Pitfalls of Early Systemic Corticosteroids Home Therapy in Older Patients with COVID-19 Pneumonia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO Director-General’s Opening Remarks at the Media Briefing on COVID-19. Available online: https://www.who.int/director-general/speeches/detail/ (accessed on 22 December 2021).
- Helmy, Y.A.; Fawzy, M.; Elaswad, A.; Sobieh, A.; Kenney, S.P.; Shehata, A.A. The COVID-19 Pandemic: A Comprehensive Review of Taxonomy, Genetics, Epidemiology, Diagnosis, Treatment, and Control. J. Clin. Med. 2020, 9, 1225. [Google Scholar] [CrossRef] [PubMed]
- Dufloo, J.; Grzelak, L.; Staropoli, I.; Madec, Y.; Tondeur, L.; Anna, F.; Pelleau, S.; Wiedemann, A.; Planchais, C.; Buchrieser, J.; et al. Asymptomatic and symptomatic SARS-CoV-2 infections elicit polyfunctional antibodies. Cell Rep. Med. 2021, 2, 100275. [Google Scholar] [CrossRef] [PubMed]
- Stockman, L.J.; Bellamy, R.; Garner, P. SARS: Systematic review of treatment effects. PLoS Med. 2006, 3, e343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arabi, Y.M.; Mandourah, Y.; Al-Hameed, F.; Sindi, A.A.; Almekhlafi, G.A.; Hussein, M.A.; Jose, J.; Pinto, R.; Al-Omari, A.; Kharaba, A.; et al. Corticosteroid Therapy for Critically Ill Patients with Middle East Respiratory Syndrome. Am. J. Respir. Crit. Care Med. 2018, 197, 757–767. [Google Scholar] [CrossRef]
- Shang, L.; Zhao, J.; Hu, Y.; Du, R.; Cao, B. On the use of corticosteroids for 2019-nCoV pneumonia. Lancet 2020, 395, 683–684. [Google Scholar] [CrossRef] [Green Version]
- Russell, C.D.; Millar, J.E.; Baillie, J.K. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet 2020, 395, 473–475. [Google Scholar] [CrossRef] [Green Version]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Mager, D.E.; Lin, S.X.; Blum, R.A.; Lates, C.D.; Jusko, W.J. Dose equivalency evaluation of major corticosteroids: Pharmacokinetics and cell trafficking and cortisol dynamics. J. Clin. Pharmacol. 2003, 43, 1216–1227. [Google Scholar] [CrossRef] [PubMed]
