Efficacy of Single Tocilizumab Administration in an 88-Year-Old Patient with Severe COVID-19 and a Mini Literature Review
Abstract
:1. Introduction
2. Case
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [Green Version]
- Zhou, P.; Yang, X.L.; Wang, X.G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.R.; Zhu, Y.; Li, B.; Huang, C.L.; et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, F.; Deng, L.; Zhang, L.; Cai, Y.; Cheung, C.W.; Xia, Z. Review of the Clinical Characteristics of Coronavirus Disease 2019 (COVID-19). J. Gen. Intern. Med. 2020, 35, 1545–1549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y.; et al. Clinical Characteristics of 138 Hospitalized Patients with 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA 2020, 323, 1061–1069. [Google Scholar] [CrossRef] [PubMed]
- Chinnadurai, R.; Ogedengbe, O.; Agarwal, P.; Money-Coomes, S.; Abdurrahman, A.Z.; Mohammed, S.; Kalra, P.A.; Rothwell, N.; Pradhan, S. Older age and frailty are the chief predictors of mortality in COVID-19 patients admitted to an acute medical unit in a secondary care setting- a cohort study. BMC Geriatr. 2020, 20, 409. [Google Scholar] [CrossRef]
- Franceschi, C.; Garagnani, P.; Parini, P.; Giuliani, C.; Santoro, A. Inflammaging: A new immune-metabolic viewpoint for age-related diseases. Nat. Rev. Endocrinol. 2018, 14, 576–590. [Google Scholar] [CrossRef]
- Schmeer, C.; Kretz, A.; Wengerodt, D.; Stojiljkovic, M.; Witte, O.W. Dissecting Aging and Senescence-Current Concepts and Open Lessons. Cells 2019, 8, 1446. [Google Scholar] [CrossRef] [Green Version]
- Terpos, E.; Ntanasis-Stathopoulos, I.; Elalamy, I.; Kastritis, E.; Sergentanis, T.N.; Politou, M.; Psaltopoulou, T.; Gerotziafas, G.; Dimopoulos, M.A. Hematological findings and complications of COVID-19. Am. J. Hematol. 2020, 95, 834–847. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Liu, L.; Zhang, D.; Xu, J.; Dai, H.; Tang, N.; Su, X.; Cao, B. SARS-CoV-2 and viral sepsis: Observations and hypotheses. Lancet 2020, 395, 1517–1520. [Google Scholar] [CrossRef]
