirium, and 197 patients suffering from other psychiatric diseases, mainly depression. No significant differences between groups were found concerning CIRS scores. Based on CT scans, DSD cases were grouped into those with cerebral atrophy only (probably pure neurodegenerative), with brain infarction, or with white matter hyperintensities (WMH), but no between-group differences regarding the MM indices could be found. Regression analysis only revealed age and dementia stage as influencing factors. Conclusion: Our results suggest that neither MM nor morphologic changes in the brain are predisposing factors for DSD.
1. Introduction
In the hospital and in nursing homes, the care of demented individuals is often complicated by delirium [1]. The prevalence of delirium superimposed on dementia (DSD) ranges from 22% to 89% of hospitalized and community populations with dementia [2,3]. There is a close interaction between delirium and dementia since dementia is a risk factor for delirium, and delirium is a known risk factor for newly developed dementia or worsening of dementia [4,5,6,7,8]. However, there is considerable overlap in the psychopathological symptomatology of both neurocognitive disorders complicating the comparison of published data on demented individuals [4,5,9].
The characteristic features of delirium are acute onset and fluctuating symptoms, including inattention, level of consciousness, and cognitive disturbances [10,11,12]. Its etiology remains poorly understood. Delirium may occur in response to a variety of noxious insults, including medications, infections, and surgery [13,14]. Individuals with preexisting diminished cognitive status may be most vulnerable to developing delirium.
Dementia is mostly caused by slowly progressive neurodegenerative disorders, suchas Alzheimer’s disease (AD). Most studies on DSD primarily investigated AD patients. AD is characterized by impaired memory and loss of ability to function independently. However, dementia and delirium is also frequently induced by vascular disorders [15,16]. The rate of DSD seems to be higher in vascular dementia than in AD [17].
However, there are different potential pathogenic pathways for vascular dementia [18]; among them, cerebral small vessel disease (cSVD) is the most frequent [19]. Evidence from neuroimaging studies shows that delirium may mainly be associated with white matter hyperintensities (WMH) [20,21]. WMH are supposed to be comprehensive expressions of the pathological changes of cSVD [19,22].
Delirium is considered to have a complex multifactorial etiology. Many predisposing and precipitating factors are known [13,14]. An important predisposing factor may be the underlying brain disorder, which in vivo can best be investigated by neuroimaging [16,21]. Despite the high frequency and clinical relevance of delirium-superimposed dementia, little is known about possible triggers. Since multimorbidity (MM) presents similar to delirium, a clear age dependence [23], the question raises whether MM may contribute to the development of DSD.
This study will assess the hypotheses: first, different kinds of underlying brain damage, such as neurodegenerative diseases, infarction, or WMH contribute to the severity of DSD. Second, DSD patients suffer from more pronounced MM than patients with dementia alone or delirium alone.
2. Materials and Methods
The complete data set of the GePsy-B study (medical records of 941 neuropsychiatric inpatients >65 years) was used for this study, described in detail in [24]. The GePsy-B study was performed in a hospital which provides acute psychiatric service for about 250,000 inhabitants of an eastern district in Berlin, Germany. Two wards are specialized for the care of neuropsychiatric patients. Only first admissions during the study period were considered.
2.1. Subjects
A total of 274 subjects had to be excluded because of incomplete data. This incompleteness was mostly caused by difficulties in performing CT scans as the patients showed agitation or other behavior disturbances. In 56 cases, CT scans revealed cerebral tumors or chronic subdural hematoma, etc. (Figure 1). A total of 45 were not included because of recent surgical operations, probable diagnoses of Parkinson’s disease, Lewy body disease, or withdrawal from psychotropic substances as a possible cause of DSD.
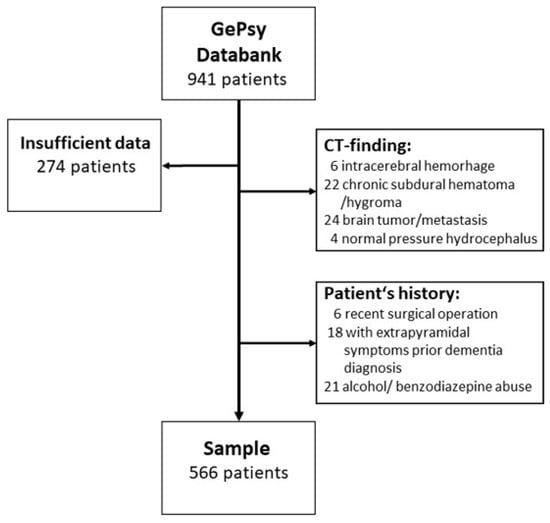
Figure 1.
