The Potential Mediation of the Effects of Physical Activity on Cognitive Function by the Gut Microbiome
Abstract
1. An Aging Population with Risk of Cognitive Decline
The Costs of Cognitive Decline
2. Physical Activity
2.1. Defining Physical Activity
2.2. Physical Activity and Cognitive Function
2.3. Potential Mechanisms for Effects of Physical Activity on Cognitive Function
2.3.1. Indirect Pathways between PA and Cognitive Function
2.3.2. Direct Pathways between PA and Cognitive Function
3. The Gut Microbiome and Cognitive Function
3.1. Direct Pathways between the Gut Microbiome and the Brain
3.2. Indirect Influences of the Gut Microbiome on Brain Health
3.2.1. Gut Microbiome and Obesity
3.2.2. Gut Microbiome and Inflammation
3.2.3. Gut Microbiome and Metabolic Disorders
3.2.4. Altering the Gut Microbiome
4. PA and the Gut Microbiome
4.1. Associations between PA and Gut Microbiome Composition
4.2. Potential Mechanisms for the Impact of PA on the Gut Microbiome
5. The Gut Microbiome and Cognitive Function
5.1. Gut Microbiome Improves Cognitive Function in Animal Models
5.2. Gut Microbiome Improves Cognitive Function in Studies with Humans
5.3. Gut Microbiome Plus Exercise for Cognitive Function
6. Previous Research on Mediation of the Effects of PA on Cognitive Function by the Gut Microbiome
6.1. Theoretical Reviews
6.2. Previous Interventional Research
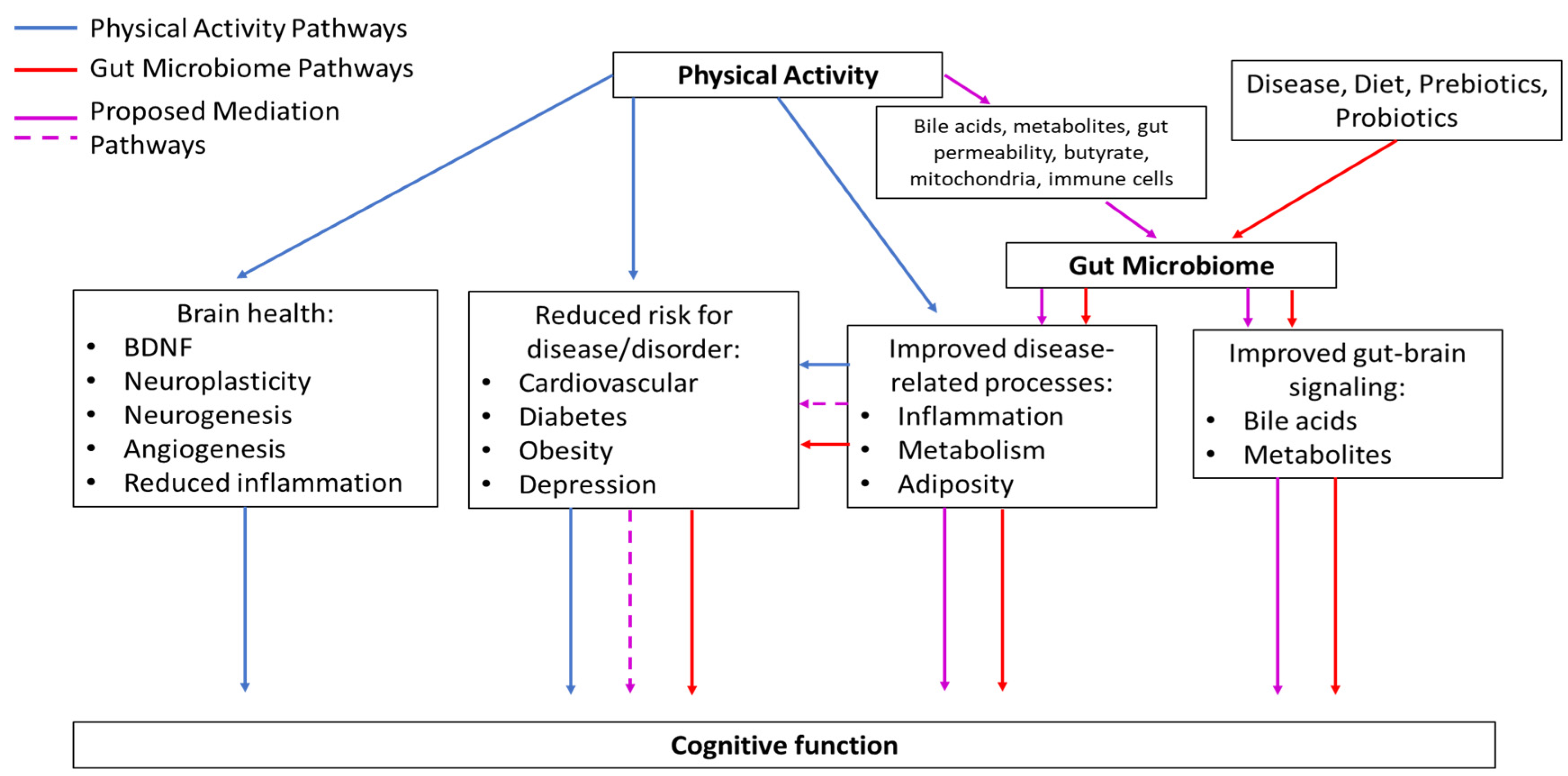
7. Proposed Mediation Model
7.1. Summary of What We Know
7.2. Rationale
7.3. Description of Proposed Mediation Model
7.3.1. PA Pathways
7.3.2. Gut Microbiome Pathways
7.3.3. Proposed Mediation Pathways
Established Pathways
Novel Pathways
7.4. Testing the Proposed Mediation Model
7.4.1. Key Features Needed to Examine the Proposed Mediation Model
7.4.2. Randomized Clinical Trial Description
8. Clinical Implications of Partial Mediation by the Gut Microbiome
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
References
- Ortman, J.M.; Velkoff, V.A.; Hogan, H. An Aging Nation: The Older Population in the United States; United States Census Bureau, Economics and Statistics Administration, US Department of Commerce: Washington, DC, USA, 2014; pp. 25–1140.
- He, W.; Goodkind, D.; Kowal, P.R. An Aging World: 2015. Available online: https://scholar.googleusercontent.com/scholar?q=cache:0924dD31EAQJ:scholar.google.com/+He+Goodkind+Kowal&hl=en&as_sdt=0,14 (accessed on 16 March 2020).
- Centers for Disease Control. Promoting Health for Older Adults. 2019. Available online: https://www.cdc.gov/chronicdisease/resources/publications/factsheets/promoting-health-for-older-adults.htm#:~:text=Age%20brings%20a%20higher%20risk,the%20risk%20increases%20with%20age (accessed on 17 March 2020).
- Harada, C.N.; Love, M.C.N.; Triebel, K.L. Normal cognitive aging. Clin. Geriatr. Med. 2013, 29, 737–752. [Google Scholar] [CrossRef]
- Salthouse, T.A. When does age-related cognitive decline begin? Neurobiol. Aging 2009, 30, 507–514. [Google Scholar] [CrossRef]
- Adamson, M.M.; Samarina, V.; Xiangyan, X.; Huynh, V.; Kennedy, Q.; Weiner, M.; Taylor, J.L. The impact of brain size on pilot performance varies with aviation training and years of education. JINS 2010, 16, 412–423. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chee, M.W.; Chen, K.H.; Zheng, H.; Chan, K.P.; Isaac, V.; Sim, S.K.; Ng, T.P. Cognitive function and brain structure correlations in healthy elderly East Asians. Neuroimage 2009, 46, 257–269. [Google Scholar] [CrossRef] [PubMed]
- Chee, M.W.L.; Zheng, H.; Goh, J.O.S.; Park, D.; Sutton, B.P. Brain structure in young and old East Asians and Westerners: Comparisons of structural volume and cortical thickness. J. Cogn. Neurosci. 2011, 23, 1065–1079. [Google Scholar] [CrossRef] [PubMed]
- Carlesimo, G.A.; Cherubini, A.; Caltagirone, C.; Spalletta, G. Hippocampal mean diffusivity and memory in healthy elderly individuals: A cross-sectional study. Neurology 2010, 74, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, K.L.; Tybjærg-Hansen, A.; Nordestgaard, B.G.; Frikke-Schmidt, R. Absolute 10-year risk of dementia by age, sex and APOE genotype: A population-based cohort study. CMAJ Can. Med. Assoc. J. 2018, 190, E1033–E1041. [Google Scholar] [CrossRef] [PubMed]
- McKhann, G.M.; Knopman, D.S.; Chertkow, H.; Hyman, B.T.; Jack Jr, C.R.; Kawas, C.H.; Mohs, R.C. The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dement. 2011, 7, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Petersen, R.C. Mild cognitive impairment as a diagnostic entity. J. Intern. Med. 2004, 256, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Roberts, R.; Knopman, D.S. Classification and epidemiology of MCI. Clin. Geriatr. Med. 2013, 29, 753–772. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Alzheimer’s Disease. 2017. Available online: https://www.cdc.gov/dotw/alzheimers/index.html (accessed on 20 March 2020).
- Wimo, A.; Jönsson, L.; Bond, J.; Prince, M.; Winblad, B.; International, A.D. The worldwide economic impact of dementia 2010. Alzheimer’s Dement. 2013, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Weiner, M.W.; Veitch, D.P.; Aisen, P.S.; Beckett, L.A.; Cairns, N.J.; Green, R.C.; Harvey, D.; Jack, C.R.; Jagust, W.; Liu, E.; et al. The Alzheimer’s disease neuroimaging initiative: A review of papers published since its inception. Alzheimer’s Dement. 2013, 9, e111–e194. [Google Scholar] [CrossRef] [PubMed]
- Plassman, B.L.; Langa, K.M.; Fisher, G.G.; Heeringa, S.G.; Weir, D.R.; Ofstedal, M.B.; Steffens, D.C. Prevalence of dementia in the United States: The aging, demographics, and memory study. Neuroepidemiology 2017, 29, 125–132. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, J.T.; Thomas, A. Vascular dementia. Lancet 2015, 386, 1698–1706. [Google Scholar] [CrossRef]
- Stebbins, G.T.; Nyenhuis, D.L.; Changsheng, W.; Cox, J.L.; Freels, S.; Bangen, K.; deToledo-Morrell, L.; Sripathirathan, K.; Moseley, M.; Turner, D.A.; et al. Gray matter atrophy in patients with ischemic stroke with cognitive impairment. Stroke 2008, 39, 785–793. [Google Scholar] [CrossRef]
- Lyketsos, C.G.; Lopez, O.; Jones, B.; Fitzpatrick, A.L.; Breitner, J.; DeKosky, S. Prevalence of neuropsychiatric symptoms in dementia and Mild Cognitive Impairment: Results from the Cardiovascular Health Study. JAMA 2002, 288, 1475–1483. [Google Scholar] [CrossRef]
- Prince, M.; Wimo, A.; Guerchet, M.; Ali, G.C.; Wu, Y.T.; Prina, M. The global impact of dementia. World Alzheimer Rep. 2015, 1–82. Available online: https://www.alz.co.uk/sites/default/files/conf2016/pl12-martin-prince-the-global-impact-of-dementia.pdf (accessed on 25 September 2020).