- Zhang, X.; Tan, Y.; Ling, Y.; Lu, G.; Liu, F.; Yi, Z.; Jia, X.; Wu, M.; Shi, B.; Xu, S.; et al. Viral and host factors related to the clinical outcome of COVID-19. Nature 2020, 583, 437–440. [Google Scholar] [CrossRef]
- Napoli, C.; Benincasa, G.; Criscuolo, C.; Faenza, M.; Liberato, C.; Rusciano, M. Immune reactivity during COVID-19: Implications for treatment. Immunol. Lett. 2021, 231, 28–34. [Google Scholar] [CrossRef]
- Ali, A.; Kamjani, M.H.; Kesselman, M.M. The Role of Tocilizumab in Cytokine Storm and Improving Outcomes in COVID-19. Recent Pat. Antiinfect. Drug Discov. 2020, 15, 104–112. [Google Scholar] [CrossRef]
- Streicher, C.; Engalenc, X.; Gaudin, M.; Vignaud, G.; Daulange, A.; Abraham, B. Could Tocilizumab be an Attractive Therapeutic Option for Elderly Patients with Severe COVID-19? A Case Report. Clin. Drug Investig. 2020, 40, 1085–1088. [Google Scholar] [CrossRef]
- Kataoka, H.; Kodama, F.; Tomita, T.; Kondo, M.; Nagasaka, A.; Nishikawa, S.; Mukai, M. Immediate Amelioration of Severe Respiratory Distress in Sjögren’s Syndrome with COVID-19 Treated with a Single Dose of Off-label Tocilizumab. Intern. Med. 2021, 60, 639–643. [Google Scholar] [CrossRef] [PubMed]
- REMAP-CAP Investigators; Gordon, A.C.; Mouncey, P.R.; Al-Beidh, F.; Rowan, K.M.; Nichol, A.D.; Arabi, Y.M.; Annane, D.; Beane, A.; van Bentum-Puijk, W.; et al. Interleukin-6 Receptor Antagonists in Critically Ill Patients with COVID-19. N. Engl. J. Med. 2021, 384, 1491–1502. [Google Scholar]
- Hermine, O.; Mariette, X.; Tharaux, P.L.; Resche-Rigon, M.; Porcher, R.; Ravaud, P.; CORIMUNO-19 Collaborative Group. Effect of Tocilizumab vs. Usual Care in Adults Hospitalized with COVID-19 and Moderate or Severe Pneumonia: A Randomized Clinical Trial. JAMA Intern. Med. 2021, 181, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Stone, J.H.; Frigault, M.J.; Serling-Boyd, N.J.; Fernandes, A.D.; Harvey, L.; Foulkes, A.S.; Horick, N.K.; Healy, B.C.; Shah, R.; Bensaci, A.M.; et al. BACC Bay Tocilizumab Trial Investigators. Efficacy of Tocilizumab in Patients Hospitalized with Covid-19. N. Engl. J. Med. 2020, 383, 2333–2344. [Google Scholar] [CrossRef] [PubMed]