- Sarzani, R.; Spannella, F.; Giulietti, F.; Di Pentima, C.; Giordano, P.; Giacometti, A. Possible harm from glucocorticoid drugs misuse in the early phase of SARS-CoV-2 infection: A narrative review of the evidence. Intern. Emerg. Med. 2021, 1–10, Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Horby, P.; Lim, W.S.; Emberson, J.R.; Mafham, M.; Bell, J.L.; Linsell, L.; Staplin, N.; Brightling, C.; Ustianowski, A.; Elmahi, E.; et al. Dexamethasone in Hospitalized Patients with Covid-19. N. Engl. J. Med. 2021, 384, 693–704. [Google Scholar] [CrossRef] [PubMed]
- Bahl, A.; Johnson, S.; Chen, N.-W. Timing of corticosteroids impacts mortality in hospitalized COVID-19 patients. Intern. Emerg. Med. 2021, 16, 1593–1603. [Google Scholar] [CrossRef] [PubMed]
- Cunha, L.L.; Perazzio, S.F.; Azzi, J.; Cravedi, P.; Riella, L.V. Remodeling of the Immune Response With Aging: Immunosenescence and Its Potential Impact on COVID-19 Immune Response. Front. Immunol. 2020, 11, 1748. [Google Scholar] [CrossRef] [PubMed]
- Shaw, A.C.; Joshi, S.; Greenwood, H.; Panda, A.; Lord, J.M. Aging of the innate immune system. Curr. Opin. Immunol. 2010, 22, 507–513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cain, D.W.; Cidlowski, J.A. Immune regulation by glucocorticoids. Nat. Rev. Immunol. 2017, 17, 233–247. [Google Scholar] [CrossRef] [PubMed]