- Testa, M.A.; Simonson, D.C. Assessment of quality-of-life outcomes. N. Engl. J. Med. 1996, 334, 835–840. [Google Scholar] [CrossRef]
- Pusswald, G.; Tropper, E.; Kryspin-Exner, I.; Moser, D.; Klug, S.; Auff, E.; Dal-Bianco, P.; Lehrner, J. Health-related quality of life in patients with subjective cognitive decline and Mild Cognitive Impairment and its relation to activities of daily living. J. Alzheimer’s Dis. 2015, 47, 479–486. [Google Scholar] [CrossRef]
- Hoe, J.; Hancock, G.; Livingston, G.; Orrell, M. Quality of life of people with dementia in residential care homes. Br. J. Psychiatry 2006, 188, 460–464. [Google Scholar] [CrossRef]
- Stites, S.D.; Karlawish, J.; Harkins, K.; Rubright, J.D.; Wolk, D. Awareness of Mild Cognitive Impairment and mild Alzheimer’s disease dementia diagnoses associated with lower self-ratings of quality of life in older adults. J. Gerontol. B Psychol. Sci. Soc. Sci. 2017, 72, 974–985. [Google Scholar] [CrossRef]
- Hurd, M.D.; Martorell, P.; Delavande, A.; Mullen, K.J.; Langa, K.M. Monetary costs of dementia in the United States. N. Engl. J. Med. 2013, 368, 1326–1334. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, M. Cost of Dementia Care in US to Double by 2040. Available online: https://www.bmj.com/content/346/bmj.f2175.full (accessed on 20 March 2020).
- Prince, M.; Wimo, A.; Guerchet, M.; Ali, G.C.; Wu, Y.T.; Prina, M. World Alzheimer Report 2015: The Global Impact of Dementia: An Analysis of Prevalence, Incidence, Cost and Trends; Alzheimer’s Disease International: London, UK, 2015. [Google Scholar]
- World Health Organization; Alzheimer’s disease International. Dementia: A Public Health Priority; World Health Organization: Geneva, Switzerland, 2012. [Google Scholar]
- Buckley, J.S.; Salpeter, S.R. A risk-benefit assessment of dementia medications: Systematic review of the evidence. Drugs Aging 2015, 32, 453–467. [Google Scholar] [CrossRef] [PubMed]
- Schott, J.M.; Aisen, P.S.; Cummings, J.L.; Howard, R.J.; Fox, N.C. Unsuccessful trials of therapies for Alzheimer’s disease. Lancet 2019, 393, 29. [Google Scholar] [CrossRef]
- Orrell, M.; Brayne, C. Dementia prevention: Call to action. Lancet 2015, 386, 1625. [Google Scholar] [CrossRef]
- Jetté, M.; Sidney, K.; Blümchen, G. Metabolic equivalents (METS) in exercise testing, exercise prescription, and evaluation of functional capacity. Clin. Cardiol. 1990, 13, 555–565. [Google Scholar] [CrossRef] [PubMed]
- Pate, R.R.; O’Neill, J.R.; Lobelo, F. The evolving definition of “Sedentary”. Exerc. Sport Sci. Rev. 2008, 36, 173–178. [Google Scholar] [CrossRef]
- Rosenberger, M. Sedentary behavior: Target for change, challenge to assess. Int. J. Obes. 2012, 2, S26–S29. [Google Scholar] [CrossRef][Green Version]
- Katzmarzyk, P.T.; Church, T.S.; Craig, C.L.; Bouchard, C. Sitting time and mortality from all causes, cardiovascular disease, and cancer. Med. Sci. Sport Exerc. 2009, 41, 998–1005. [Google Scholar] [CrossRef]
- Hamilton, M.T.; Hamilton, D.G.; Zderic, T.W. Role of low energy expenditure and sitting in obesity, metabolic syndrome, type 2 diabetes, and cardiovascular disease. Diabetes 2007, 56, 2655–2667. [Google Scholar] [CrossRef]
- Caspersen, C.J.; Powell, K.E.; Christenson, G.M. Physical activity, exercise, and physical fitness: Definitions and distinctions for health-related research. Public Health Rep. 1985, 100, 126–131. [Google Scholar]
- Loprinzi, P.D. Light-intensity physical activity and all-cause mortality. Am. J. Health Promot. 2017, 31, 340–342. [Google Scholar] [CrossRef] [PubMed]
- Healy, G.N.; Dunstan, D.W.; Salmon, J.; Cerin, E.; Shaw, J.E.; Zimmet, P.Z.; Owen, N. Objectively measured light-intensity physical activity is independently associated with 2-h plasma glucose. Diabetes Care 2007, 30, 1384–1389. [Google Scholar] [CrossRef]
- Yuko, G.; Kenta, Y.; Haruka, M.; Yumi, O.; Ryoko, K.; Kiyoshi, S.; Mitsuru, H.; Izumi, T.; Motohiko, M. Longer time spent in light physical activity is associated with reduced arterial stiffness in older adults. Hypertension 2010, 56, 540–546. [Google Scholar] [CrossRef]
- Loprinzi, P.D.; Lee, H.; Cardinal, B.J. Evidence to support including lifestyle light-intensity recommendations in physical activity guidelines for older adults. Am. J. Health Promot. 2015, 29, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Hupin, D.; Roche, F.; Gremeaux, V.; Chatard, J.-C.; Oriol, M.; Gaspoz, J.-M.; Barthélémy, J.-C.; Edouard, P. Even a low-dose of moderate-to-vigorous physical activity reduces mortality by 22% in adults aged ≥60 years: A systematic review and meta-analysis. Br. J. Sports Med. 2015, 49, 1262–1267. [Google Scholar] [CrossRef]
- Edwards, M.K.; Loprinzi, P.D. The association between sedentary behavior and cognitive function among older adults may be attenuated with adequate physical activity. J. Phys. Act. Health 2017, 14, 52–58. [Google Scholar] [CrossRef]
- Falck, R.S.; Davis, J.C.; Liu-Ambrose, T. What is the association between sedentary behaviour and cognitive function? A systematic review. Br. J. Sports Med. 2017, 51, 800–811. [Google Scholar] [CrossRef]
- Vásquez, E.; Strizich, G.; Isasi, C.R.; Echeverria, S.E.; Sotres-Alvarez, D.; Evenson, K.R.; Tarraf, W. Is there a relationship between accelerometer-assessed physical activity and sedentary behavior and cognitive function in US Hispanic/Latino adults? The Hispanic Community Health Study/Study of Latinos (HCHS/SOL). Prev. Med. 2017, 103, 43–48. [Google Scholar]
- Gardiner, P.A.; Eakin, E.G.; Healy, G.N.; Owen, N. Feasibility of reducing older adults’ sedentary time. Prev. Med. 2011, 41, 174–177. [Google Scholar] [CrossRef]
- Rosenberger, M.E.; Buman, M.P.; Haskell, W.L.; McConnell, M.V.; Carstensen, L.L. 24 hours of sleep, sedentary behavior, and physical activity with nine wearable devices. Med. Sci. Sport Exerc. 2016, 48, 457. [Google Scholar]
- Lewis, L.K.; Rowlands, A.V.; Gardiner, P.A.; Standage, M.; English, C.; Olds, T. Small Steps: Preliminary effectiveness and feasibility of an incremental goal-setting intervention to reduce sitting time in older adults. Maturitas 2016, 85, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Copeland, J.L.; Ashe, M.C.; Biddle, S.J.; Brown, W.J.; Buman, M.P.; Chastin, S.; Gardiner, P.A.; Inoue, S.; Jefferis, B.J.; Oka, K.; et al. Sedentary time in older adults: A critical review of measurement, associations with health, and interventions. Br. J. Sports Med. 2017, 51, 1539. [Google Scholar] [CrossRef]
- De Souto Barreto, P.; Delrieu, J.; Andrieu, S.; Vellas, B.; Rolland, Y. Physical activity and cognitive function in middle-aged and older adults: An analysis of 104,909 people from 20 countries. Mayo Clin. Proc. 2016, 91, 1515–1524. [Google Scholar] [CrossRef] [PubMed]
- Alosco, M.L.; Spitznagel, M.B.; Cohen, R.; Raz, N.; Sweet, L.H.; Josephson, R.; Hughes, J.; Rosneck, J.; Gunstad, J. Decreased physical activity predicts cognitive dysfunction and reduced cerebral blood flow in heart failure. J. Neurol. Sci. 2014, 339, 169–175. [Google Scholar] [CrossRef]
- Halloway, S.; Schoeny, M.E.; Wilbur, J.; Barnes, L.L. Interactive effects of physical activity and cognitive activity on cognition in older adults without mild cognitive impairment or dementia. J. Aging Health 2019. [Google Scholar] [CrossRef]
- Zhu, W.; Wadley, V.G.; Howard, V.J.; Hutto, B.; Blair, S.N.; Hooker, S.P. Objectively measured physical activity and cognitive function in older adults. Med. Sci. Sport Exerc. 2017, 49, 47–53. [Google Scholar] [CrossRef]
- Angevaren, M.; Vanhees, L.; Nooyens, A.C.J.; Wendel-Vos, C.G.W.; Verschuren, W.M.M. Physical activity and 5-year cognitive decline in the Doetinchem cohort study. Ann. Epidemiol. 2019, 20, 473–479. [Google Scholar] [CrossRef]
- Espeland, M.A.; Lipska, K.; Miller, M.E.; Rushing, J.; Cohen, R.A.; Verghese, J.; Pahor, M. Effects of physical activity intervention on physical and cognitive function in sedentary adults with and without diabetes. J. Gerontol. A Biol. Sci. Med. Sci. 2017, 72, 861–866. [Google Scholar] [CrossRef]
- Zlatar, Z.Z.; Godbole, S.; Takemoto, M.; Crist, K.; Sweet, C.M.C.; Kerr, J.; Rosenberg, D.E. Changes in moderate intensity physical activity are associated with better cognition in the Multilevel Intervention for Physical Activity in Retirement Communities (MIPARC) study. Am. J. Geriatr. Psychiatry 2019, 27, 1110–1121. [Google Scholar] [CrossRef]
- Northey, J.M.; Cherbuin, N.; Pumpa, K.L.; Smee, D.J.; Rattray, B. Exercise interventions for cognitive function in adults older than 50: A systematic review with meta-analysis. Br. J. Sports Med. 2018, 52, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Weuve, J.; Kang, J.H.; Manson, J.E.; Breteler, M.M.B.; Ware, J.H.; Grodstein, F. Physical activity, including walking, and cognitive function in older women. JAMA 2004, 292, 1454–1461. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, S.; Wallis, M.; Polit, D.; Steele, M.; Shum, D.; Morris, N. The effects of multimodal exercise on cognitive and physical functioning and brain-derived neurotrophic factor in older women: A randomised controlled trial. Age Ageing 2014, 43, 623–629. [Google Scholar] [CrossRef]
