Inter-Relationships between Frailty, Sarcopenia, Undernutrition and Dysphagia in Older People Who Are Admitted to Acute Frailty and Medical Wards: Is There an Older Adult Quartet?
Abstract
1. Introduction
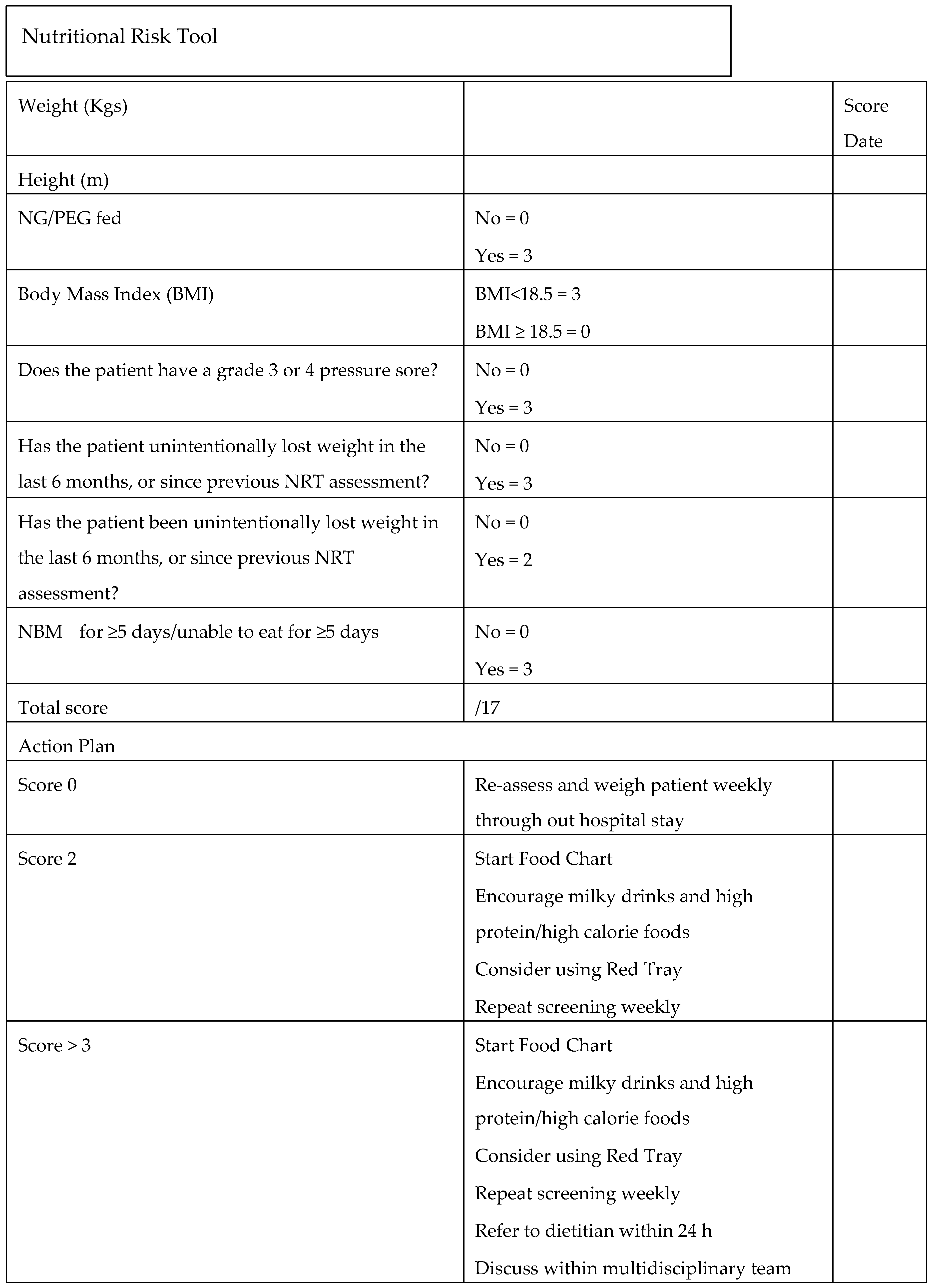
2. Methodology
3. Statistical Analysis