- Qian, Z.; Travanty, E.A.; Oko, L.; Edeen, K.; Berglund, A.; Wang, J.; Ito, Y.; Holmes, K.V.; Mason, R.J. Innate immune response of human alveolar type II cells infected with severe acute respiratory syndrome-coronavirus. Am. J. Respir. Cell Mol. Biol. 2013, 48, 742–748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Zhang, S.; Dong, X.; Li, Z.; Xu, Q.; Feng, H.; Cai, J.; Huang, S.; Guo, J.; Zhang, L.; et al. Corticosteroid treatment in severe COVID-19 patients with acute respiratory distress syndrome. J. Clin. Investig. 2020, 130, 6417–6428. [Google Scholar] [CrossRef] [PubMed]
| All Patients N = 90 | ECT N = 33 | Controls N = 57 | p-Value | |
|---|---|---|---|---|
| Gender (male) | 47 (52.3) | 16 (48.5) | 31 (54.4) | 0.58 |
| Age (years) | 82.3 (6.7) | 84.5 (2.4) | 81.3 (6.1) | 0.02 |
| SOFA score | 2 (2) | 2 (2) | 2 (2) | 0.6 |
| Arterial Hypertension | 58 (64.4) | 15 (42.8) | 33 (57.1) | 0.18 |
| Diabetes Mellitus | 19 (21.5) | 3 (9.0) | 16 (29.2) | 0.05 |
| Chronic Heart Failure | 32 (35.0) | 12 (34.9) | 20 (35.8) | 0.92 |
| COPD | 33 (36.9) | 12 (37.5) | 21 (36.5) | 0.94 |
| Dementia | 33 (36.9) | 12 (37.5) | 21 (36.5) | 0.94 |
| Charlson Comorbidity Index | 5 (2) | 5 (3) | 5 (2) | 0.45 |
| Shortness of breath | 44 (49) | 19 (57.5) | 25 (43.8) | 0.21 |
| Cough | 22 (24) | 10 (30.3) | 12 (21.3) | 0.32 |
| Fever | 42 (46) | 15 (45.5) | 27 (47.3) | 0.86 |
| CT bilateral patchy shadow | 41 (45.5) | 23 (69.5) | 18 (32.3) | 0.005 |
| CT pleural effusion | 27 (30) | 4 (13) | 23 (40.4) | 0.024 |
| CT pulmonary consolidations | 42 (47) | 14 (43.3) | 28 (48.6) | 0.69 |
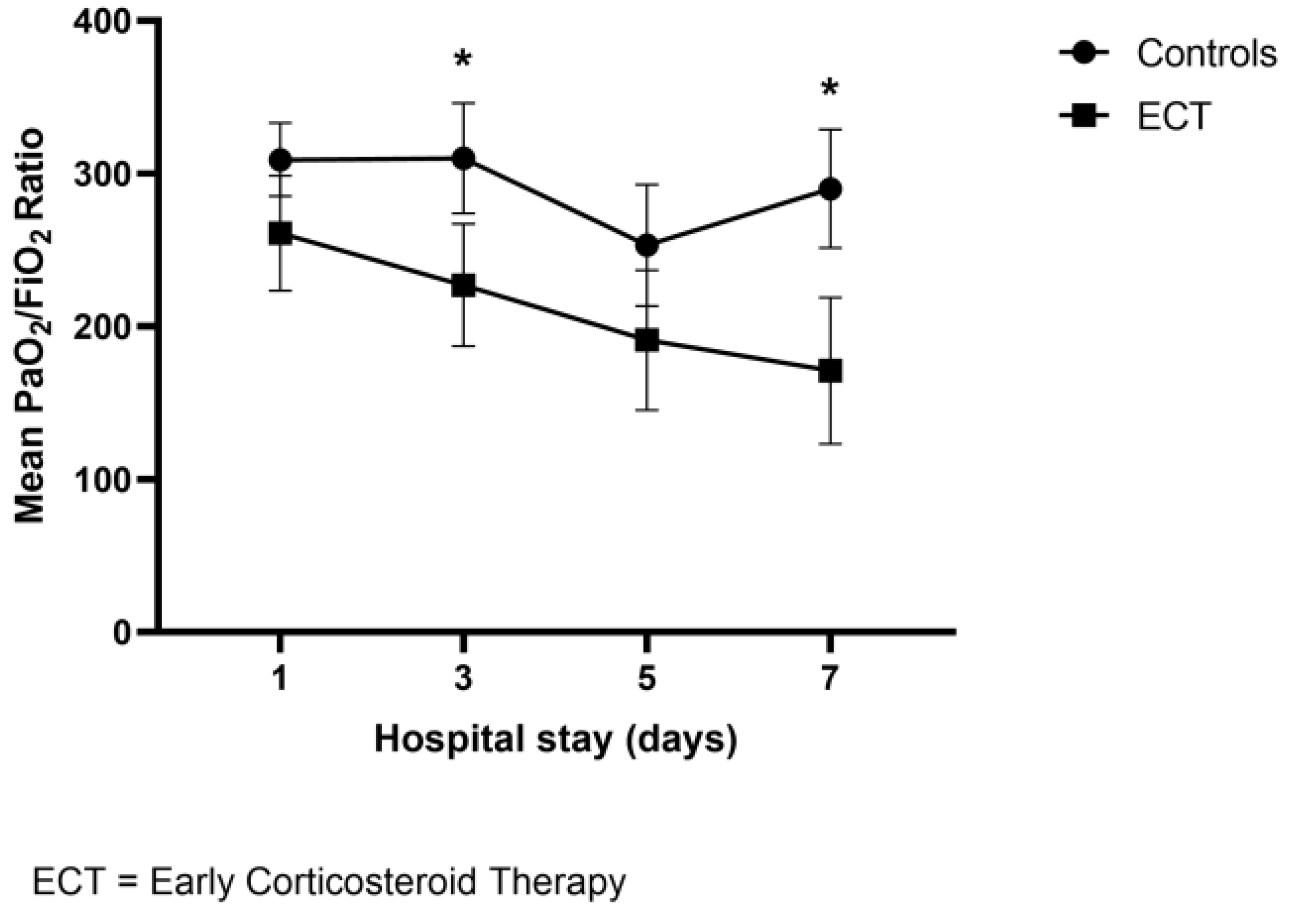
| Median PaO2/FiO2 baseline | 297 (109) | 261 (138) | 304 (106) | 0.06 |
| Median PaO2/Fio2 nadir | 215 (201) | 138 (128) | 252 (191) | 0.017 |
| White blood cells count/mm3 | 7827 (3626) | 9254 (2561) | 6992 (3915) | 0.014 |
| Baseline Lymphocytes /mm3 | 1258 (2364) | 851 (579) | 1477 (2890) | 0.32 |
| Baseline C-reactive protein (mg/dL) | 8.1 (6.3) | 11.3 (7) | 6.1 (5.1) | 0.002 |