- Gregory, M.A.; Boa Sorte Silva, N.C.; Gill, D.P.; McGowan, C.L.; Liu-Ambrose, T.; Shoemaker, J.K.; Petrella, R.J. Combined dual-task gait training and aerobic exercise to improve cognition, mobility, and vascular health in community-dwelling older adults at risk for future cognitive decline 1. J. Alzheimer’s Dis. 2017, 57, 747–763. [Google Scholar] [CrossRef] [PubMed]
- Heyn, P.; Abreu, B.C.; Ottenbacher, K.J. The effects of exercise training on elderly persons with cognitive impairment and dementia: A meta-analysis11No commercial party having a direct financial interest in the results of the research supporting this article has or will confer a benefit upon the author(s) or upon any organization with which the author(s) is/are associated. Arch. Phys. Med. Rehabil. 2004, 85, 1694–1704. [Google Scholar] [CrossRef]
- De Asteasu, M.L.S.; Martinez-Velilla, N.; Zambom-Ferraresi, F.; Casas-Herrero, A.; Izquierdo, M. Role of physical exercise on cognitive function in healthy older adults: A systematic review of randomized clinical trials. Ageing Res. Rev. 2017, 37, 117–134. [Google Scholar] [CrossRef]
- Herting, M.M.; Keenan, M.F.; Nagel, B.J. Aerobic fitness linked to cortical brain development in adolescent males: Preliminary findings suggest a possible role of BDNF genotype. Front. Hum. Neurosci. 2016, 10. [Google Scholar] [CrossRef]
- Colcombe, S.; Kramer, A.F. Fitness effects on the cognitive function of older adults: A meta-analytic study. Psychol. Sci. 2003, 14, 125–130. [Google Scholar] [CrossRef]
- American Heart Association. High Blood Pressure; American Heart Association: Dallas, IL, USA, 2013. [Google Scholar]
- Kuo, H.K.; Sorond, F.; Iloputaife, I.; Gagnon, M.; Milberg, W.; Lipsitz, L.A. Effect of blood pressure on cognitive functions in elderly persons. J. Gerontol. A Biol. Sci. Med. Sci. 2004, 59, 1191–1194. [Google Scholar] [CrossRef]
- Knopman, D.S.; Mosley, T.H.; Catellier, D.J.; Coker, L.H. Fourteen-year longitudinal study of vascular risk factors, APOE genotype, and cognition: The ARIC MRI Study. Alzheimer’s Dement. 2009, 5, 207–214. [Google Scholar] [CrossRef]
- Abete, P.; Della-Morte, D.; Gargiulo, G.; Basile, C.; Langellotto, A.; Galizia, G.; Cacciatore, F. Cognitive impairment and cardiovascular diseases in the elderly. A heart–brain continuum hypothesis. Ageing Res. Rev. 2014, 18, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.S.; Vidal, J.S.; Masaki, K.; Petrovitch, H.; Ross, G.W.; Tilley, C.; Launer, L.J. Midlife blood pressure, plasma β-amyloid, and the risk for Alzheimer disease: The Honolulu Asia Aging Study. Hypertension 2012, 59, 780–786. [Google Scholar] [CrossRef] [PubMed]
- Cononie, C.C.; Graves, J.E.; Pollock, M.L.; Phillips, M.I.; Sumners, C.; Hagberg, J.M. Effect of exercise training on blood pressure in 70-to 79-yr-old men and women. Med. Sci. Sport Exerc. 1991, 23, 505–511. [Google Scholar] [CrossRef]
- Simão, R.; Fleck, S.J.; Polito, M.; Monteiro, W.; Farinatti, P. Effects of resistance training intensity, volume, and session format on the postexercise hypotensive response. J. Strength Cond. Res. 2005, 19, 853. [Google Scholar]
- Fagard, R.H. Exercise is good for your blood pressure: Effects of endurance training and resistance training. Clin. Exp. Pharmacol. Physiol. 2006, 33, 853–856. [Google Scholar] [CrossRef]
- Cornelissen, V.A.; Smart, N.A. Exercise training for blood pressure: A systematic review and meta-analysis. Am. Heart J. 2013, 2, e004473. [Google Scholar] [CrossRef]
- Fernando, D.; Nikolaos, P.; Felix, S.; Robert, A.; Walter, Z.; Timm, W.H. Aerobic exercise reduces blood pressure in resistant hypertension. Hypertension 2012, 60, 653–658. [Google Scholar] [CrossRef]
- Monnink, S.H.; Tio, R.A.; van Boven, A.J.; van Gilst, W.H.; van Veldhuisen, D.J. The role of coronary endothelial function testing in patients suspected for angina pectoris. Int. J. Cardiol. 2004, 96, 123–129. [Google Scholar] [CrossRef]
- Pal, S.; Radavelli-Bagatini, S.; Ho, S. Potential benefits of exercise on blood pressure and vascular function. Am. J. Hypertens. 2013, 7, 494–506. [Google Scholar] [CrossRef]
- Lusis, A.J. Atherosclerosis. Nature 2000, 407, 233–241. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Benjamin, E.J.; Go, A.S.; Arnett, D.K.; Blaha, M.J.; Cushman, M.; Huffman, M.D. Executive summary: Heart disease and stroke statistics—2015 update: A report from the American Heart Association. Circulation 2015, 131, 434–441. [Google Scholar] [CrossRef]
- Ihle-Hansen, H.; Vigen, T.; Berge, T.; Hagberg, G.; Engedal, K.; Rønning, O.M.; Tveit, A. Carotid Atherosclerosis and cognitive function in a general population aged 63–65 years: Data from the akershus cardiac examination (ACE) 1950 study. J. Alzheimer’s Dis. 2019, 70, 1041–1049. [Google Scholar] [CrossRef] [PubMed]
- Pase, M.P.; Herbert, A.; Grima, N.A.; Pipingas, A.; O’Rourke, M.F. Arterial stiffness as a cause of cognitive decline and dementia: A systematic review and meta-analysis. Intern. Med. J. 2012, 42, 808–815. [Google Scholar] [CrossRef] [PubMed]
- Triantafyllidi, H.; Arvaniti, C.; Lekakis, J.; Ikonomidis, I.; Siafakas, N.; Tzortzis, S.; Kremastinos, D.T. Cognitive impairment is related to increased arterial stiffness and microvascular damage in patients with never-treated essential hypertension. Am. J. Hypertens. 2009, 22, 525–530. [Google Scholar] [CrossRef]
- Kozakova, M.; Paterni, M.; Bartolomucci, F.; Morizzo, C.; Rossi, G.; Galetta, F.; Palombo, C. Epicardial coronary artery size in hypertensive and physiologic left ventricular hypertrophy. Am. J. Hypertens. 2005, 20, 279–284. [Google Scholar] [CrossRef]
- Casey, D.P.; Pierce, G.L.; Howe, K.S.; Mering, M.C.; Braith, R.W. Effect of resistance training on arterial wave reflection and brachial artery reactivity in normotensive postmenopausal women. Eur. J. Appl. Physiol. 2007, 100, 403–408. [Google Scholar] [CrossRef]
- Maeda, S.; Miyauchi, T.; Kakiyama, T.; Sugawara, J.; Iemitsu, M.; Irukayama-Tomobe, Y.; Matsuda, M. Effects of exercise training of 8 weeks and detraining on plasma levels of endothelium-derived factors, endothelin-1 and nitric oxide, in healthy young humans. Life Sci. 2001, 69, 1005–1016. [Google Scholar] [CrossRef]
- Miyaki, A.; Maeda, S.; Yoshizawa, M.; Misono, M.; Saito, Y.; Sasai, H.; Ajisaka, R. Effect of habitual aerobic exercise on body weight and arterial function in overweight and obese men. Am. J. Cardiol. 2009, 104, 823–828. [Google Scholar] [CrossRef]
- American Diabetes Association. 2. Classification and diagnosis of diabetes. Diabetes Care 2015, 38, S8–S16. [Google Scholar] [CrossRef]
- Kirkman, M.S.; Briscoe, V.J.; Clark, N.; Florez, H.; Haas, L.B.; Halter, J.B.; Pratley, R.E. Diabetes in older adults. Diabetes Care 2012, 35, 2650–2664. [Google Scholar] [CrossRef]
- Cheng, G.; Huang, C.; Deng, H.; Wang, H. Diabetes as a risk factor for dementia and mild cognitive impairment: A meta-analysis of longitudinal studies. Intern. Med. J. 2012, 42, 484–491. [Google Scholar] [CrossRef] [PubMed]
- Nooyens, A.C.J.; Baan, C.A.; Spijkerman, A.M.W.; Verschuren, W.M.M. Type 2 diabetes and cognitive decline in middle-aged men and women: The Doetinchem Cohort Study. Diabetes Care 2010, 33, 1964–1969. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, A.M.; Musen, G.; Ryan, C.M.; Silvers, N.; Cleary, P.; Waberski, B.; Harth, J. Long-term effect of diabetes and its treatment on cognitive function. N. Engl. J. Med. 2007, 356, 1842–1852. [Google Scholar] [PubMed]
- Brands, A.M.; Biessels, G.J.; De Haan, E.H.; Kappelle, L.J.; Kessels, R.P. The effects of type 1 diabetes on cognitive performance: A meta-analysis. Diabetes Care 2005, 28, 726–735. [Google Scholar] [CrossRef] [PubMed]
- Musen, G.; Tinsley, L.J.; Marcinkowski, K.A.; Pober, D.; Sun, J.K.; Khatri, M.; Keenan, H.A. Cognitive function deficits associated with long-duration type 1 diabetes and vascular complications. Diabetes Care 2018, 41, 1749–1756. [Google Scholar] [CrossRef]
- Castaneda, C.; Layne, J.E.; Munoz-Orians, L.; Gordon, P.L.; Walsmith, J.; Foldvari, M.; Nelson, M.E. A randomized controlled trial of resistance exercise training to improve glycemic control in older adults with type 2 diabetes. Diabetes Care 2002, 25, 2335–2341. [Google Scholar] [CrossRef]
- Mavros, Y.; Kay, S.; Anderberg, K.A.; Baker, M.K.; Wang, Y.; Zhao, R.; Baune, B.T. Changes in insulin resistance and HbA1c are related to exercise-mediated changes in body composition in older adults with type 2 diabetes: Interim outcomes from the GREAT2DO trial. Diabetes Care 2013, 36, 2372–2379. [Google Scholar] [CrossRef]
- Fakhouri, T.H. Prevalence of Obesity among Older Adults in the United States, 2007–2010 (No. 106); US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics: Hyattsville, MD, USA, 2012.