Flow diagram showing the selection of study patients.
Thus, our sample consists of 566 patients, mean age: 79.5 + 7.7 years. On average, the 382 female patients were significantly older than the 184 male patients (80.1 + 7.8 vs. 78.1 + 7.4 years) (U-test p = 0.004).
2.2. Measures
Delirium and dementia, according to DSM IV TR criteria [25], were diagnosed by a senior psychiatrist. Delirium was assessed at admission, then daily during the first week by using the Confusion Assessment Method (CAM-S) [26] in the validated German translation [27] and the Delirium Rating Scale-Revised-98 (DRS-R-98) [28] 2001). The maximum values were calculated. The stage of dementia was assessed by the Clinical Dementia Rating (CDR) [29].
Multimorbidity was measured retrospectively by the Cumulative Illness Rating Scale (CIRS) [30] as described in detail by Salvi et al., 2008 [31]. To isolate any effect of dementia or delirium, the neuropsychiatric item of the CIRS was eliminated, leaving 13 systems to comprise a total score, referred to henceforth as CIRS-13. The count of ICD-10 diagnoses was a simple and universally available comorbidity index. Furthermore, the nutritional status was evaluated by using the Mini Nutritional Assessment short-form (MNA-SF) [32].
As outcome variables, the days of inpatient treatment, the mortality rate, the Charlson Comorbidity Index (CCI) [33], and the Clinical Global Impressions (CGI) Scale [34] at discharge were used. The CCI was primarily developed to prospectively assess mortality risks due to comorbid conditions.
Since MRI scans were conducted only in a small portion of the patients, the findings of cerebral CT scans were used. All other data, such as routine laboratory values, length of stay, etc. were gathered from the anonymized medical records. The cut-off values of laboratory parameters are given in Tables 4 and 5.
2.3. Statistical Analysis
The statistical calculations were conducted with the IBM-SPSS program, version 27.0G. One-step ANOVA was computed. The homogeneity of variances was tested by Levene’s test. In the case of inhomogeneity, nonparametric tests, such as the Mann–Whitney U-test or the Kruskal–Wallis H-test, were performed. Correlations were calculated as Pearson’s r. The level of significance was given in the text as * p < 0.05, ** p < 0.01, and *** p < 0.001.
3. Results
A three steps analysis was performed. In the first step, we calculated the data of all patients. The degree of dementia as measured by CDR showed a clear age dependence: no dementia (CDR < 1) 243 cases, mean age 76.8 + 7.6 years; mild dementia (CDR = 1) 103, mean age 81.2 + 7.5 years; moderate dementia (CDR = 2) 120 mean age: 81.5 + 6.3 years; and severe dementia (CDR = 3) 100, mean age: 81.8 + 7.8 years (H-test 79.9 ***).
The number of typical delirium symptoms as assessed by CAM-S or DSR-98 scores showed a significant age dependence (r = 0.195 *** or resp. 0.201 ***), as did the numbers of somatic ICD-10 diagnoses (r = 0.175 **). The CAM-S and DSR-98 scores correlated with the number of ICD-10 diagnoses (r = 0.099 * or resp. 0.119 **) and strongly with the severity of dementia measured by CDR (r = 0.530 *** or resp. 0.581 ***).
In 160 subjects, CT-findings consisted of atrophy only (age: 77.2 + 7.0 years); in 82 subjects (mostly non-territorial) infarcts could be seen (age: 81.2 + 7.6 years) and 324 patients had WMH (age: 80.2 + 7.8 years) (ANOVA df 2 F10.8 ***).