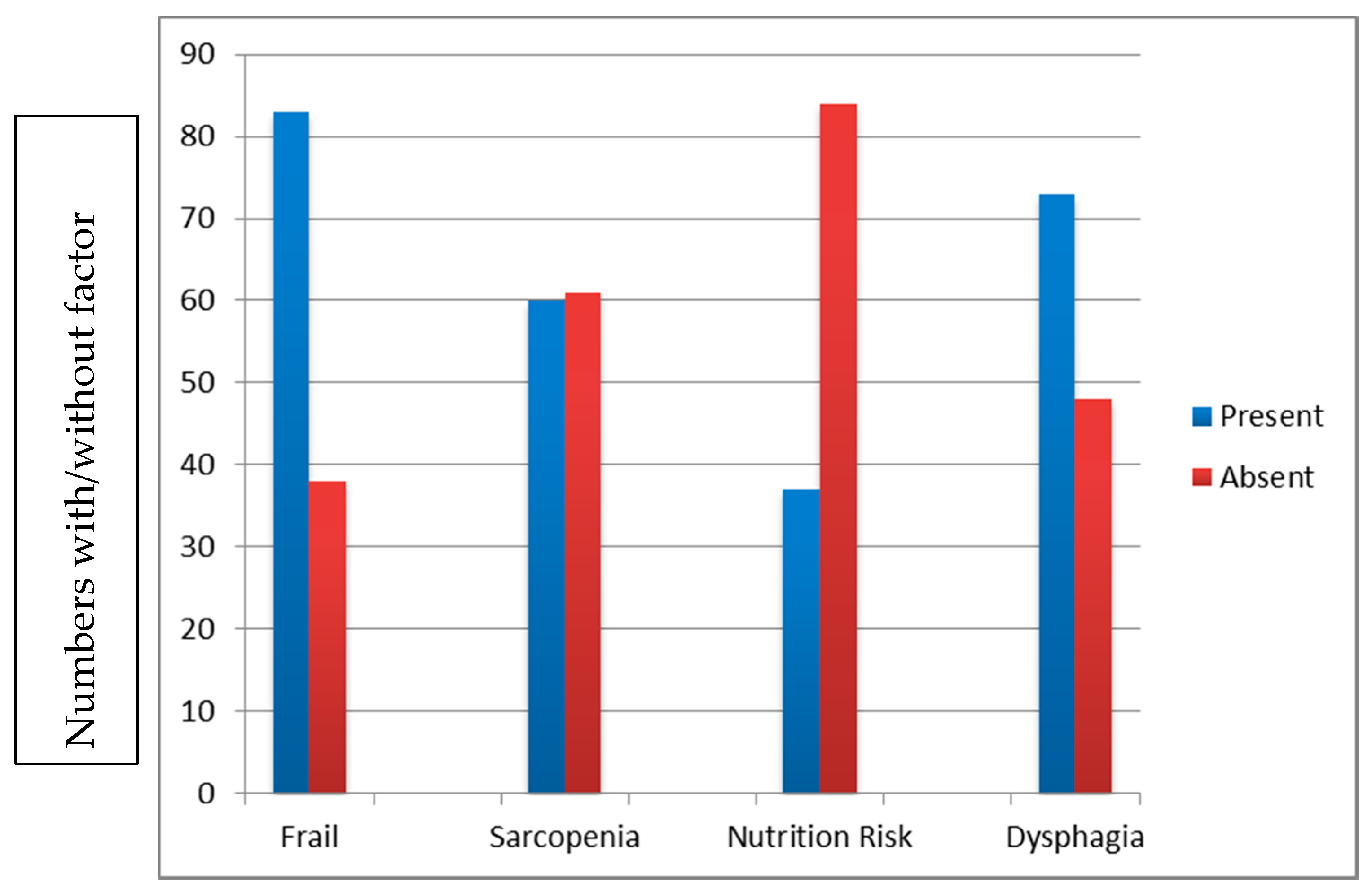
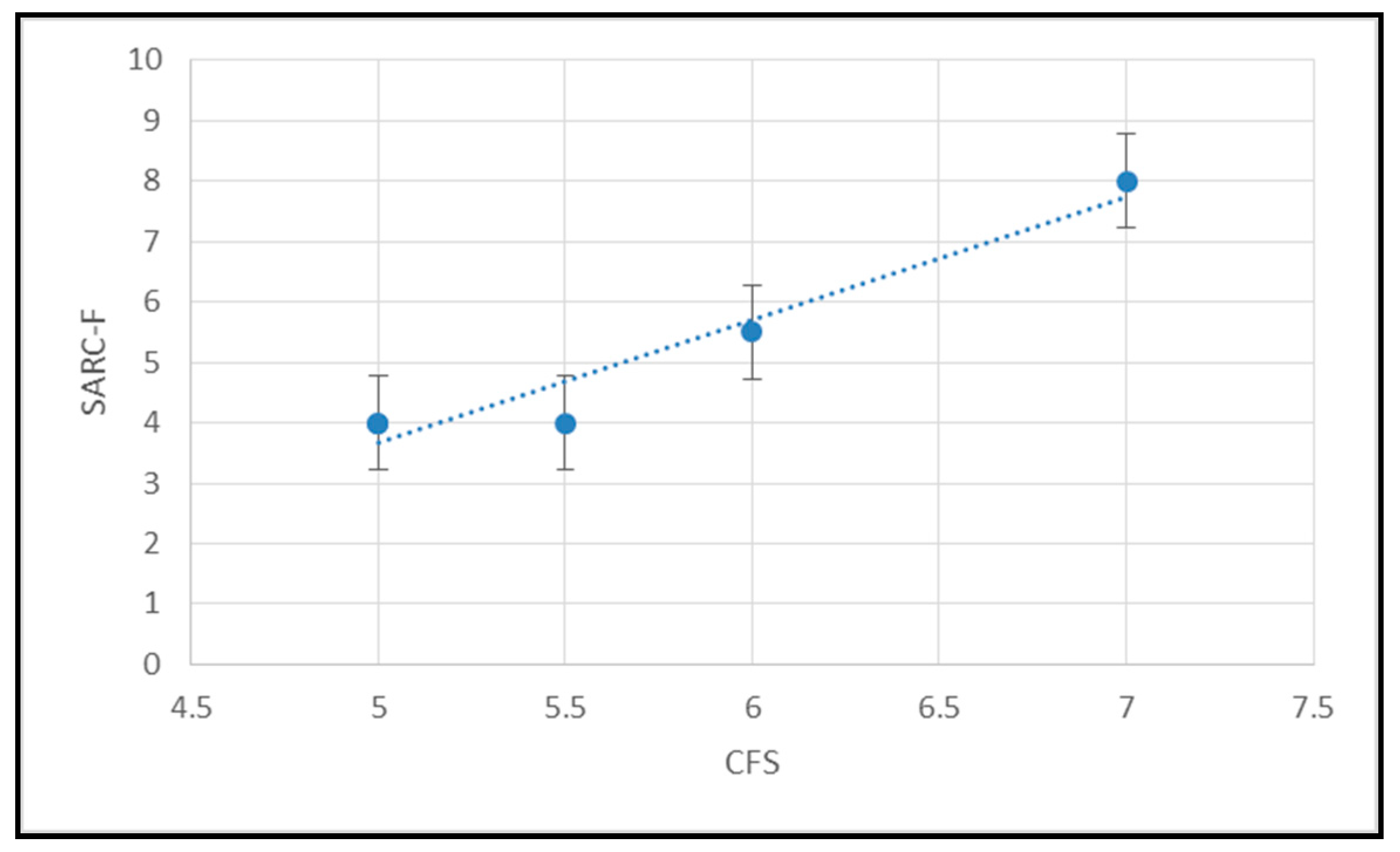
4. Results
5. Discussion
6. Limitations
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- World Bank Statistics. Available online: https://data.worldbank.org/indicator/SP.POP.65UP.TO.ZS?view=chart (accessed on 9 December 2019).
- Eurostats. European Health Information Gateway European Mortality Database. Available online: https://gateway.euro.who.int/en/datasets/european-mortality-database/#population-and-icd-used (accessed on 9 December 2019).