- Ogden, C.L.; Flegal, K.M. Prevalence of obesity among adults and youth: United States, 2011–2014. NCHS Data Brief 2015, 219, 8. [Google Scholar]
- McLaren, L. Socioeconomic status and obesity. Epidemiol. Rev. 2007, 29, 29–48. [Google Scholar] [CrossRef]
- Malandrino, N.; Capristo, E.; Taveira, T.H.; Mingrone, G.; Wu, W.-C. Cognitive function in individuals with normal weight obesity: Results from the third national health and nutrition examination survey (NHANES III). J. Alzheimer’s Dis. 2018, 65, 125–135. [Google Scholar] [CrossRef]
- Whitmer, R.A.; Gunderson, E.P.; Barrett-Connor, E.; Quesenberry, C.P.; Yaffe, K. Obesity in middle age and future risk of dementia: A 27year longitudinal population based study. BMJ 2005, 330, 1360. [Google Scholar] [CrossRef] [PubMed]
- Gunstad, J.; Paul, R.H.; Cohen, R.A.; Tate, D.F.; Spitznagel, M.B.; Grieve, S.; Gordon, E. Relationship between body mass index and brain volume in healthy adults. J. Neurosci. 2008, 118, 1582–1593. [Google Scholar] [CrossRef] [PubMed]
- Verstynen, T.D.; Weinstein, A.M.; Schneider, W.W.; Jakicic, J.M.; Rofey, D.L.; Erickson, K.I. Increased body mass index is associated with a global and distributed decrease in white matter microstructural integrity. Psychosom. Med. 2012, 74, 682–690. [Google Scholar] [CrossRef] [PubMed]
- Bischof, G.N.; Park, D.C. Obesity and aging: Consequences for cognition, brain structure and brain function. Psychosom. Med. 2015, 77, 697–709. [Google Scholar] [CrossRef] [PubMed]
- Sturman, M.T.; de Leon, C.M.; Bienias, J.L.; Morris, M.C.; Wilson, R.S.; Evans, D.A. Body mass index and cognitive decline in a biracial community population. Neurology 2008, 70, 360–367. [Google Scholar] [CrossRef] [PubMed]
- Villareal, D.T.; Aguirre, L.; Gurney, A.B.; Waters, D.L.; Sinacore, D.R.; Colombo, E.; Armamento-Villareal, R.; Qualls, C. Aerobic or resistance exercise, or both, in dieting obese older adults. N. Engl. J. Med. 2017, 376, 1943–1955. [Google Scholar] [CrossRef]
- Shibata, A.I.; Oka, K.; Sugiyama, T.; Salmon, J.O.; Dunstan, D.W.; Owen, N. Physical activity, television viewing time, and 12-year changes in waist circumference. Med. Sci. Sport Exerc. 2016, 48, 633. [Google Scholar] [CrossRef]
- Dumuid, D.; Lewis, L.K.; Olds, T.S.; Maher, C.; Bondarenko, C.; Norton, L. Relationships between older adults’ use of time and cardio-respiratory fitness, obesity and cardio-metabolic risk: A compositional isotemporal substitution analysis. Maturitas 2018, 110, 104–110. [Google Scholar] [CrossRef]
- Brewster, G.S.; Peterson, L.; Roker, R.; Ellis, M.L.; Edwards, J.D. Depressive symptoms, cognition and everyday function among community-residing older adults. J. Aging Health 2017, 29, 367–388. [Google Scholar] [CrossRef]
- Donovan, N.J.; Wu, Q.; Rentz, D.M.; Sperling, R.A.; Marshall, G.A.; Glymour, M.M. Loneliness, depression and cognitive function in older U.S. adults. Int. J. Geriatr. Psychiatry 2017, 32, 564–573. [Google Scholar] [CrossRef]
- Potter, G.; Steffens, D. Contribution of depression to cognitive impairment and dementia in older adults. Neurologist 2007, 13, 105–117. [Google Scholar] [CrossRef] [PubMed]
- Vance, D.E.; Marson, D.C.; Triebel, K.L.; Ball, K.; Wadley, V.G.; Humphrey, S.C. Physical activity and cognitive function in older adults: The mediating effect of depressive symptoms. J. Neurosci. Nurs. 2016, 48, E2. [Google Scholar] [CrossRef] [PubMed]
- Bridle, C.; Spanjers, K.; Patel, S.; Atherton, N.M.; Lamb, S.E. Effect of exercise on depression severity in older people: Systematic review and meta-analysis of randomised controlled trials. Br. J. Psychiatry 2012, 201, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Schuch, F.B.; Vancampfort, D.; Rosenbaum, S.; Richards, J.; Ward, P.B.; Veronese, N.; Stubbs, B. Exercise for depression in older adults: A meta-analysis of randomized controlled trials adjusting for publication bias. Braz. J. Psychiatry 2016, 38, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Huang, E.J.; Reichardt, L.F. Neurotrophins: Roles in neuronal development and function. Annu. Rev. Neurosci. 2001, 24, 677–736. [Google Scholar] [CrossRef] [PubMed]
- Miranda, M.; Morici, J.F.; Zanoni, M.B.; Bekinschtein, P. Brain-derived neurotrophic factor: A key molecule for memory in the healthy and the pathological brain. Front. Cell Neurosci 2019, 13. [Google Scholar] [CrossRef]
- Erickson, K.I.; Prakash, R.S.; Voss, M.W.; Chaddock, L.; Heo, S.; McLaren, M.; Pence, B.D.; Martin, S.A.; Vieira, V.J.; Woods, J.A.; et al. Brain-derived neurotrophic factor is associated with age-related decline in hippocampal volume. J. Neurosci. 2010, 30, 5368–5375. [Google Scholar] [CrossRef]
- Shimada, H.; Makizako, H.; Doi, T.; Yoshida, D.; Tsutsumimoto, K.; Anan, Y.; Uemura, K.; Lee, S.; Park, H.; Suzuki, T. A large, cross-sectional observational study of serum BDNF, cognitive function, and Mild Cognitive Impairment in the elderly. Front. Aging Neurosci. 2014, 6. [Google Scholar] [CrossRef]
- Hock, C.; Heese, K.; Hulette, C.; Rosenberg, C.; Otten, U. Region-specific neurotrophin imbalances in Alzheimer disease: Decreased levels of brain-derived neurotrophic factor and increased levels of nerve growth factor in hippocampus and cortical areas. Arch. Neurol. 2000, 57, 846–851. [Google Scholar] [CrossRef]
- Peng, S.; Wuu, J.; Mufson, E.J.; Fahnestock, M. Precursor form of brain-derived neurotrophic factor and mature brain-derived neurotrophic factor are decreased in the pre-clinical stages of Alzheimer’s disease. J. Neurochem. 2005, 93, 1412–1421. [Google Scholar] [CrossRef]
- Komulainen, P.; Pedersen, M.; Hänninen, T.; Bruunsgaard, H.; Lakka, T.A.; Kivipelto, M.; Hassinen, M.; Rauramaa, T.H.; Pedersen, B.K.; Rauramaa, R. BDNF is a novel marker of cognitive function in ageing women: The DR’s EXTRA Study. Neurobiol. Learn. Mem. 2008, 90, 596–603. [Google Scholar] [CrossRef]
- Gomez-Pinilla, F.; Zhuang, Y.; Feng, J.; Ying, Z.; Fan, G. Exercise impacts brain-derived neurotrophic factor plasticity by engaging mechanisms of epigenetic regulation. Eur. J. Neurosci. 2011, 33, 383–390. [Google Scholar] [CrossRef] [PubMed]
- De Melo Coelho, F.G.; Gobbi, S.; Andreatto, C.A.A.; Corazza, D.I.; Pedroso, R.V.; Santos-Galduróz, R.F. Physical exercise modulates peripheral levels of brain-derived neurotrophic factor (BDNF): A systematic review of experimental studies in the elderly. Arch. Gerontol. Geriatr. 2013, 56, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Engeroff, T.; Füzéki, E.; Vogt, L.; Fleckenstein, J.; Schwarz, S.; Matura, S.; Banzer, W. Is Objectively Assessed Sedentary Behavior, Physical Activity and Cardiorespiratory Fitness Linked to Brain Plasticity Outcomes in Old Age? Neuroscience 2018, 388, 384–392. [Google Scholar] [CrossRef]
- Håkansson, K.; Ledreux, A.; Daffner, K.; Terjestam, Y.; Bergman, P.; Carlsson, R.; Kivipelto, M.; Winblad, B.; Granholm, A.-C.; Mohammed, A.K.H. BDNF responses in healthy older persons to 35 minutes of physical exercise, cognitive training, and mindfulness: Associations with working memory function. J. Alzheimer’s Dis. JAD 2017, 55, 645–657. [Google Scholar] [CrossRef]
- Phillips, C. Lifestyle modulators of neuroplasticity: How physical activity, mental engagement, and diet promote cognitive health during aging. Neural Plast. 2017. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.W.; Tsai, S.F.; Kuo, Y.M. Physical exercise enhances neuroplasticity and delays Alzheimer’s disease. Brain Plast. 2018, 4, 95–110. [Google Scholar] [CrossRef] [PubMed]
- Eadie, B.D.; Redila, V.A.; Christie, B.R. Voluntary exercise alters the cytoarchitecture of the adult dentate gyrus by increasing cellular proliferation, dendritic complexity, and spine density. J. Comp. Neurol. 2005, 486, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Soulé, J.; Messaoudi, E.; Bramham, C.R. Brain-derived neurotrophic factor and control of synaptic consolidation in the adult brain. Biochem. Soc. Trans. 2006, 34, 600–604. [Google Scholar] [CrossRef]
- Pereira, A.C.; Huddleston, D.E.; Brickman, A.M.; Sosunov, A.A.; Hen, R.; McKhann, G.M.; Small, S.A. An in vivo correlate of exercise-induced neurogenesis in the adult dentate gyrus. Proc. Natl. Acad. Sci. USA 2007, 104, 5638–5643. [Google Scholar] [CrossRef]
- Lee, J.; Duan, W.; Mattson, M.P. Evidence that brain-derived neurotrophic factor is required for basal neurogenesis and mediates, in part, the enhancement of neurogenesis by dietary restriction in the hippocampus of adult mice. J. Neurochem. 2002, 82, 1367–1375. [Google Scholar] [CrossRef] [PubMed]
- Cotman, C.W.; Berchtold, N.C.; Christie, L.-A. Exercise builds brain health: Key roles of growth factor cascades and inflammation. Trends Neurosci. 2007, 30, 464–472. [Google Scholar] [CrossRef] [PubMed]