In the second step, the sample was divided into four groups according to their current psychopathological symptomatology: dementia alone, delirium alone, DSD, and other diagnoses (i.e., depression). The comparison of the groups (Table 1) again showed significant age differences. The pure dementia cases were about three years older than those of the other groups (ANOVA df 3, F = 20.8 ***). Gender was evenly distributed. 218 of 323 dementia cases (67.5%) had DSD. Only 46 of 264 delirium cases (17.4%) had no dementia symptomatology. The four groups did not differ regarding the extent of infarcts or WMH.

Table 1.
Sample.
The comparison of predisposing factors for delirium in patients with dementia alone and DSD reveals some significant differences (Table 2). The DSD patients were younger than those with pure dementia (ANOVA df 1 F = 5.97 *) and were more severely demented as assessed by CDR (ANOVA df 1 F = 44.9 ***). Furthermore, their nutritional status is lower (ANOVA df = 1 F = 18.3 ***). No significant differences are found for disabilities (visual impairment, hearing loss, or restricted mobility) or CT findings. Logistic regression, including the variables number of ICD-10 diagnoses, CIRS-13, and MNA score, is performed controlling for age, gender, and dementia stage. Only the dementia stage and younger age significantly contributed to the differentiation between pure dementia and DSD. CIRS-13 data measuring MM revealed no differences between neurocognitive disorders (Table 1) nor in DSD patients with probably different underlying brain damage (Table 3).

Table 2.
Comparison of predisposing factors for delirium in patients with dementia alone and DSD.

Table 3.
Predisposing factors for delirium in DSD patients with different underlying brain damage.
In the third step, only the 218 cases classified as DSD were considered with regard to the predisposing factors. They were subdivided into three groups according to the CT scans: no changes or atrophy only (=probably neurodegenerative dementia), with infarcts (=probably vascular dementia), and those with WMH (=probably cerebral microangiopathy). As shown in Table 3, those cases without signs of a vascular etiology were significantly younger (ANOVA df 2 F 4.0 *). Those with infarcts had a trend to more severe MM as estimated by the CIRS-13 (n.s.) and a higher rate of visual impairment (Chi2 8.99 *) and restricted mobility (n.s). The cases with WMH suffered from less severe dementia as estimated by CDR (ANOVA F = 3.25 *)
In Table 4, the potentially precipitating factors in demented patients with and without delirium were presented. A lot of laboratory parameters indicating, i.e., diabetes mellitus (Hba1c) anemia, etc., showed slightly elevated odds ratios, but the corresponding confidence intervals started at values clearly below one. Thus, no elevated risk can be concluded. Only a current infection can be estimated as a trigger of DSD. The number of administered psychotropic drugs and of other medications at admission had no effect on the development of DSD.

Table 4.
Potentially precipitating factors in demented patients with and without delirium.
Considering the potentially underlying morphological brain changes of DSD (Table 5), the comparison revealed no differences in the tested laboratory parameters. The number of applied drugs at admission showed inconsistencies that are hard to interpret. While the number of administered psychotropic drugs was significantly higher in DSD patients without vascular signs (ANOVA F = 6.10 **), the number of other medications was highest in DSD patients showing WMH (ANOVA F = 6.10 **).

Table 5.
Potentially precipitating factors in DSD-patients.
To evaluate the outcome, the CCI score, the length of stay, and the general status at discharge as assessed by CGI were evaluated. The calculation revealed no differences between the DSD groups besides a higher CCI score in DSD patients with brain infarction (ANOVA F = 3.88 *).
4. Discussion
Dementia and delirium have been conceptualized as distinct and mutually exclusive conditions according to common guidelines [10,11]. Both diagnostic guidelines state that dementia should not be diagnosed in the face of delirium. Furthermore, delirium should not be diagnosed when symptoms can be “better accounted for by a pre-existing, established, or evolving dementia”. Distinguishing the two diagnoses in the clinical setting can be difficult, however, even for experienced clinicians [6].