- Smithard, D.G. Dysphagia: A geriatric giant? Med. Clin. Rev. 2016, 2, 1–7. [Google Scholar] [CrossRef]
- Morley, J.E. Frailty and sarcopenia: The new geriatric giants. Rev. Investig. Clin. 2016, 68, 59–67. [Google Scholar]
- Rockwood, K.; Song, X.; MacKnight, C.; Bergman, H.; Hogan, D.B.; McDowell, I.; Mitnitski, A. A global clinical measure of fitness and frailty in elderly people. Can. Med. Assoc. J. 2005, 30, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in Older adults: Evidence for a phenotype. J. Gerontol. Med. Sci. 2001, 56A, M146–M156. [Google Scholar] [CrossRef]
- Clegg, A.; Young, J.; Illife, S.; Rikkert, M.O.; Rockwood, K. Frailty in Older people. Lancet 2013, 381, 752–762. [Google Scholar] [CrossRef]
- Clegg, A.; Bates, C.; Young, J.; Ryan, R.; Nichols, L.; Teal, E.A.; Mohmmed, M.A.; Patty, J.; Marshall, T. Development and validation of an electronic frailty index using routine primary care electronic health record data. Age Ageing 2016, 45, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Rooks, D. Sarcopenia: A muscle disease with decreased functional capacity and an increased risk of adverse health outcomes. Curr. Phys. Med. Rehabil. Rep. 2019, 7, 290–296. [Google Scholar] [CrossRef]
- Van Kan, A.G. Epidemiology and consequences of sarcopenia. J. Nutr. Health Aging 2009, 13, 708–712. [Google Scholar] [CrossRef]
- Malstromm, T.K.; Morely, J.E. SARC-F: A simple questionnaire to rapidly diagnose sarcopenia. J. Am. Med. Direct. Assoc. 2013, 14, 531–532. [Google Scholar] [CrossRef]
- Ida, S.; Kaneko, R.; Murata, K. SARC-F for screening of sarcopenia among older adults: A meta-analysis of screening test accuracy. Am. Med. Direct. Assoc. 2018, 19, 685–689. [Google Scholar] [CrossRef]
- Carrion, S.; Roca, M.; Costa, A.; Arreola, V.; Ortega, O.; Palomera, E.; Serra-Pratt, M.; Cabre, M.; Clave, P. Nutritional status of older people with oropharyngeal dysphagia in a chronic vs acute clinical situation. Clin. Nutr. 2017, 36, 1110–1116. [Google Scholar] [CrossRef]
- Cabre, M.; Serra-Prat, M.; Palomera, E.; Almirall, J.; Pallares, R.; Clave, P. Prevalence and prognostic implications of dysphagia in elderly patients with pneumonia. Age Ageing 2010, 39, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Baijens, L.W.J.; Clave, P.; Cras, P.; Ekberg, O.; Forster, A.; Kolb, G.F.; Leners, J.C.; Masiero, S.; Mateos-Nozal, J.; Ortega, O.; et al. European Society for Swallowing Disorders—European Union Geriatric Medicine Society white paper: Oropharyngeal dysphagia as a geriatric syndrome. CIA 2016, 11, 1403–1428. [Google Scholar] [CrossRef]
- Tsang, K.; Lau, E.S.Y.; Shazzra, M.; Eyres, R.; Hansjee, D.; Smithard, D.G. A new simple screening tool-4QT: Can it identify those with swallowing problems? A pilot study. Geriatrics 2020, 5, 11. [Google Scholar] [CrossRef] [PubMed]
- Uhm, K.E.; Kim, M.; Lee, Y.M.; Kim, B.R.; Kim, Y.S.; Choi, J.; Han, S.H.; Kim, H.J.; Yoo, K.H.; Lee, J. The easy dysphagia symptom questionnaire for older adults. Eur. Geriatr. Med. 2019, 10, 47–52. [Google Scholar] [CrossRef]
- Mori, T.; Fujishima, I.; Wakabayashi, H.; Oshima, F.; Itoda, M.; Kunieda, K.; Kayashita, S.; Sonoda, A.; Kuroda, Y.; Yamada, M.; et al. Development, reliability, and validity of a diagnostic algorithm for sarcopenic dysphagia. J. Cachexia Sarcopenia Muscle Clin. Rep. 2017, 2, e00017. [Google Scholar]