- Trejo, J.L.; Carro, E.; Torres-Alemán, I. Circulating insulin-like growth factor I mediates exercise-induced increases in the number of new neurons in the adult hippocampus. J. Neurosci. 2001, 21, 1628–1634. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Zhang, L.; Hu, Z. Cerebral insulin, insulin signaling pathway, and brain angiogenesis. Neurol. Sci. 2016, 37, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Lopez, C.; LeRoith, D.; Torres-Aleman, I. Insulin-like growth factor I is required for vessel remodeling in the adult brain. Proc. Natl. Acad. Sci. USA 2004, 101, 9833–9838. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, N. Role of vascular endothelial growth factor in the regulation of angiogenesis. Kidney Int. 1999, 56, 794–814. [Google Scholar] [CrossRef]
- Ding, Y.H.; Li, J.; Zhou, Y.; Rafols, J.A.; Clark, J.C.; Ding, Y. Cerebral angiogenesis and expression of angiogenic factors in aging rats after exercise. Curr. Neurovasc. Res. 2006, 3, 15–23. [Google Scholar] [CrossRef]
- Zhang, H.; DiBaise, J.K.; Zuccolo, A.; Kudrna, D.; Braidotti, M.; Yu, Y.; Parameswaran, P.; Crowell, M.D.; Wing, R.; Rittmann, B.E.; et al. Human gut microbiota in obesity and after gastric bypass. Proc. Natl. Acad. Sci. USA 2009, 106, 2365–2370. [Google Scholar] [CrossRef]
- Rhyu, I.J.; Bytheway, J.A.; Kohler, S.J.; Lange, H.; Lee, K.J.; Boklewski, J.; McCormick, K.; Williams, N.I.; Stanton, G.B.; Greenough, W.T.; et al. Effects of aerobic exercise training on cognitive function and cortical vascularity in monkeys. Neuroscience 2010, 167, 1239–1248. [Google Scholar] [CrossRef]
- Bullitt, E.; Rahman, F.N.; Smith, J.K.; Kim, E.; Zeng, D.; Katz, L.M.; Marks, B.L. The effect of exercise on the cerebral vasculature of healthy aged subjects as visualized by MR angiography. Am. J. AJNR Am. J. Neuroradiol. 2009, 30, 1857–1863. [Google Scholar] [CrossRef]
- Cunningham, C.; Hennessy, E. Co-morbidity and systemic inflammation as drivers of cognitive decline: New experimental models adopting a broader paradigm in dementia research. Alzheimer’s Res. Ther. 2015, 7, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Perry, V.H.; Holmes, C. Microglial priming in neurodegenerative disease. Nat. Rev. Neurol. 2014, 10, 217–224. [Google Scholar] [CrossRef]
- Trapero, I.; Cauli, O. Interleukin 6 and cognitive dysfunction. Metab. Brain Dis. 2014, 29, 593–608. [Google Scholar] [CrossRef] [PubMed]
- Marsland, A.L.; Gianaros, P.J.; Kuan, D.C.-H.; Sheu, L.K.; Krajina, K.; Manuck, S.B. Brain morphology links systemic inflammation to cognitive function in midlife adults. Brain Behav. Immun. 2015, 48, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Bettcher, B.M.; Kramer, J.H. Longitudinal inflammation, cognitive decline, and Alzheimer’s disease: A mini-review. Clin. Pharmacol. Ther. 2014, 96, 464–469. [Google Scholar] [CrossRef]
- Marsland, A.L.; Petersen, K.L.; Sathanoori, R.; Muldoon, M.F.; Neumann, S.A.; Ryan, C.; Flory, J.D.; Manuck, S.B. Interleukin-6 covaries inversely with cognitive performance among middle-aged community volunteers. Psychosom. Med. 2006, 68, 895–903. [Google Scholar] [CrossRef]
- Yirmiya, R.; Goshen, I. Immune modulation of learning, memory, neural plasticity and neurogenesis. Brain Behav. Immun. 2011, 25, 181–213. [Google Scholar] [CrossRef]
- Colbert, L.H.; Visser, M.; Simonsick, E.M.; Tracy, R.P.; Newman, A.B.; Kritchevsky, S.B.; Harris, T.B. Physical activity, exercise, and inflammatory markers in older adults: Findings from the Health, Aging and Body Composition Study. J. Am. Geriatr. Soc. 2004, 52, 1098–1104. [Google Scholar] [CrossRef]
- Kohut, M.L.; McCann, D.A.; Russell, D.W.; Konopka, D.N.; Cunnick, J.E.; Franke, W.D.; Vanderah, E. Aerobic exercise, but not flexibility/resistance exercise, reduces serum IL-18, CRP, and IL-6 independent of β-blockers, BMI, and psychosocial factors in older adults. Brain Behav. Immun. 2006, 20, 201–209. [Google Scholar] [CrossRef]
- Woods, J.A.; Ceddia, M.A.; Wolters, B.W.; Evans, J.K.; Lu, Q.; McAuley, E. Effects of 6 months of moderate aerobic exercise training on immune function in the elderly. Mech. Ageing Dev. 1999, 109, 1–19. [Google Scholar] [CrossRef]
- Ursell, L.K.; Metcalf, J.L.; Parfrey, L.W.; Knight, R. Defining the human microbiome. Nutr. Rev. 2012, 70, S38–S44. [Google Scholar] [CrossRef] [PubMed]
- Turnbaugh, P.J.; Ley, R.E.; Hamady, M.; Fraser-Liggett, C.M.; Knight, R.; Gordon, J.I. The Human Microbiome Project. Nature 2007, 449, 804–810. [Google Scholar] [CrossRef] [PubMed]
- Mariat, D.; Firmesse, O.; Levenez, F.; Guimarăes, V.; Sokol, H.; Doré, J.; Corthier, G.; Furet, J.-P. The Firmicutes/Bacteroidetes ratio of the human microbiota changes with age. BMC Microbiol. 2009, 9, 123. [Google Scholar] [CrossRef] [PubMed]
- Tilg, H.; Kaser, A. Gut microbiome, obesity, and metabolic dysfunction. J. Clin. Investig. 2011, 121, 2126–2132. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Seo, J.H.; Youn, H.S. Gut microbiota and clinical disease: Obesity and nonalcoholic Fatty liver disease. J. Pediatric Gastroenterol. Nutr. 2013, 16, 22–27. [Google Scholar] [CrossRef]
- O’Mahony, S.M.; Marchesi, J.R.; Scully, P.; Codling, C.; Ceolho, A.M.; Quigley, E.M.; Dinan, T.G. Early life stress alters behavior, immunity, and microbiota in rats: Implications for irritable bowel syndrome and psychiatric illnesses. Biol. Psychiatry 2009, 65, 263–267. [Google Scholar] [CrossRef]
- Leblhuber, F.; Strasser, B.; Steiner, K.; Gostner, J.; Schuetz, B.; Fuchs, D. On the role of intestinal microbiota in patients with cognitive decline. J. Pharm. Pharmacol. 2017, 5, 648–653. [Google Scholar]
- Li, B.; He, Y.; Ma, J.; Huang, P.; Du, J.; Cao, L.; Chen, S. Mild cognitive impairment has similar alterations as Alzheimer’s disease in gut microbiota. Alzheimer’s Dement. 2019, 15, 1357–1366. [Google Scholar] [CrossRef]
- Biagi, E.; Nylund, L.; Candela, M.; Ostan, R.; Bucci, L.; Pini, E.; Brigidi, P. Through ageing, and beyond: Gut microbiota and inflammatory status in seniors and centenarians. PLoS ONE 2010, 5, e10667. [Google Scholar] [CrossRef]
- O’Toole, P.W.; Jeffery, I.B. Gut microbiota and aging. Science 2015, 350, 1214–1215. [Google Scholar] [CrossRef]
- Junges, V.M.; Closs, V.E.; Nogueira, G.M.; Gottlieb, M.G.V. Crosstalk between Gut Microbiota and Central Nervous System: A Focus on Alzheimer’s Disease [Text]; Bentham Science Publishers: Sharjah, UAE, 2018. [Google Scholar] [CrossRef]
- Nankova, B.B.; Agarwal, R.; MacFabe, D.F.; Gamma, E.F.L. Enteric bacterial metabolites propionic and butyric acid modulate gene expression, including CREB-dependent catecholaminergic neurotransmission, in PC12 cells—Possible relevance to autism spectrum disorders. PLoS ONE 2014, 9, e103740. [Google Scholar] [CrossRef] [PubMed]
- Wilkins, J.M.; Trushina, E. Application of metabolomics in Alzheimer’s disease. Front. Neurol. 2018, 8. [Google Scholar] [CrossRef] [PubMed]
- Ackerman, H.D.; Gerhard, G.S. Bile acids in neurodegenerative disorders. Front. Aging Neurosci. 2016, 8. [Google Scholar] [CrossRef]
- Mapstone, M.; Cheema, A.K.; Fiandaca, M.S.; Zhong, X.; Mhyre, T.R.; MacArthur, L.H.; Hall, W.J.; Fisher, S.G.; Peterson, D.R.; Haley, J.M.; et al. Plasma phospholipids identify antecedent memory impairment in older adults. Nat. Med. 2014, 20, 415–418. [Google Scholar] [CrossRef] [PubMed]
- Sherwin, E.; Sandhu, K.V.; Dinan, T.G.; Cryan, J.F. May the force be with tou: The light and dark sides of the microbiota–gut–brain axis in neuropsychiatry. CNS Drugs 2016, 30, 1019–1041. [Google Scholar] [CrossRef]
- De Vadder, F.; Kovatcheva-Datchary, P.; Goncalves, D.; Vinera, J.; Zitoun, C.; Duchampt, A.; Bäckhed, F.; Mithieux, G. Microbiota-generated metabolites promote metabolic benefits via gut-brain neural circuits. Cell 2014, 156, 84–96. [Google Scholar] [CrossRef]
- Schroeder, F.A.; Lin, C.L.; Crusio, W.E.; Akbarian, S. Antidepressant-like effects of the histone deacetylase inhibitor, sodium butyrate, in the mouse. Biol. Psychiatry 2007, 62, 55–64. [Google Scholar] [CrossRef]
- MacFabe, D.F.; Cain, D.P.; Rodriguez-Capote, K.; Franklin, A.E.; Hoffman, J.E.; Boon, F.; Taylor, A.R.; Kavaliers, M.; Ossenkopp, K.-P. Neurobiological effects of intraventricular propionic acid in rats: Possible role of short chain fatty acids on the pathogenesis and characteristics of autism spectrum disorders. Behav. Brain Res. 2007, 176, 149–169. [Google Scholar] [CrossRef]
- Santacruz, A.; Collado, M.C.; García-Valdés, L.; Segura, M.T.; Martín-Lagos, J.A.; Anjos, T.; Martí-Romero, M.; Lopez, R.M.; Florido, J.; Campoy, C.; et al. Gut microbiota composition is associated with body weight, weight gain and biochemical parameters in pregnant women. Br. J. Nutr. 2010, 104, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006, 444, 1027–1031. [Google Scholar] [CrossRef]
- Han, K.; Bose, S.; Kim, Y.M.; Chin, Y.W.; Kim, B.S.; Wang, J.H.; Kim, H. Rehmannia glutinosa reduced waist circumferences of Korean obese women possibly through modulation of gut microbiota. Food Funct. 2015, 6, 2684–2692. [Google Scholar] [CrossRef] [PubMed]
- Dicksved, J.; Halfvarson, J.; Rosenquist, M.; Järnerot, G.; Tysk, C.; Apajalahti, J.; Engstrand, L.; Jansson, J.K. Molecular analysis of the gut microbiota of identical twins with Crohn’s disease. ISME J. 2008, 2, 716–727. [Google Scholar] [CrossRef] [PubMed]