Delirium can be thought of as “acute brain failure”, a multifactorial syndrome analogous to acute heart failure. The onset of delirium involves a complex interaction between the patient’s baseline vulnerability (predisposing factors) and precipitating factors or noxious insults. The precipitating factors vary due to the investigated sample of patients [13,14]. Most studies concerning DSD were conducted in perioperative or ICU patients [35]. Only a few studies reported data collected from general medicine [21,27,36,37]. In this investigation, based on the GePsy B databank, all patients admitted after stroke, surgical operation, or suffering from probable Parkinson’s or Lewy body disease or alcohol- or medication-withdrawal were excluded. Thus, the investigated sample differs in important aspects from most of the previous studies concerning DSD.
Moreover, the comparison of the published studies is hindered by partly different scales to assess delirium, i.e., CAM-S [26] or DRS-98 [28]. However, these measures have a high degree of correlation in hospitalized patients [37,38], also with the DSM-IV or ICD-10 criteria [37]. Previous studies suggested that DSD patients may have a more severe course of delirium [36]. However, in our sample, delirium severity does not differ between delirium alone and DSD, nor between the DSD groups subdivided according to CT scans. The CDR scores even show less severe dementia stages in DSD patients with WMH. These results suggest that the psychopathology of DSD may be influenced by the probably underlying type of brain disorder.
Since many predisposing factors of delirium are known [13,14], it seemed obvious that physical MM may be an essential contributor to delirium and DSD. However, our CIRS-13 data measuring MM revealed no differences between delirium and DSD. The number of ICD-10 diagnoses used as an indicator for MM showed no significant differences. In summary, MM indices do not provide a simple way to predict the development of DSD.
A systematic review and meta-analysis supported the existence of a relationship between frailty and delirium, although there was notable methodological heterogeneity between studies [39]. In our study corresponding, some parameters, such as malnutrition and physical disability, were assessed. The frequency of visual impairment, hearing loss, and restricted mobility showed no significant differences between dementia and DSD. These results agree with those in very old patients (>80 years [37]. However, impaired sight and restricted mobility seemed to be more frequent in DSD patients with brain infarction.
Many risk factors for delirium are known. However, there is a disparity concerning the investigated sample of patients (i.e., ICU, after surgical operation) [13]. The data of our sample of hospitalized medical patients suggest that infections and malnutrition may influence the development of DSD, but that MM does not. The influence of WMH ([18], this study) has to be confirmed by further studies.
While previous studies revealed that DSD patients may have poorer outcomes, including increased length of hospitalization, poorer functional status, and higher mortality [36,40], our data showed no such poorer outcome, not even in the CCI or CGI at discharge.
5. Limitations
There are several limitations to this study. First, our study was based on the retrospective analysis of clinical data of patients consecutively admitted to only one hospital.
Moreover, there may be selection bias. About 20% of our patients were transferred from medical or surgical wards or the ICU of our hospital. About 30% were primarily admitted to the central emergency room and transferred to our ward after excluding life-threatening diseases. This is likely to have reduced the number of patients with delirium, in whose case medical comorbidities could be identified.
Second, there is a possible selection bias as many cases had to be excluded because in compliance and agitation made CT scans impossible. It can be assumed that these cases were more mentally impaired.
As no established biomarkers for delirium exist, DSD is diagnosed clinically. The differentiation of the DSD patients according to CT scans into atrophy only, with infarction and with WMH, does not allow a well-grounded pathophysiological categorization. However, all patients showing in CT scans brain damage (such as a tumor or subdural hematoma, etc.) and all patients with a history of extrapyramidal symptoms prior to dementia were excluded from analysis in order to focus on typical cerebral changes associated with dementia since the frequency of delirium and its relation to dementia in Parkinson’s disease [41] or Lewy body disease remains unclear [42]. CSF levels of β-42 amyloid, total tau, and phospho-tau protein and apolipoprotein E were not routinely measured, such as in some other studies [43], but studies of these AD biomarkers in delirium revealed mixed results [44].
In summary, despite the limitations, we think that the chosen categorization of DSD according to morphological findings in CT scans might help to elucidate the underlying brain disorders of DSD.
6. Conclusions
Our results suggest that MM and the underlying brain disorder are not predisposing factors of DSD in patients living with dementia. Younger age and higher dementia stage affect the development of DSD. The only potentially precipitating factor for delirium in demented patients found in this study is current infections.