- Shiozu, H.; Higashijima, M.; Koga, T. Association of sarcopenia with swallowing problems, related to nutrition and activities of daily living of elderly individuals. J. Phys. 2015, 27, 393–396. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Li, Z.; Tang, Z.; Sun, F.; Diao, L.; Wang, J.; Zhao, X.; Ge, G. Use of the frailty index in evaluating the prognosis of older people in Beijing: A cohort study with an 8-year follow up. Arch. Gerontol. Geriatr. 2016, 64, 172–177. [Google Scholar] [CrossRef]
- Gilbert, T.; Neuburger, J.; Krandler, J.; Keeble, E.; Smith, P.; Ariti, C.; Arora, S.; Street, A.; Parker, S.; Roberts, H.C.; et al. Development and validation of a hospital frailty risk score focusing on older people in acute care settings using electronic hospital records: An observational study. Lancet 2018, 392, 1775–1782. [Google Scholar] [CrossRef]
- Wallis, S.J.; Wall, J.; Birman, R.W.S.; Romero-Ortuno, R. Association of the clinical frailty scale with hospital outcomes. QJM 2015, 108, 943–949. [Google Scholar] [CrossRef]
- Morely, J.E.; Vellas, B.; van Khan, A.; Anker, S.D.; Bauer, J.M.; Bernabei, R.; Cesari, M.; Chumlea, W.C.; Doehner, W.; Evans, J.; et al. Frailty consensus; A call to action. J. Am. Med. Direct. Assoc. 2013, 14, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Joosten, E.; Demuynck, M.; DFetroyer, E.; Milisen, K. Prevalence of frailty and its ability to predict in hospital delirium, falls, and 6 month mortality in hospitalized older patients. BVMC Geriatr. 2014, 14, 1. [Google Scholar] [CrossRef] [PubMed]
- Ekerstad, N.; Swahn, E.; Janzon, M.; Alfredsson, J.; Lofmark, R.; Lindenberger, M.; Carlsson, P. Frailty is independently associated with short-term outcomes in elderly patients with non-ST-Segment elevation myocardial infarction. Circulation 2011, 124, 1397–2404. [Google Scholar] [CrossRef] [PubMed]
- Andela, R.M.; Dijkstra, A.; Slaets, J.P.J.; Sanderman, R. Prevalence of frailty on clinical wards: Description and implications. Int. J. Nurs. Pr. 2010, 16, 14–19. [Google Scholar] [CrossRef]
- Patel, K.V.; Brennan, K.L.; Brennan, M.L.; Jupiter, D.C.; Shar, A.; David, L. Association of a modified frailty index with mortality after femoral neck fracture in patients aged 60 years and older. Clin. Orthop. Relat. Res. 2014, 472, 1010–1017. [Google Scholar] [CrossRef]
- Morandi, A.; Onder, G.; Fodri, L.; Sanniti, A.; Schnelle, J.; Simmons, S.; Lamdi, F.; Gentile, S.; Trabucchi, M.; Bellelli, G. The association between the probability of sarcopenia and functional outcomes in older patients undergoing in-hospital rehabilitation. J. Am. Direct. Assoc. 2015, 16, 951–956. [Google Scholar] [CrossRef]
- Perez-Zepeda, M.U.; Sgaravatti, A.; Dent, E. Sarcopenia and post hospital outcomes in older adults: A longitudinal study. Arch. Gerontol Geriatr. 2017, 69, 105–109. [Google Scholar] [CrossRef]
- McWhirter, J.P.; Pennington, C.R. Incidence and recognition of malnutrition in hospital. BMJ 1994, 308, 945–948. [Google Scholar] [CrossRef]
- Somachi, M.; Tao, X.; Mullin, G.E. The facilitated early enteral nutrition and dietary management effectiveness trial in hospitalized patients with malnutrition. J. Parenter. Enter. Nutr. 2011, 35, 209–2016. [Google Scholar] [CrossRef]
- Axelsson, K.; Asplun, K.; Eriksson, S. Eating problems and nutritional status during hospital stay of patients with severe stroke. J. Am. Diet. Ass. 1989, 89, 1092–1096. [Google Scholar]
- Dignity and Nutrition for Older People; Care Quality Commission: London, UK, 2017; Available online: https://www.cqc.org.uk/publications/themed-inspection/dignity-and-nutrition-older-people (accessed on 19 April 2020).