| In-hospital death or ICU admission (%) | 22 (24) | 9 (27.1) | 13 (22.8) | 0.63 |
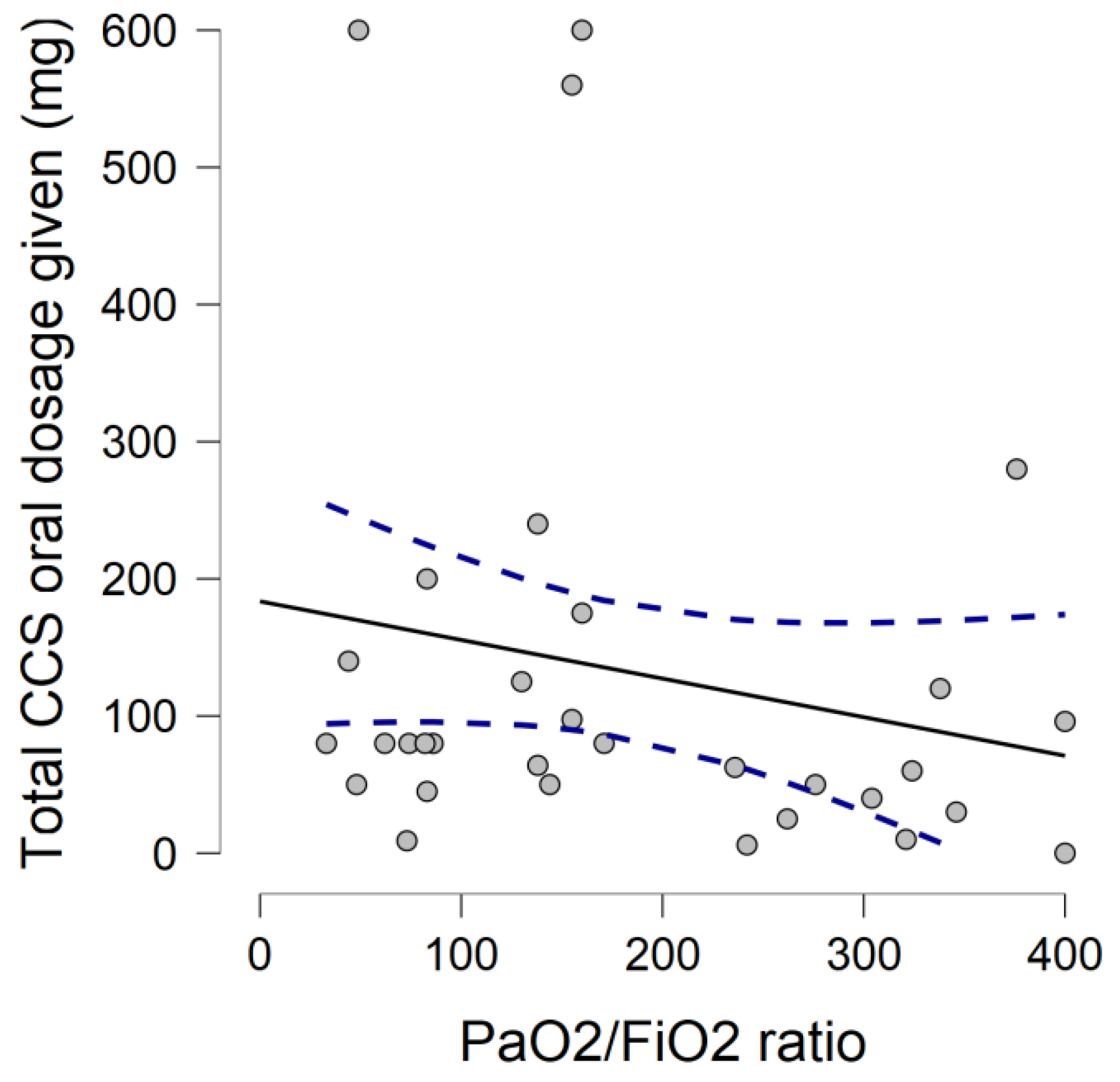
| Corticosteroid (CCS) Home Therapy | Observed Frequency (%) | Total CCS Given Dose in mg (n° of Subjects) |
|---|---|---|
| Dexamethasone 4 mg tabs | 3/33 (9.1) | 50–100 (3) |
| Methylprednisolone sodium succinate 16 mg | 29/33 (87.9) | 50–100 (6) 100–300 (1) >300 (9) |
| Prednisone 25 mg po tabs | 1/33 (3.0) | 50–100 (3) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okoye, C.; Rogani, S.; Franchi, R.; Pompilii, I.M.; Calabrese, A.M.; Mazzarone, T.; Bianchi, E.; Lemmi, B.; Calsolaro, V.; Monzani, F. Pitfalls of Early Systemic Corticosteroids Home Therapy in Older Patients with COVID-19 Pneumonia. Geriatrics 2022, 7, 21. https://doi.org/10.3390/geriatrics7010021
Okoye C, Rogani S, Franchi R, Pompilii IM, Calabrese AM, Mazzarone T, Bianchi E, Lemmi B, Calsolaro V, Monzani F. Pitfalls of Early Systemic Corticosteroids Home Therapy in Older Patients with COVID-19 Pneumonia. Geriatrics. 2022; 7(1):21. https://doi.org/10.3390/geriatrics7010021
Chicago/Turabian StyleOkoye, Chukwuma, Sara Rogani, Riccardo Franchi, Igino Maria Pompilii, Alessia Maria Calabrese, Tessa Mazzarone, Elena Bianchi, Bianca Lemmi, Valeria Calsolaro, and Fabio Monzani. 2022. "Pitfalls of Early Systemic Corticosteroids Home Therapy in Older Patients with COVID-19 Pneumonia" Geriatrics 7, no. 1: 21. https://doi.org/10.3390/geriatrics7010021
APA StyleOkoye, C., Rogani, S., Franchi, R., Pompilii, I. M., Calabrese, A. M., Mazzarone, T., Bianchi, E., Lemmi, B., Calsolaro, V., & Monzani, F. (2022). Pitfalls of Early Systemic Corticosteroids Home Therapy in Older Patients with COVID-19 Pneumonia. Geriatrics, 7(1), 21. https://doi.org/10.3390/geriatrics7010021