- Giacomin, P.; Croese, J.; Krause, L.; Loukas, A.; Cantacessi, C. Suppression of inflammation by helminths: A role for the gut microbiota? Philos. Trans. R. Soc. Lond. B Biol. Sci. 2015, 370, 20140296. [Google Scholar] [CrossRef] [PubMed]
- Collado, M.C.; Donat, E.; Ribes-Koninckx, C.; Calabuig, M.; Sanz, Y. Specific duodenal and faecal bacterial groups associated with paediatric coeliac disease. Am. J. Clin. Pathol. 2009, 62, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Sanz, Y.; Sánchez, E.; Marzotto, M.; Calabuig, M.; Torriani, S.; Dellaglio, F. Differences in faecal bacterial communities in coeliac and healthy children as detected by PCR and denaturing gradient gel electrophoresis. FEMS Immunol. Med. Microbiol. 2007, 51, 562–568. [Google Scholar] [CrossRef] [PubMed]
- Collins, K.H.; Paul, H.A.; Reimer, R.A.; Seerattan, R.A.; Hart, D.A.; Herzog, W. Relationship between inflammation, the gut microbiota, and metabolic osteoarthritis development: Studies in a rat model. Osteoarthr. Cartil. 2015, 23, 1989–1998. [Google Scholar] [CrossRef] [PubMed]
- Velloso, L.A.; Folli, F.; Saad, M.J. TLR4 at the crossroads of nutrients, gut microbiota, and metabolic inflammation. Endocr. Rev. 2015, 36, 245–271. [Google Scholar] [CrossRef]
- Murri, M.; Leiva, I.; Gomez-Zumaquero, J.M.; Tinahones, F.J.; Cardona, F.; Soriguer, F.; Queipo-Ortuño, M.I. Gut microbiota in children with type 1 diabetes differs from that in healthy children: A case-control study. BMC Med. 2013, 11, 46. [Google Scholar] [CrossRef]
- Giongo, A.; Gano, K.A.; Crabb, D.B.; Mukherjee, N.; Novelo, L.L.; Casella, G.; Drew, J.C.; Ilonen, J.; Knip, M.; Hyöty, H.; et al. Toward defining the autoimmune microbiome for type 1 diabetes. ISME J. 2011, 5, 82–91. [Google Scholar] [CrossRef]
- Qin, J.; Li, Y.; Cai, Z.; Li, S.; Zhu, J.; Zhang, F.; Liang, S.; Zhang, W.; Guan, Y.; Shen, D.; et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature 2012, 490, 55–60. [Google Scholar] [CrossRef]
- Ruan, Y.; Sun, J.; He, J.; Chen, F.; Chen, R.; Chen, H. Effect of probiotics on glycemic control: A systematic review and meta-analysis of randomized, controlled trials. PLoS ONE 2015, 10, e0132121. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.; Nicolucci, A.C.; Virtanen, H.; Schick, A.; Meddings, J.; Reimer, R.A.; Huang, C. Effect of prebiotic on microbiota, intestinal permeability, and glycemic control in children with Type 1 diabetes. J. Clin. Endocrinol. Metab. 2019, 104, 4427–4440. [Google Scholar] [CrossRef]
- Gaulke, C.A.; Sharpton, T.J. The influence of ethnicity and geography on human gut microbiome composition. Nat. Med. 2018, 24, 1495–1496. [Google Scholar] [CrossRef]
- Deschasaux, M.; Bouter, K.E.; Prodan, A.; Levin, E.; Groen, A.K.; Herrema, H.; Tremaroli, V.; Bakker, G.J.; Attaye, I.; Pinto-Sietsma, S.-J.; et al. Depicting the composition of gut microbiota in a population with varied ethnic origins but shared geography. Nat. Med. 2018, 24, 1526–1531. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Wu, W.; Zheng, H.M.; Li, P.; McDonald, D.; Sheng, H.F.; Chen, X.J. Regional variation limits applications of healthy gut microbiome reference ranges and disease models. Nat. Med. 2018, 24, 1532–1535. [Google Scholar] [CrossRef] [PubMed]
- Jie, Z.; Xia, H.; Zhong, S.L.; Feng, Q.; Li, S.; Liang, S.; Zhang, D. The gut microbiome in atherosclerotic cardiovascular disease. Nat. Commun. 2017, 8, 1–12. [Google Scholar] [CrossRef]
- Shreiner, A.B.; Kao, J.Y.; Young, V.B. The gut microbiome in health and in disease. Curr. Opin. Gastroenterol. 2015, 31, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Naia, L.; Cunha-Oliveira, T.; Rodrigues, J.; Rosenstock, T.R.; Oliveira, A.; Ribeiro, M.; Carmo, C.; Oliveira-Sousa, S.I.; Duarte, A.I.; Hayden, M.R.; et al. Histone deacetylase inhibitors protect against pyruvate dehydrogenase dysfunction in Huntington’s Disease. J. Neurosci. 2017, 37, 2776–2794. [Google Scholar] [CrossRef] [PubMed]
- Da Zhou, Q.P.; Xin, F.Z.; Zhang, R.N.; He, C.X.; Chen, G.Y.; Liu, C.; Chen, Y.W.; Fan, J.G. Sodium butyrate attenuates high-fat diet-induced steatohepatitis in mice by improving gut microbiota and gastrointestinal barrier. World J. Gastroenterol. 2017, 23, 60–75. [Google Scholar] [CrossRef]
- Gao, F.; Lv, Y.; Long, J.; Chen, J.; He, J.; Ruan, X.; Zhu, H. Butyrate improves the metabolic disorder and gut microbiome dysbiosis in mice induced by a high-fat diet. Front. Pharmacol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Tu, B.P. Acetyl-CoA and the regulation of metabolism: Mechanisms and consequences. Curr. Opin. Cell Biol. 2015, 33, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.K.; Chang, H.-W.; Yan, D.; Lee, K.M.; Ucmak, D.; Wong, K.; Abrouk, M.; Farahnik, B.; Nakamura, M.; Zhu, T.H.; et al. Influence of diet on the gut microbiome and implications for human health. J. Transl. Med. 2017, 15. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.D.; Chen, J.; Hoffmann, C.; Bittinger, K.; Chen, Y.Y.; Keilbaugh, S.A.; Bewtra, M.; Knights, D.; Walters, W.A.; Knight, R.; et al. Linking long-term dietary patterns with gut microbial enterotypes. Science 2011, 334, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Fava, F.; Gitau, R.; Griffin, B.A.; Gibson, G.R.; Tuohy, K.M.; Lovegrove, J.A. The type and quantity of dietary fat and carbohydrate alter faecal microbiome and short-chain fatty acid excretion in a metabolic syndrome ‘at-risk’ population. Int. J. Obes. 2013, 37, 216–223. [Google Scholar] [CrossRef]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Biddinger, S.B. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef]
- Cotillard, A.; Kennedy, S.P.; Kong, L.C.; Prifti, E.; Pons, N.; Le Chatelier, E.; Gougis, S. Dietary intervention impact on gut microbial gene richness. Nature 2013, 500, 585–588. [Google Scholar] [CrossRef]
- Eid, N.; Enani, S.; Walton, G.; Corona, G.; Costabile, A.; Gibson, G.; Rowland, I.; Spencer, J.P.E. The impact of date palm fruits and their component polyphenols, on gut microbial ecology, bacterial metabolites and colon cancer cell proliferation. J. Nutr. Sci. 2014, 3. [Google Scholar] [CrossRef] [PubMed]
- Francavilla, R.; Calasso, M.; Calace, L.; Siragusa, S.; Ndagijimana, M.; Vernocchi, P.; Brunetti, L.; Mancino, G.; Tedeschi, G.; Guerzoni, E.; et al. Effect of lactose on gut microbiota and metabolome of infants with cow’s milk allergy. Pediatric Allergy Immunol. 2012, 23, 420–427. [Google Scholar] [CrossRef] [PubMed]
- Costabile, A.; Klinder, A.; Fava, F.; Napolitano, A.; Fogliano, V.; Leonard, C.; Gibson, G.R.; Tuohy, K.M. Whole-grain wheat breakfast cereal has a prebiotic effect on the human gut microbiota: A double-blind, placebo-controlled, crossover study. Br. J. Nutr. 2008, 99, 110–120. [Google Scholar] [CrossRef] [PubMed]
- Carvalho-Wells, A.L.; Helmolz, K.; Nodet, C.; Molzer, C.; Leonard, C.; McKevith, B.; Thielecke, F.; Jackson, K.G.; Tuohy, K.M. Determination of the in vivo prebiotic potential of a maize-based whole grain breakfast cereal: A human feeding study. Br. J. Nutr. 2010, 104, 1353–1356. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Roberfroid, M.B. Dietary modulation of the human colonic microbiota: Introducing the concept of prebiotics. J. Nutr. 1995, 125, 1401–1412. [Google Scholar] [CrossRef] [PubMed]
- Gulati, G.; Mulryan, D. The Psychobiotic Revolution: Mood, Food and the New Science of the Gut-Brain Connection Edited by Scott C. Anderson John F. Cryan and Ted Dinan 320 pp. ISBN 9781426218460. National Geographic, Washington, DC, 2017. Ir. J. Psychol. Med. 2017, 1. [Google Scholar] [CrossRef]
- Connolly, M.L.; Lovegrove, J.A.; Tuohy, K.M. In Vitro Fermentation Characteristics of Whole Grain Wheat Flakes and the Effect of Toasting on Prebiotic Potential. J. Med. Food 2011, 15, 33–43. [Google Scholar] [CrossRef]
- Hoseinifar, S.H.; Khalili, M.; Khoshbavar Rostami, H.; Esteban, M.Á. Dietary galactooligosaccharide affects intestinal microbiota, stress resistance, and performance of Caspian roach (Rutilus rutilus) fry. Fish. Shellfish Immunol. 2013, 35, 1416–1420. [Google Scholar] [CrossRef]
- Gori, A.; Rizzardini, G.; Land, B.; van’t, Amor, K.B.; van Schaik, J.; Torti, C.; Quirino, T.; Tincati, C.; Bandera, A.; Knol, J.; et al. Specific prebiotics modulate gut microbiota and immune activation in HAART-naive HIV-infected adults: Results of the “COPA” pilot randomized trial. Mucosal Immunol. 2011, 4, 554–563. [Google Scholar] [CrossRef] [PubMed]
- Dewulf, E.M.; Cani, P.D.; Claus, S.P.; Fuentes, S.; Puylaert, P.G.; Neyrinck, A.M.; Bindels, L.B.; de Vos, W.M.; Gibson, G.R.; Thissen, J.-P.; et al. Insight into the prebiotic concept: Lessons from an exploratory, double blind intervention study with inulin-type fructans in obese women. Gut 2013, 62, 1112–1121. [Google Scholar] [CrossRef] [PubMed]
- Butel, M.-J. Probiotics, gut microbiota and health. Médecine Mal. Infect. 2014, 44, 1–8. [Google Scholar] [CrossRef]
- Rajkumar, H.; Mahmood, N.; Kumar, M.; Varikuti, S.R.; Challa, H.R.; Myakala, S.P. Effect of probiotic (VSL#3) and omega-3 on lipid profile, insulin sensitivity, inflammatory markers, and gut colonization in overweight adults: A randomized, controlled trial [Research article]. Mediat. Inflamm. 2014. [Google Scholar] [CrossRef]