- Tappenden, K.A.; Quatrara, B.; Parkhurst, M.L.; Malone, A.M.; Fanjiang, G.; Ziegler, T.R. Critical role of nutrition in improving quality of care: An interdisciplinary call lto action to address adult hospital malnutrition. J. Parenter. Enter. Nutr. 2013, 37, 482–497. [Google Scholar] [CrossRef]
- Agarwal, E.; Ferguson, M.; Banks, M.; Batterham, M.; Bauer, J.; Capra, S.; Isenring, E. Malnutrition and poor food intake are associated with prolonged hospital stay, frequent readmissions, and greater in-hospital mortality: Results from the nutrition day care survey 2010. Clin. Nutr. 2013, 32, 737–745. [Google Scholar] [CrossRef] [PubMed]
- Schilp, J.; Kruizenga, H.M.; Wijinhoven, H.A.H.; Leistra, E. High prevalence of undernutrition in Dutch community-dwelling older individuals. Nutrition 2012, 28, 1151–1156. [Google Scholar] [CrossRef] [PubMed]
- Bloem, B.R.; Lagaay, A.M.; van Beck, W.; Haan, J.; Roos, R.A.; Wintzen, A.R. Prevalence of subjective dysphagia in community residents aged over 87. Br. Med. J. 1990, 300, 721–722. [Google Scholar] [CrossRef] [PubMed]
- Wilkins, T.; Gillies, R.A.; Thomas, A.M.; Wagner, P.J. The prevalence of dysphagia in a primary care patients: A Hames Net research network study. J. Am. Board. Fam. Med. 2007, 20, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.H.; Golub, J.S.; Hapner, E.R.; Johns, I.I.I.M. Prevalence of perceived dysphagia and quality of life impairment in a geriatric population. Dysphagia 2009, 24, 1–67. [Google Scholar] [CrossRef]
- Altman, K.W.; Yu, G.-P.; Schaefer, S.D. Consequences of dysphagia in the hospitalized patient. Arch. Otolarngol. Head Neck Surg. 2010, 13, 784–789. [Google Scholar] [CrossRef] [PubMed]
- Smithard, D.; Westmark, S.; Melgaard, D. Evaluation of the prevalence of screening for dysphagia among older people admitted to medical services—An international survey. Geriatrics 2019, 3, 1–8. [Google Scholar] [CrossRef]
- Chung, C.J.; Wu, C.; Jones, M.; Kato, T.; Dam, T.T.; Givens, R.C.; Templeton, D.L.; Maurer, M.S.; Naka, Y.; Takayama, H.; et al. Reduced handgrip strength as a marker of frailty predicts clinical outcomes in patients with heart failure undergoing ventricular assist device placement. J. Card. Fail. 2014, 20, 310–315. [Google Scholar] [CrossRef]
- Maeda, K.; Akaji, J. Sarcopenia is an independent risk factor of dysphagia in hospitalized older people. Geriatr. Gerontol. Int. 2016, 16, 515–521. [Google Scholar] [CrossRef]
- Yoshimura, Y.; Wakabayashi, H.; Bise, T.; Nagano, F.; Shimazu, S.; Shiraishi, A.; Yamaga, M.; Koga, H. Sarcopenia is associated with worse recovery of physical function and dysphagia and a lower rate of home discharge in Japanese hospitalized adults undergoing convalescent rehabilitation. Nutrition 2019, 61, 111–118. [Google Scholar] [CrossRef]
- Wakabayeshi, H.; Sakuma, K. Rehabilitation nutrition for sarcopenia with disability: A combination of both rehabilitation and nutrition care management. J. Cachexia Sarcopenia Muscle 2014, 5, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Atrill, S.; White, S.; Murray, J.; Hammond, S.; Doeltgen, S. Impact of oropharyngeal dysphagia on healthcare cost and length of stay in hospital: A systematic review. BMC Health Serv. Res. 2018, 18, 594. [Google Scholar] [CrossRef]
- Garcia-Nogueras, L.; Aranda-Reneo, L.; Pena-Longobardo, L.M.; Oliva-Moreno, J.; Abizanda, P. Use of health resources and healthcare costs associated with frailty: The FRADEA study. J. Nutr. Health Ageing 2017, 21, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Bock, J.-O.; Konig, H.-H.; Brenner, H.; Haefeli, W.E.; Quinzler, R.; Matschinger, H.; Saum, K.-U.; Schottker, B.; Heider, D. Associations of frailty with healthcare costs- results of the Esther cohort study. BMC Health Serv. Res. 2016, 16, 128. [Google Scholar] [CrossRef] [PubMed]