- Salvarani, C.; Dolci, G.; Massari, M.; Merlo, D.F.; Cavuto, S.; Savoldi, L.; Bruzzi, P.; Boni, F.; Braglia, L.; Turrà, C.; et al. RCT-TCZ-COVID-19 Study Group. Effect of Tocilizumab vs. Standard Care on Clinical Worsening in Patients Hospitalized with COVID-19 Pneumonia: A Randomized Clinical Trial. JAMA Intern. Med. 2021, 181, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Salama, C.; Han, J.; Yau, L.; Reiss, W.G.; Kramer, B.; Neidhart, J.D.; Criner, G.J.; Kaplan-Lewis, E.; Baden, R.; Pandit, L.; et al. Tocilizumab in Patients Hospitalized with Covid-19 Pneumonia. N. Engl. J. Med. 2021, 384, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Tsai, A.; Diawara, O.; Nahass, R.G.; Brunetti, L. Impact of tocilizumab administration on mortality in severe COVID-19. Sci. Rep. 2020, 10, 19131. [Google Scholar] [CrossRef]
- Mariette, X.; Hermine, O.; Tharaux, P.L.; Resche-Rigon, M.; Steg, P.G.; Porcher, R.; Ravaud, P. Effectiveness of Tocilizumab in Patients Hospitalized with COVID-19: A Follow-up of the CORIMUNO-TOCI-1 Randomized Clinical Trial. JAMA Intern. Med. 2021, 181, 1241–1243. [Google Scholar] [CrossRef] [PubMed]
- Al-Baadani, A.; Eltayeb, N.; Alsufyani, E.; Albahrani, S.; Basheri, S.; Albayat, H.; Batubara, E.; Ballool, S.; Al Assiri, A.; Faqihi, F.; et al. Efficacy of tocilizumab in patients with severe COVID-19: Survival and clinical outcomes. J. Infect. Public. Health. 2021, 14, 1021–1027. [Google Scholar] [CrossRef] [PubMed]
- Duarte-Millán, M.A.; Mesa-Plaza, N.; Guerrero-Santillán, M.; Morales-Ortega, A.; Bernal-Bello, D.; Farfán-Sedano, A.I.; García de Viedma-García, V.; Velázquez-Ríos, L.; Frutos-Pérez, B.; De Ancos-Aracil, C.L.; et al. Prognostic factors and combined use of tocilizumab and corticosteroids in a Spanish cohort of elderly COVID-19 patients. J. Med. Virol. 2021, in press. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Cui, W.; Tian, B.P. Efficacy of tocilizumab treatment in severely ill COVID-19 patients. Crit. Care 2020, 24, 524. [Google Scholar] [CrossRef]

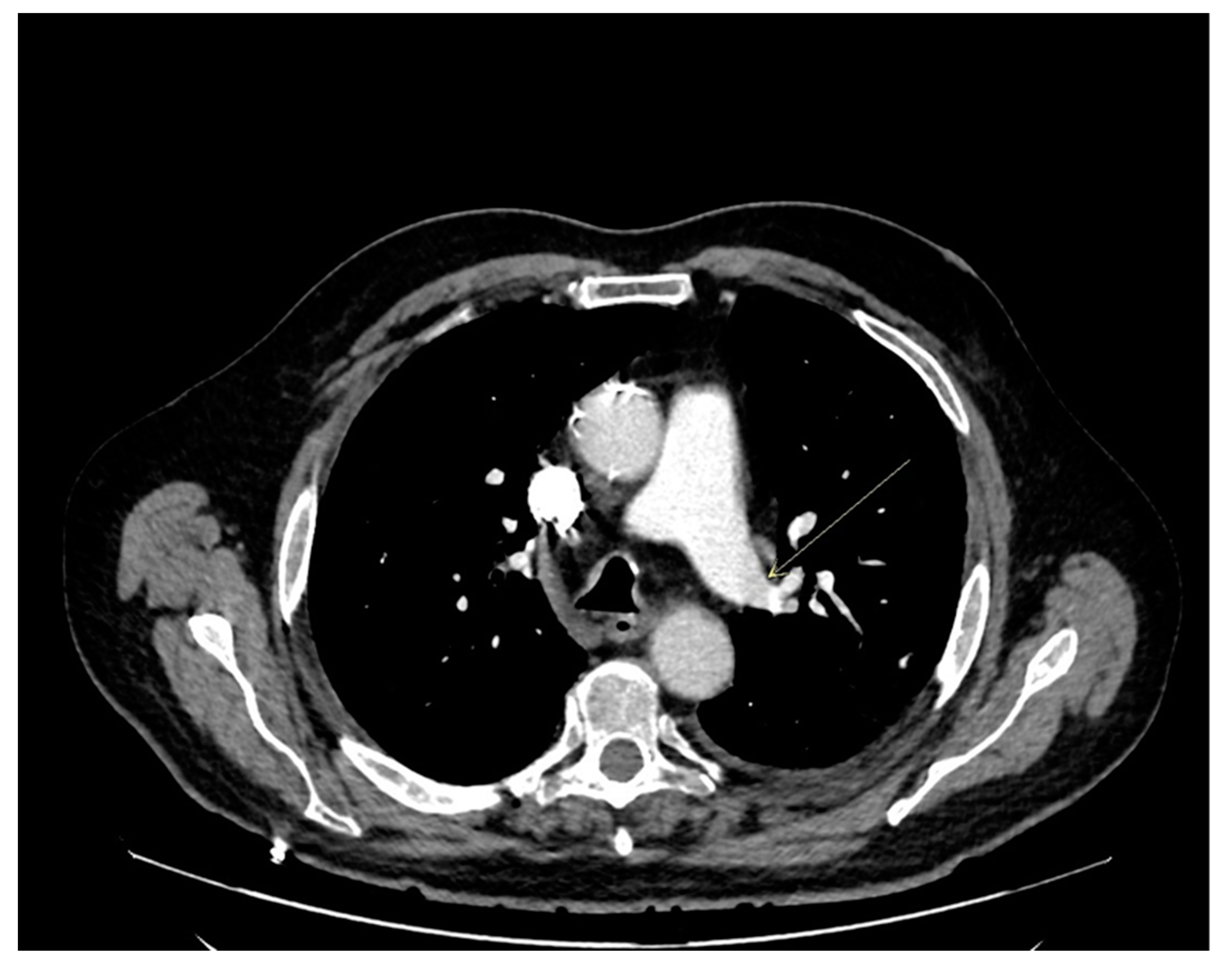
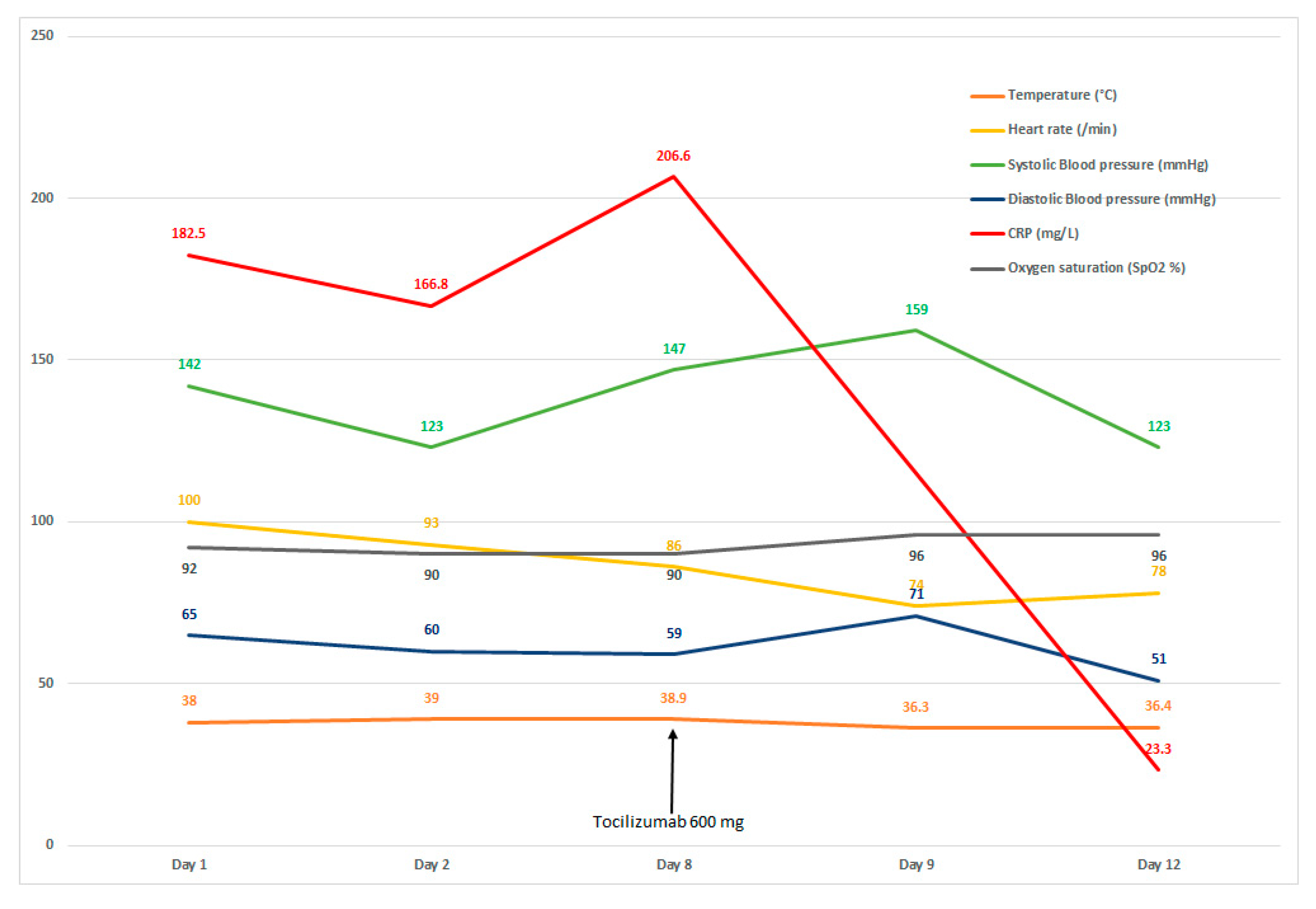