Author Contributions
Conceptualization, T.W.; methodology, T.W.; validation, T.W. and K.J.; formal analysis, T.W. and K.J.; investigation, T.W.; resources, T.W.; data curation, T.W.; writing—original draft preparation, T.W.; writing—review and editing, T.W. and K.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki for studies involving humans. Ethical review and approval were waived for this study since only anonymized routine clinical data were used. The permittance to use these data was included in informed consent protocol. No examination was conducted for this study.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study or, respectively, their legal custodian.
Data Availability Statement
No new data were created or analyzed in this study. The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Morandi, A.; Lucchi, E.; Turco, R.; Morghen, S.; Guerini, F.; Santi, R.; Gentile, S.; Meagher, D.; Voyer, P.; Fick, D.M.; et al. Delirium superimposed on dementia: A quantitative and qualitative evaluation of informal caregivers and health care staff experience. J. Psychosom. Res. 2015, 79, 272–280. [Google Scholar] [CrossRef]
- Fick, D.M.; Agostini, J.V.; Inouye, S.K. Delirium superimposed on dementia: A systematic review. J. Am. Geriatr. Soc. 2002, 50, 1723–1732. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.Y.C.; Rodrigues, N.G.; Klainin-Yobas, P.; Haugan, G.; Wu, X.V. Prevalence, risk factors, and Iipact of delirium on hospitalized older adults with dementia: A systematic review and meta-analysis. J. Am. Med. Dir. Assoc. 2022, 23, 23–32. [Google Scholar] [CrossRef]
- Fong, T.G.; Inouye, S.K. The inter-relationship between delirium and dementia: The importance of delirium prevention. Nat. Rev. Neurol. 2022, 18, 579–596. [Google Scholar] [CrossRef]
- Goldberg, T.E.; Chen, C.; Wang, Y.; Jung, E.; Swanson, A.; Ing, C.; Garcia, P.S.; Whittington, R.A.; Moitra, V. Association of delirium with long-term cognitive decline: A meta-analysis. JAMA Neurol. 2020, 77, 1373–1381. [Google Scholar] [CrossRef]
- Morandi, A.; Bellelli, G. Delirium superimposed on dementia. Eur. Geriatr. Med. 2020, 11, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Pereira, J.V.; Aung Thein, M.Z.; Nitchingham, A.; Caplan, G.A. Delirium in older adults is associated with development of new dementia: A systematic review and meta-analysis. Int. J. Geriatr. Psychiatry 2021, 36, 993–1003. [Google Scholar] [CrossRef]
- Tsui, A.; Searle, S.D.; Bowden, H.; Hoffmann, K.; Hornby, J.; Goslett, A.; Weston-Clarke, M.; Howes, L.H.; Street, R.; Perera, R.; et al. The effect of baseline cognition and delirium on long-term cognitive impairment and mortality: A prospective population-based study. Lancet Healthy Longev. 2022, 3, e232–e241. [Google Scholar] [CrossRef]
- Nitchingham, A.; Caplan, G.A. Current challenges in the recognition and management of delirium superimposed on dementia. Neuropsychiatr. Dis. Treat. 2021, 17, 1341–1352. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-5, 5th ed.; American Psychiatric Press: Washington, DC, USA, 2013. [Google Scholar]
- WHO. ICD-10. Geneve. Available online: https://apps.who.int/iris/handle/10665/37958 (accessed on 12 April 2023).
- WHO. ICD-11. Geneve. Available online: https://icd.who.int/browse11/l-m/en (accessed on 12 April 2023).