- Gibson, G.R. Dietary modulation of the human gut microflora using prebiotics. Br. J. Nutr. 1998, 80, S209–S212. [Google Scholar] [CrossRef]
- Mack, D.R.; Michail, S.; Wei, S.; McDougall, L.; Hollingsworth, M.A. Probiotics inhibit enteropathogenic E. coli adherence in vitro by inducing intestinal mucin gene expression. Am. J. Physiol. Gastrointest Liver Physiol. 1999, 276, G941–G950. [Google Scholar] [CrossRef]
- Yang, Y.-J.; Sheu, B.-S. Probiotics-containing yogurts suppress Helicobacter pylori load and modify immune response and intestinal microbiota in the Helicobacter pylori-infected children. Helicobacter 2012, 17, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Estaki, M.; Pither, J.; Baumeister, P.; Little, J.P.; Gill, S.K.; Ghosh, S.; Ahmadi-Vand, Z.; Marsden, K.R.; Gibson, D.L. Cardiorespiratory fitness as a predictor of intestinal microbial diversity and distinct metagenomic functions. Microbiome 2016, 4, 42. [Google Scholar] [CrossRef] [PubMed]
- Durk, R.P.; Castillo, E.; Márquez-Magaña, L.; Grosicki, G.J.; Bolter, N.D.; Lee, C.M.; Bagley, J.R. Gut microbiota composition is related to cardiorespiratory fitness in healthy young adults. Int. J. Sport Nutr. Exerc. Metab. 2019, 29, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Shi, Y.; Wiklund, P.; Tan, X.; Wu, N.; Zhang, X.; Tikkanen, O.; Zhang, C.; Munukka, E.; Cheng, S. The association between cardiorespiratory fitness and gut microbiota composition in premenopausal women. Nutrients 2017, 9, 792. [Google Scholar] [CrossRef]
- Luo, B.; Xiang, D.; Nieman, D.C.; Chen, P. The effects of moderate exercise on chronic stress-induced intestinal barrier dysfunction and antimicrobial defense. Brain Behav. Immun. 2014, 39, 99–106. [Google Scholar] [CrossRef]
- Campbell, S.C.; Wisniewski, P.J.; Noji, M.; McGuinness, L.R.; Häggblom, M.M.; Lightfoot, S.A.; Kerkhof, L.J. The effect of diet and exercise on intestinal integrity and microbial diversity in mice. PLoS ONE 2016, 11, e0150502. [Google Scholar] [CrossRef]
- Evans, C.C.; LePard, K.J.; Kwak, J.W.; Stancukas, M.C.; Laskowski, S.; Dougherty, J.; Moulton, L.; Glawe, A.; Wang, Y.; Leone, V.; et al. Exercise prevents weight gain and alters the gut microbiota in a mouse model of high fat diet-induced obesity. PLoS ONE 2014, 9, e92193. [Google Scholar] [CrossRef]
- Allen, J.M.; Mailing, L.J.; Niemiro, G.M.; Moore, R.; Cook, M.D.; White, B.A.; Holscher, H.D.; Woods, J.A. Exercise alters gut microbiota composition and function in lean and obese humans. Med. Sci. Sport Exerc. 2018, 50, 747. [Google Scholar] [CrossRef]
- Codella, R.; Luzi, L.; Terruzzi, I. Exercise has the guts: How physical activity may positively modulate gut microbiota in chronic and immune-based diseases. Dig. Liver Dis. 2018, 50, 331–341. [Google Scholar] [CrossRef]
- Mailing, L.J.; Allen, J.M.; Buford, T.W.; Fields, C.J.; Woods, J.A. Exercise and the gut microbiome: A review of the evidence, potential mechanisms, and implications for human health. Exerc. Sport Sci. Rev. 2019, 47, 75–85. [Google Scholar] [CrossRef]
- Worthmann, A.; John, C.; Rühlemann, M.C.; Baguhl, M.; Heinsen, F.-A.; Schaltenberg, N.; Heine, M.; Schlein, C.; Evangelakos, I.; Mineo, C.; et al. Cold-induced conversion of cholesterol to bile acids in mice shapes the gut microbiome and promotes adaptive thermogenesis. Nat. Med. 2017, 23, 839–849. [Google Scholar] [CrossRef]
- Kakiyama, G.; Pandak, W.M.; Gillevet, P.M.; Hylemon, P.B.; Heuman, D.M.; Daita, K.; Takei, H.; Muto, A.; Nittono, H.; Ridlon, J.M.; et al. Modulation of the fecal bile acid profile by gut microbiota in cirrhosis. J. Hepatol. 2013, 58, 949–955. [Google Scholar] [CrossRef] [PubMed]
- Meissner, M.; Lombardo, E.; Havinga, R.; Tietge, U.J.F.; Kuipers, F.; Groen, A.K. Voluntary wheel running increases bile acid as well as cholesterol excretion and decreases atherosclerosis in hypercholesterolemic mice. Atherosclerosis 2011, 218, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Morville, T.; Sahl, R.E.; Trammell, S.A.J.; Svenningsen, J.S.; Gillum, M.P.; Helge, J.W.; Clemmensen, C. Divergent effects of resistance and endurance exercise on plasma bile acids, FGF19, and FGF21 in humans. JCI Insight 2018, 3. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhang, Z.; Hu, B.; Huang, W.; Yuan, C.; Zou, L. Response of gut microbiota to metabolite changes induced by endurance exercise. Front. Microbiol. 2018, 9, 765. [Google Scholar] [CrossRef]
- Levy, M.; Thaiss, C.A.; Elinav, E. Metabolites: Messengers between the microbiota and the immune system. Genes Dev. 2016, 30, 1589–1597. [Google Scholar] [CrossRef]
- Sakaguchi, C.A.; Nieman, D.C.; Signini, E.F.; Abreu, R.M.; Catai, A.M. Metabolomics-based studies assessing exercise-induced alterations of the human metabolome: A systematic review. Metabolites 2019, 9, 164. [Google Scholar] [CrossRef]
- Kelly, J.R.; Kennedy, P.J.; Cryan, J.F.; Dinan, T.G.; Clarke, G.; Hyland, N.P. Breaking down the barriers: The gut microbiome, intestinal permeability and stress-related psychiatric disorders. Front. Cell Neurosci. 2015, 9. [Google Scholar] [CrossRef]
- Liu, H.; Dicksved, J.; Lundh, T.; Lindberg, J.E. Heat shock proteins: Intestinal gatekeepers that are influenced by dietary components and the gut microbiota. Pathogens 2014, 3, 187–210. [Google Scholar] [CrossRef]
- Ren, H.; Musch, M.W.; Kojima, K.; Boone, D.; Ma, A.; Chang, E.B. Short-chain fatty acids induce intestinal epithelial heat shock protein 25 expression in rats and IEC 18 cells. Gastroenterology 2001, 121, 631–639. [Google Scholar] [CrossRef]
- Fehrenbach, E.; Passek, F.; Niess, A.M.; Pohla, H.; Weinstock, C.; Dickhuth, H.H.; Northoff, H. HSP expression in human leukocytes is modulated by endurance exercise. Med. Sci. Sport Exerc. 2001, 32, 592–600. [Google Scholar] [CrossRef] [PubMed]
- Lira, F.S.; Rosa, J.C.; Pimentel, G.D.; Souza, H.A.; Caperuto, E.C.; Carnevali, L.C.; Seelaender, M.; Damaso, A.R.; Oyama, L.M.; de Mello, M.T.; et al. Endotoxin levels correlate positively with a sedentary lifestyle and negatively with highly trained subjects. Lipids Health Dis. 2010, 9, 82. [Google Scholar] [CrossRef]
- Yu, C.; Liu, S.; Chen, L.; Shen, J.; Niu, Y.; Wang, T.; Zhang, W.; Fu, L. Effect of exercise and butyrate supplementation on microbiota composition and lipid metabolism. J. Endocrinol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Anderson, G.; Maes, M. Gut dysbiosis dysregulates central and systemic homeostasis via suboptimal mitochondrial function: Assessment, treatment and classification implications. Curr. Top. Med. Chem. 2020, 20, 524–539. [Google Scholar] [CrossRef]
- Jin, C.J.; Engstler, A.J.; Sellmann, C.; Ziegenhardt, D.; Landmann, M.; Kanuri, G.; Lounis, H.; Schröder, M.; Vetter, W.; Bergheim, I. Sodium butyrate protects mice from the development of the early signs of non-alcoholic fatty liver disease: Role of melatonin and lipid peroxidation. Br. J. Nutr. 2016, 23, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Polyakova, V.O.; Kvetnoy, I.M.; Anderson, G.; Rosati, J.; Mazzoccoli, G.; Linkova, N.S. Reciprocal interactions of mitochondria and the neuroimmunoendocrine system in neurodegenerative sisorders: An important role for melatonin regulation. Front. Physiol. 2018, 12, 9199. [Google Scholar] [CrossRef]
- Gómez-Rubio, P.; Trapero, I. The beneficial effect of physical exercise on inflammatory makers in older individuals. Endocr. Metab. Immune Disord. Drug Targets 2020, 6. [Google Scholar] [CrossRef]
- Ciolac, E.G.; Rodrigues da Silva, J.M.; Vieira, R.P. Physical exercise as an immunomodulator of chronic diseases in aging. J. Phys. Act. Health 2020, 11, 1–11. [Google Scholar] [CrossRef]
- Clark, A.; Mach, N. The crosstalk between the gut microbiota and mitochondria during exercise. Front. Physiol. 2017, 8, 319. [Google Scholar] [CrossRef]
- Saint-Georges-Chaumet, Y.; Edeas, M. Microbiota–mitochondria inter-talk: Consequence for microbiota–host interaction. FEMS Pathog. Dis. 2016. [Google Scholar] [CrossRef]
- Ma, Y.H.; Xu, C.; Wang, W.S.; Sun, L.; Yang, S.; Lu, D.; Liu, Y.; Yang, H. Role of SIRT1 in the protection of intestinal epithelial barrier under hypoxia and its mechanism. Zhonghua Wei Chang Wai Ke Za Zhi Chin. J. Gastrointest Surg. 2014, 17, 602–606. [Google Scholar]
- Ticinesi, A.; Nouvenne, A.; Cerundolo, N.; Catania, P.; Prati, B.; Tana, C.; Meschi, T. Gut microbiota, muscle mass and function in aging: A focus on physical frailty and sarcopenia. Nutrients 2019, 11, 1633. [Google Scholar] [CrossRef] [PubMed]
- Lobet, E.; Letesson, J.J.; Arnould, T. Mitochondria: A target for bacteria. Biochem. Pharmacol. 2015, 94, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, M.; Guarente, L. mTORC1 and SIRT1 cooperate to foster expansion of gut adult stem cells during calorie restriction. Cell 2016, 166, 436–450. [Google Scholar] [CrossRef]
- Peng, L.; Li, Z.R.; Green, R.S.; Holzman, I.R.; Lin, J. Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP-activated protein kinase in Caco-2 cell monolayers. J. Nutr. 2009, 139, 1619–1625. [Google Scholar] [CrossRef]