| Parameters (Laboratory Reference) | Admission | Day 2 | Day 8 | Day 13 |
|---|---|---|---|---|
| CRP (<5.0 mg/L) | 182.5 | 176.8 | 206.6 | 23.3 |
| Hemoglobin (12.9–16.7 g/dL) | 10.8 | 10.7 | 9.9 | 10.0 |
| Natremia (136–145 mmol/L) | 132 | 135 | 137 | 139 |
| Kalemia (3.40–4.50 mmol/L) | 4.53 | 4.56 | 4.38 | 3.75 |
| Lymphocytes (1.070–4.100 g/L), | 1.470 | 1.010 | 0.690 | 0.48 |
| Blood creatinine (62.0–106.0 µmol/L) | 99.9 | 103.3 | 78.4 | 88.1 |
| Blood urea (2.86–8.21 mmol/L) | 11.8 | 12.9 | 8.2 | 7.1 |
| MDRD 1 (>90) | 62 | 60 | 82 | 72 |
| NT-proBNP 2 (50.0–125.0 pg/mL) | - | 9118.0 | 3819.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ould Ouali, C.; Ladjouzi, N.; Tamas, K.; Raveloson, H.; Ben Hassen, J.; El Omeiri, N.; Zouloumis, G.; Al Zoabi, M.M.; Asadi, M.; Jhouri, A.; et al. Efficacy of Single Tocilizumab Administration in an 88-Year-Old Patient with Severe COVID-19 and a Mini Literature Review. Geriatrics 2022, 7, 22. https://doi.org/10.3390/geriatrics7010022
Ould Ouali C, Ladjouzi N, Tamas K, Raveloson H, Ben Hassen J, El Omeiri N, Zouloumis G, Al Zoabi MM, Asadi M, Jhouri A, et al. Efficacy of Single Tocilizumab Administration in an 88-Year-Old Patient with Severe COVID-19 and a Mini Literature Review. Geriatrics. 2022; 7(1):22. https://doi.org/10.3390/geriatrics7010022
Chicago/Turabian StyleOuld Ouali, Cid, Nadia Ladjouzi, Khidher Tamas, Hendriniaina Raveloson, Jihene Ben Hassen, Nesrine El Omeiri, Georges Zouloumis, Mohamed Moataz Al Zoabi, Muneer Asadi, Aziza Jhouri, and et al. 2022. "Efficacy of Single Tocilizumab Administration in an 88-Year-Old Patient with Severe COVID-19 and a Mini Literature Review" Geriatrics 7, no. 1: 22. https://doi.org/10.3390/geriatrics7010022
APA StyleOuld Ouali, C., Ladjouzi, N., Tamas, K., Raveloson, H., Ben Hassen, J., El Omeiri, N., Zouloumis, G., Al Zoabi, M. M., Asadi, M., Jhouri, A., & Schlatter, J. (2022). Efficacy of Single Tocilizumab Administration in an 88-Year-Old Patient with Severe COVID-19 and a Mini Literature Review. Geriatrics, 7(1), 22. https://doi.org/10.3390/geriatrics7010022