- Inouye, S.K.; Westendorp, R.G.; Saczynski, J.S. Delirium in elderly people. Lancet 2014, 383, 911–922. [Google Scholar] [CrossRef]
- Marcantonio, E.R. Delirium in hospitalized older adults. N. Engl. J. Med. 2017, 377, 1456–1466. [Google Scholar] [CrossRef] [PubMed]
- Iadecola, C.; Duering, M.; Hachinski, V.; Joutel, A.; Pendlebury, S.T.; Schneider, J.A.; Dichgans, M. Vascular cognitive impairment and dementia: JACC Scientific Expert Panel. J. Am. Coll. Cardiol. 2019, 73, 3326–3344. [Google Scholar] [CrossRef] [PubMed]
- Kalvas, L.B.; Monroe, T.B. Structural brain changes in delirium: An integrative review. Biol. Res. Nurs. 2019, 21, 355–365. [Google Scholar] [CrossRef]
- Hasegawa, N.; Hashimoto, M.; Yuuki, S.; Honda, K.; Yatabe, Y.; Araki, K.; Ikeda, M. Prevalence of delirium among outpatients with dementia. Int. Psychogeriatr. 2013, 25, 1877–1883. [Google Scholar] [CrossRef]
- Bir, S.C.; Khan, M.W.; Javalkar, V.; Toledo, E.G.; Kelley, R.E. Emerging concepts in vascular dementia: A review. J. Stroke Cerebrovasc. Dis. 2021, 30, 105864. [Google Scholar] [CrossRef] [PubMed]
- Cannistraro, R.J.; Badi, M.; Eidelman, B.H.; Dickson, D.W.; Middlebrooks, E.H.; Meschia, J.F. CNS small vessel disease: A clinical review. Neurology 2019, 92, 1146–1156. [Google Scholar] [CrossRef]
- Clancy, U.; Gilmartin, D.; Jochems, A.C.C.; Knox, L.; Doubal, F.N.; Wardlaw, J.M. Neuropsychiatric symptoms associated with cerebral small vessel disease: A systematic review and meta-analysis. Lancet Psychiatry 2021, 8, 225–236. [Google Scholar] [CrossRef]
- Nitchingham, A.; Kumar, V.; Shenkin, S.; Ferguson, K.J.; Caplan, G.A. A systematic review of neuroimaging in delirium: Predictors, correlates and consequences. Int. J. Geriatr. Psychiatry 2018, 33, 1458–1478. [Google Scholar] [CrossRef]
- Pantoni, L. Cerebral small vessel disease: From pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol. 2010, 9, 689–701. [Google Scholar] [CrossRef]
- Violan, C.; Foguet-Boreu, Q.; Flores-Mateo, G.; Salisbury, C.; Blom, J.; Freitag, M.; Glynn, L.; Muth, C.; Valderas, J.-M. Prevalence, determinants and patterns of multimorbidity in primary care: A systematic review of observational studies. PLoS ONE 2014, 9, e102149. [Google Scholar] [CrossRef]
- Wetterling, T.; Junghanns, K. Does multimorbidity in older psychiatric patients lead to higher transfer rates between psychiatric and somatic departments? Z. Gerontol. Geriatr. 2019, 52, 568–574. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical MANUAL of Mental Disorders: DSM IV TR, 4th ed.; American Psychiatric Press: Washington, DC, USA, 2000. [Google Scholar]
- Inouye, S.K.; van Dyck, C.H.; Alessi, C.A.; Balkin, S.; Siegal, A.P.; Horwitz, R.I. Clarifying confusion: The confusion assessment method. A new method for detection of delirium. Ann. Intern. Med. 1990, 113, 941–948. [Google Scholar] [CrossRef] [PubMed]
- Hestermann, U.; Backenstrass, M.; Gekle, I.; Hack, M.; Mundt, C.; Oster, P.; Thomas, C. Validation of a German version of the Confusion Assessment Method for delirium detection in a sample of acute geriatric patients with a high prevalence of dementia. Psychopathology 2009, 42, 270–276. [Google Scholar] [CrossRef]
- Trzepacz, P.T.; Mittal, D.; Torres, R.; Kanary, K.; Norton, J.; Jimerson, N. Validation of the Delirium Rating Scale-revised-98: Comparison with the delirium rating scale and the cognitive test for delirium. J. Neuropsychiatry Clin. Neurosci. 2001, 3, 229–242. [Google Scholar] [CrossRef]
- Hughes, C.P.; Berg, L.; Danziger, W.L.; Coben, L.A.; Martin, R.L. A new clinical scale for the staging of dementia. Br. J. Psychiatry 1982, 140, 566–572. [Google Scholar] [CrossRef] [PubMed]