- Hooper, L.V.; Littman, D.R.; Macpherson, A.J. Interactions between the microbiota and the immune system. Science 2012, 336, 1268–1273. [Google Scholar] [CrossRef]
- Marchesi, J.R.; Adams, D.H.; Fava, F.; Hermes, G.D.; Hirschfield, G.M.; Hold, G.; Quraishi, M.N.; Kinross, J.; Smidt, H.; Tuohy, K.M.; et al. The gut microbiota and host health: A new clinical frontier. Gut 2016, 65, 330–339. [Google Scholar] [CrossRef]
- Hood, D.A.; Uguccioni, G.; Vainshtein, A.; D’souza, D. Mechanisms of exercise-induced mitochondrial biogenesis in skeletal muscle: Implications for health and disease. Compr. Physiol 2011, 1, 1119–1134. [Google Scholar]
- Safdar, A.; Bourgeois, J.M.; Ogborn, D.I.; Little, J.P.; Hettinga, B.P.; Akhtar, M.; Thompson, J.E.; Melov, S.; Mocellin, N.J.; Kujoth, G.C.; et al. Endurance exercise rescues progeroid aging and induces systemic mitochondrial rejuvenation in mtDNA mutator mice. Proc. Natl. Acad. Sci. USA 2011, 108, 4135–4140. [Google Scholar] [CrossRef]
- Radak, Z.; Zhao, Z.; Koltai, E.; Ohno, H.; Atalay, M. Oxygen consumption and usage during physical exercise: The balance between oxidative stress and ROS-dependent adaptive signaling. Antioxid. Redox Signal. 2013, 18, 1208–1246. [Google Scholar] [CrossRef] [PubMed]
- Nieman, D.C.; Wentz, L.M. The compelling link between physical activity and the body’s defense system. J. Sport Health Sci. 2019, 8, 201–217. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.-D.; Wang, L.-K.; Wu, H.-Y.; Jiao, L. Effects of prebiotic galacto-oligosaccharide on postoperative cognitive dysfunction and neuroinflammation through targeting of the gut-brain axis. BMC Anesthesiol. 2018, 18, 177. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.; Sugahara, H.; Shimada, K.; Mitsuyama, E.; Kuhara, T.; Yasuoka, A.; Xiao, J.Z. Therapeutic potential of Bifidobacterium breve strain A1 for preventing cognitive impairment in Alzheimer’s disease. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Chunchai, T.; Thunapong, W.; Yasom, S.; Wanchai, K.; Eaimworawuthikul, S.; Metzler, G.; Lungkaphin, A.; Pongchaidecha, A.; Sirilun, S.; Chaiyasut, C.; et al. Decreased microglial activation through gut-brain axis by prebiotics, probiotics, or synbiotics effectively restored cognitive function in obese-insulin resistant rats. J. Neuroinflamm. 2018, 15, 11. [Google Scholar] [CrossRef]
- Valls-Pedret, C.; Sala-Vila, A.; Serra-Mir, M.; Corella, D.; de la Torre, R.; Martínez-González, M.Á.; Martínez-Lapiscina, E.H.; Fitó, M.; Pérez-Heras, A.; Salas-Salvadó, J.; et al. Mediterranean diet and age-related cognitive decline: A randomized clinical trial. JAMA Intern. Med. 2015, 175, 1094. [Google Scholar] [CrossRef]
- Nagpal, R.; Neth, B.J.; Wang, S.; Craft, S.; Yadav, H. Modified Mediterranean-ketogenic diet modulates gut microbiome and short-chain fatty acids in association with Alzheimer’s disease markers in subjects with mild cognitive impairment. EBioMedicine 2019, 47, 529–542. [Google Scholar] [CrossRef]
- Roman, P.; Estévez, A.F.; Miras, A.; Sánchez-Labraca, N.; Cañadas, F.; Vivas, A.B.; Cardona, D. A pilot randomized controlled trial to explore cognitive and emotional effects of probiotics in fibromyalgia. Sci. Rep. 2018, 8. [Google Scholar] [CrossRef]
- Messaoudi, M.; Lalonde, R.; Violle, N.; Javelot, H.; Desor, D.; Nejdi, A.; Bisson, J.-F.; Rougeot, C.; Pichelin, M.; Cazaubiel, M.; et al. Assessment of psychotropic-like properties of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in rats and human subjects. Br. J. Nutr. 2011, 105, 755–764. [Google Scholar] [CrossRef]
- Rao, A.V.; Bested, A.C.; Beaulne, T.M.; Katzman, M.A.; Iorio, C.; Berardi, J.M.; Logan, A.C. A randomized, double-blind, placebo-controlled pilot study of a probiotic in emotional symptoms of chronic fatigue syndrome. Gut Pathog. 2009, 1, 6. [Google Scholar] [CrossRef]
- Abraham, D.; Feher, J.; Scuderi, G.L.; Szabo, D.; Dobolyi, A.; Cservenak, M.; Vina, J. Exercise and probiotics attenuate the development of Alzheimer’s disease in transgenic mice: Role of microbiome. Exp. Gerontol. 2019, 115, 122–131. [Google Scholar] [CrossRef]
- Inoue, T.; Kobayashi, Y.; Mori, N.; Sakagawa, M.; Xiao, J.-Z.; Moritani, T.; Sakane, N.; Nagai, N. Effect of combined bifidobacteria supplementation and resistance training on cognitive function, body composition and bowel habits of healthy elderly subjects. Benef. Microbes 2018, 9, 843–853. [Google Scholar] [CrossRef] [PubMed]
- Yuan, T.-F.; Ferreira Rocha, N.B.; Paes, F.; Arias-Carrión, O.; Machado, S.; de Sá Filho, A.S. Neural mechanisms of exercise: Effects on gut miccrobiota and depression. CNS Neurol. Disord. Drug Targets 2015, 14, 1312–1314. [Google Scholar] [CrossRef] [PubMed]
- Schlegel, P.; Novotny, M.; Klimova, B.; Valis, M. “Muscle-gut-brain axis”: Can physical activity help patients with Alzheimer’s disease due to microbiome modulation? J. Alzheimer’s Dis. 2019, 71, 861–878. [Google Scholar] [CrossRef]
- Elli, M.; Callegari, M.L.; Ferrari, S.; Bessi, E.; Cattivelli, D.; Soldi, S.; Antoine, J.M. Survival of yogurt bacteria in the human gut. Appl. Environ. Microbiol. 2006, 72, 5113–5117. [Google Scholar] [CrossRef] [PubMed]
- Ahlskog, J.E.; Geda, Y.E.; Graff-Radford, N.R.; Petersen, R.C. Physical exercise as a preventive or disease-modifying treatment of dementia and brain aging. In Mayo Clinic Proceedings; Elsevier: Amsterdam, The Netherlands, 2011; Volume 86, pp. 876–884. [Google Scholar]
- Uc, E.Y.; Doerschug, K.C.; Magnotta, V.; Dawson, J.D.; Thomsen, T.R.; Kline, J.N.; Bruss, J. Phase I/II randomized trial of aerobic exercise in Parkinson disease in a community setting. Neurology 2014, 83, 413–425. [Google Scholar] [CrossRef]
- Steenland, K.; Goldstein, F.C.; Levey, A.; Wharton, W. A meta-analysis of Alzheimer’s disease incidence and prevalence comparing African-Americans and Caucasians. J. Alzheimer’s Dis. 2016, 50, 71–76. [Google Scholar] [CrossRef]
- Samper-Ternent, R.; Kuo, Y.F.; Ray, L.A.; Ottenbacher, K.J.; Markides, K.S.; Al Snih, S. Prevalence of health conditions and predictors of mortality in oldest old Mexican Americans and non-Hispanic whites. J. Am. Med. Dir. Assoc. 2012, 13, 254–259. [Google Scholar] [CrossRef]
- Joseph, J.J.; Echouffo Tcheugui, J.B.; Carnethon, M.R.; Bertoni, A.G.; Shay, C.M.; Ahmed, H.M.; Blumenthal, R.S.; Cushman, M.; Golden, S.H. The association of ideal cardiovascular health with incident type 2 diabetes mellitus: The Multi-Ethnic Study of Atherosclerosis. Diabetologia 2016, 59, 1893–1903. [Google Scholar] [CrossRef]
- Fei, K.; Rodriguez-Lopez, J.S.; Ramos, M.; Islam, N.; Trinh-Shevrin, C.; Yi, S.S.; Chernov, C.; Perlman, S.E.; Thorpe, L.E. Racial and ethnic subgroup disparities in hypertension prevalence, New York City Health and Nutrition Examination Survey, 2013–2014. Prev. Chronic Dis. 2017, 14. [Google Scholar] [CrossRef]
- King, A.C.; King, D.K. Physical activity for an aging population. Public Health Rev. 2010, 32, 401–426. [Google Scholar] [CrossRef]
- Doyle, S.; Kelly-Schwartz, A.; Schlossberg, M.; Stockard, J. Active community environments and health: The relationship of walkable and safe communities to individual health. JAPA 2006, 72, 19–31. [Google Scholar] [CrossRef]
- Gordon-Larsen, P.; Nelson, M.C.; Page, P.; Popkin, B.M. Inequality in the built environment underlies key health disparities in physical activity and obesity. Pediatrics 2006, 117, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Kirby, J.B.; Liang, L.; Chen, H.-J.; Wang, Y. Race, place, and obesity: The complex relationships among community racial/ethnic composition, individual race/ethnicity, and obesity in the United States. Am. J. Public Health 2012, 102, 1572–1578. [Google Scholar] [CrossRef] [PubMed]
- Bowser, J.; Martinez-Donate, A.P.; Carrel, A.; Allen, D.B.; Moberg, D.P. Disparities in fitness and physical activity among children. WMJ 2016, 115, 245–250. [Google Scholar]
- Mayeda, E.R.; Glymour, M.M.; Quesenberry, C.P.; Whitmer, R.A. Inequalities in dementia incidence between six racial and ethnic groups over 14 years. Alzheimer’s Dement. 2016, 12, 216–224. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanborn, V.; Gunstad, J. The Potential Mediation of the Effects of Physical Activity on Cognitive Function by the Gut Microbiome. Geriatrics 2020, 5, 63. https://doi.org/10.3390/geriatrics5040063
Sanborn V, Gunstad J. The Potential Mediation of the Effects of Physical Activity on Cognitive Function by the Gut Microbiome. Geriatrics. 2020; 5(4):63. https://doi.org/10.3390/geriatrics5040063
Chicago/Turabian StyleSanborn, Victoria, and John Gunstad. 2020. "The Potential Mediation of the Effects of Physical Activity on Cognitive Function by the Gut Microbiome" Geriatrics 5, no. 4: 63. https://doi.org/10.3390/geriatrics5040063
APA StyleSanborn, V., & Gunstad, J. (2020). The Potential Mediation of the Effects of Physical Activity on Cognitive Function by the Gut Microbiome. Geriatrics, 5(4), 63. https://doi.org/10.3390/geriatrics5040063