- Peters, L.L.; Burgerhorf, J.G.M.; Boter, H.; Wild, B.; Buskerns, E.; Slaets, J.P.J. Predictive validity of a frailty measure (GFI) and a case complexity measure (IM-E-SA) on health care costs in an elderly population. J. Psychosom. Res. 2015, 79, 404–411. [Google Scholar] [CrossRef] [PubMed]
- Westmark, S.; Melgaard, D.; Rethmeier, L.O.; Ehlers, L.H. The cost of dysphagia in geriatric patients. Clinico-Econ. Outcomes Res. 2018, 10, 321–326. [Google Scholar] [CrossRef]
- Sousa, A.S.; Guerra, R.S.; Fonesca, I.; Pichel, F.; Ferreira, S. Amaral TF Finacial impact of sarcopenia on hospitalization costs. Eur. J. Clin. Nutr. 2016, 70, 1046–1051. [Google Scholar] [CrossRef]
- Janssen, I.; Shepard, D.S.; Katzmarzyk, P.T.; Roubenoff, R. The healthcare costs of sarcopenia in the United States. J. Am. Geriatr. Soc. 2004, 52, 80–85. [Google Scholar] [CrossRef]
- Soysal, P.; Stubbs, B.; Lucato, P.; Luchini, C.; Solmi, M.; Peluso, R.; Sergi, G.; Isik, A.T.; Manzato, E.; Maggi, S.; et al. Inflammation and frailty in the elderly: A systematic review and meta-analysis. Aging Res. Rev. 2016, 31, 1–8. [Google Scholar] [CrossRef]
- Li, H.; Manwani, B.; Leng, S.X. Frailty, inflammation, and immunity. Aging Dis. 2011, 6, 466–473. [Google Scholar]
- Smithard, D.G.; O’Neill, P.A.; Park, C.; Morris, J.; Wyatt, R.; England, R.; Martin, D.F. Complications and outcome following acute stroke: Does dysphagia matter? Stroke 1996, 27, 1200–1204. [Google Scholar] [CrossRef] [PubMed]
- Gingrich, A.; Vollert, D.; Kiesswetter, E.; Thomanek, M.; Bach, S.; Sieber, C.; Zopf, Y. Prevalence and overlap of sarcopenia, cachexia and malnutrition in older medical patients. BMC Geriatr. 2019, 19, 120. [Google Scholar] [CrossRef] [PubMed]
| NRT | SARC-F | CFS | |
|---|---|---|---|
| NRT | x | x | x |
| SARC-F | 0.0991 (NS) | x | x |
| CFS | 0.0005 (NS) | 0.6111 (p ≤ 0.00001) | x |
| 4QT | 0.3842 (≤0.0001) | 0.1424 (NS) | 0.2308 (p ≤ 0.0109) |
| (1) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smithard, D.; Hansjee, D.; Henry, D.; Mitchell, L.; Sabaharwal, A.; Salkeld, J.; Yeung, E.; Younus, O.; Swaine, I. Inter-Relationships between Frailty, Sarcopenia, Undernutrition and Dysphagia in Older People Who Are Admitted to Acute Frailty and Medical Wards: Is There an Older Adult Quartet? Geriatrics 2020, 5, 41. https://doi.org/10.3390/geriatrics5030041
Smithard D, Hansjee D, Henry D, Mitchell L, Sabaharwal A, Salkeld J, Yeung E, Younus O, Swaine I. Inter-Relationships between Frailty, Sarcopenia, Undernutrition and Dysphagia in Older People Who Are Admitted to Acute Frailty and Medical Wards: Is There an Older Adult Quartet? Geriatrics. 2020; 5(3):41. https://doi.org/10.3390/geriatrics5030041
Chicago/Turabian StyleSmithard, David, Dharinee Hansjee, Darrien Henry, Laura Mitchell, Arjun Sabaharwal, Jo Salkeld, Eirene Yeung, Osman Younus, and Ian Swaine. 2020. "Inter-Relationships between Frailty, Sarcopenia, Undernutrition and Dysphagia in Older People Who Are Admitted to Acute Frailty and Medical Wards: Is There an Older Adult Quartet?" Geriatrics 5, no. 3: 41. https://doi.org/10.3390/geriatrics5030041
APA StyleSmithard, D., Hansjee, D., Henry, D., Mitchell, L., Sabaharwal, A., Salkeld, J., Yeung, E., Younus, O., & Swaine, I. (2020). Inter-Relationships between Frailty, Sarcopenia, Undernutrition and Dysphagia in Older People Who Are Admitted to Acute Frailty and Medical Wards: Is There an Older Adult Quartet? Geriatrics, 5(3), 41. https://doi.org/10.3390/geriatrics5030041