- Linn, B.S.; Linn, M.W.; Gurel, L. Cumulative illness rating scale. J. Am. Geriatr. Soc. 1968, 16, 622–626. [Google Scholar] [CrossRef] [PubMed]
- Salvi, F.; Miller, M.D.; Grilli, A.; Giorgi, R.; Towers, A.L.; Morichi, V.; Spazzafumo, L.; Mancinelli, L.; Espinosa, E.; Rappelli, A.; et al. A manual of guidelines to score the modified cumulative illness rating scale and its validation in acute hospitalized elderly patients. J. Am. Geriatr. Soc. 2008, 56, 1926–1931. [Google Scholar] [CrossRef]
- Guigoz, Y.; Lauque, S.; Vellas, B.J. Identifying the elderly at risk for malnutrition. The Mini Nutritional Assessment. Clin. Geriatr. Med. 2002, 18, 737–757. [Google Scholar] [CrossRef]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Guy, W. Clinical global impression scale. In ECDEU Assessment Manual for Psychopharmacology; U.S. Department of Health, Education, and Welfare: Rockville, MD, USA, 1976. [Google Scholar]
- Krzych, Ł.J.; Rachfalska, N.; Putowski, Z. Delirium superimposed on dementia in perioperative period and intensive care. J. Clin. Med. 2020, 9, 3279. [Google Scholar] [CrossRef]
- Fick, D.M.; Steis, M.R.; Waller, J.L.; Inouye, S.K. Delirium superimposed on dementia is associated with prolonged length of stay and poor outcomes in hospitalized older adults. J. Hosp. Med. 2013, 8, 500–505. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.; Kreisel, S.H.; Oster, P.; Driessen, M.; Arolt, V.; Inouye, S.K. Diagnosing delirium in older hospitalized adults with dementia: Adapting the confusion assessment method to international classification of diseases, tenth revision, diagnostic criteria. J. Am. Geriatr. Soc. 2012, 60, 1471–1477. [Google Scholar] [CrossRef] [PubMed]
- Gross, A.L.; Tommet, D.; D’Aquila, M.; Schmitt, E.; Marcantonio, E.R.; Helfand, B.; Inouye, S.K.; Jones, R.N.; BASIL Study Group. Harmonization of delirium severity instruments: A comparison of the DRS-R-98, MDAS, and CAM-S using item response theory. BMC Med. Res. Methodol. 2018, 18, 92. [Google Scholar] [CrossRef]
- Persico, I.; Cesari, M.; Morandi, A.; Haas, J.; Mazzola, P.; Zambon, A.; Annoni, G.; Bellelli, G. Frailty and delirium in older adults: A systematic review and meta-analysis of the literature. J. Am. Geriatr. Soc. 2018, 66, 2022–2030. [Google Scholar] [CrossRef]
- Morandi, A.; Davis, D.; Fick, D.M.; Turco, R.; Boustani, M.; Lucchi, E.; Guerini, F.; Morghen, S.; Torpilliesi, T.; Gentile, S.; et al. Delirium superimposed on dementia strongly predicts worse outcomes in older rehabilitation inpatients. J. Am. Med. Dir. Assoc. 2014, 15, 349–354. [Google Scholar] [CrossRef]
- Lawson, R.A.; McDonald, C.; Burn, D.J. Defining delirium in idiopathic Parkinson’s disease: A systematic review. Park. Relat. Disord. 2019, 64, 29–39. [Google Scholar] [CrossRef]
- Richardson, S.; Lawson, R.A.; Price, A.; Taylor, J.P. Challenges in diagnosis and management of delirium in Lewy body disease. Acta Psychiatr. Scand. 2022, 147, 475–480. [Google Scholar] [CrossRef]
- Wang, S.; Lindroth, H.; Chan, C.; Greene, R.; Serrano-Andrews, P.; Khan, S.; Rios, G.; Jabbari, S.; Lim, J.; Saykin, A.J.; et al. A systematic review of delirium biomarkers and their alignment with the NIA-AA research framework. J. Am. Geriatr. Soc. 2021, 69, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Fong, T.G.; Vasunilashorn, S.M.; Libermann, T.; Marcantonio, E.R.; Inouye, S.K. Delirium and Alzheimer disease: A proposed model for shared pathophysiology. Int. J. Geriatr. Psychiatry 2019, 34, 781–789. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).