Review of Therapeutic Options for the Prevention of VTE in Total Joint Arthroplasty
Abstract
1. Introduction
1.1. General Principles
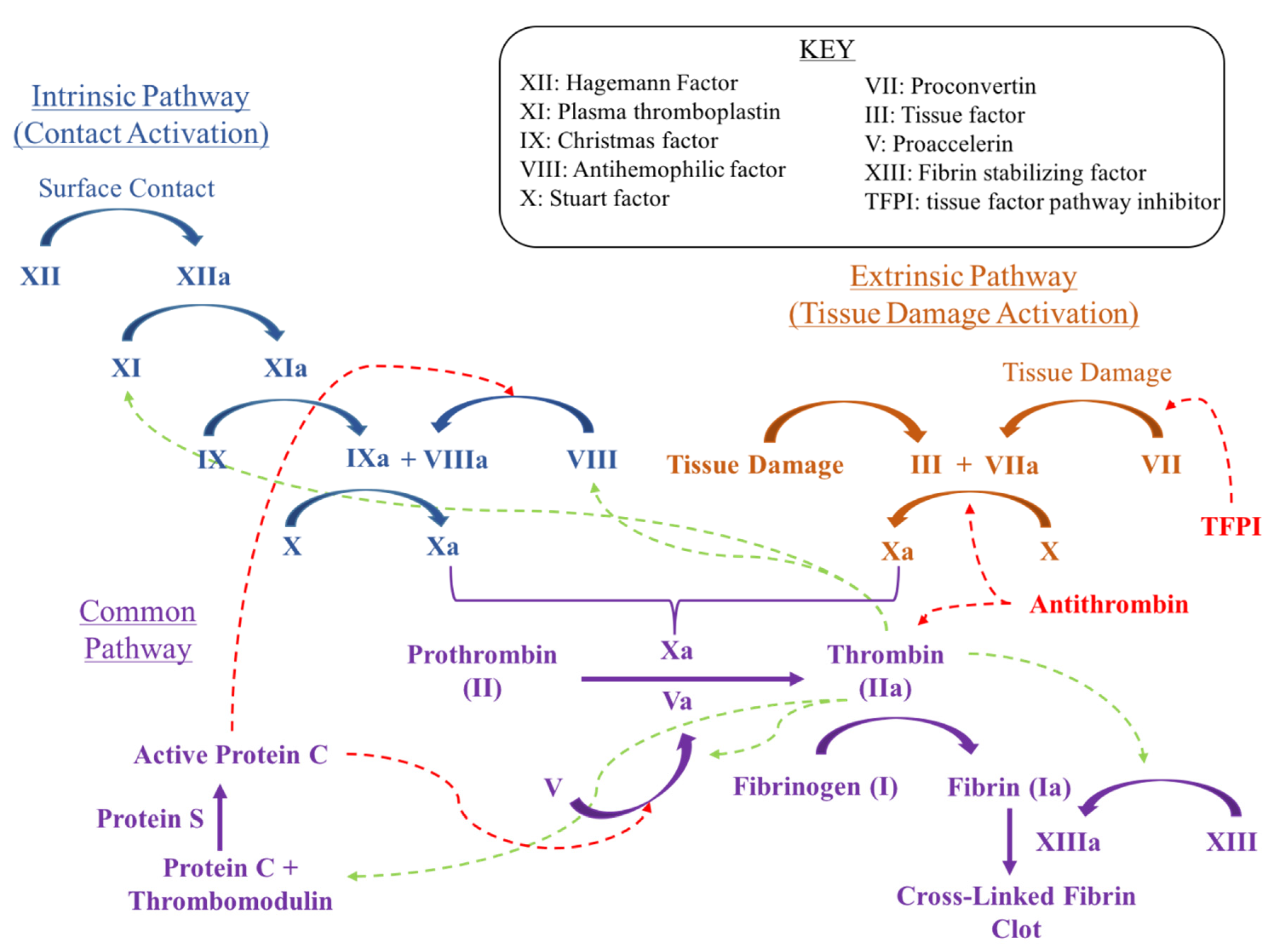
1.2. Mechanisms for Intervention
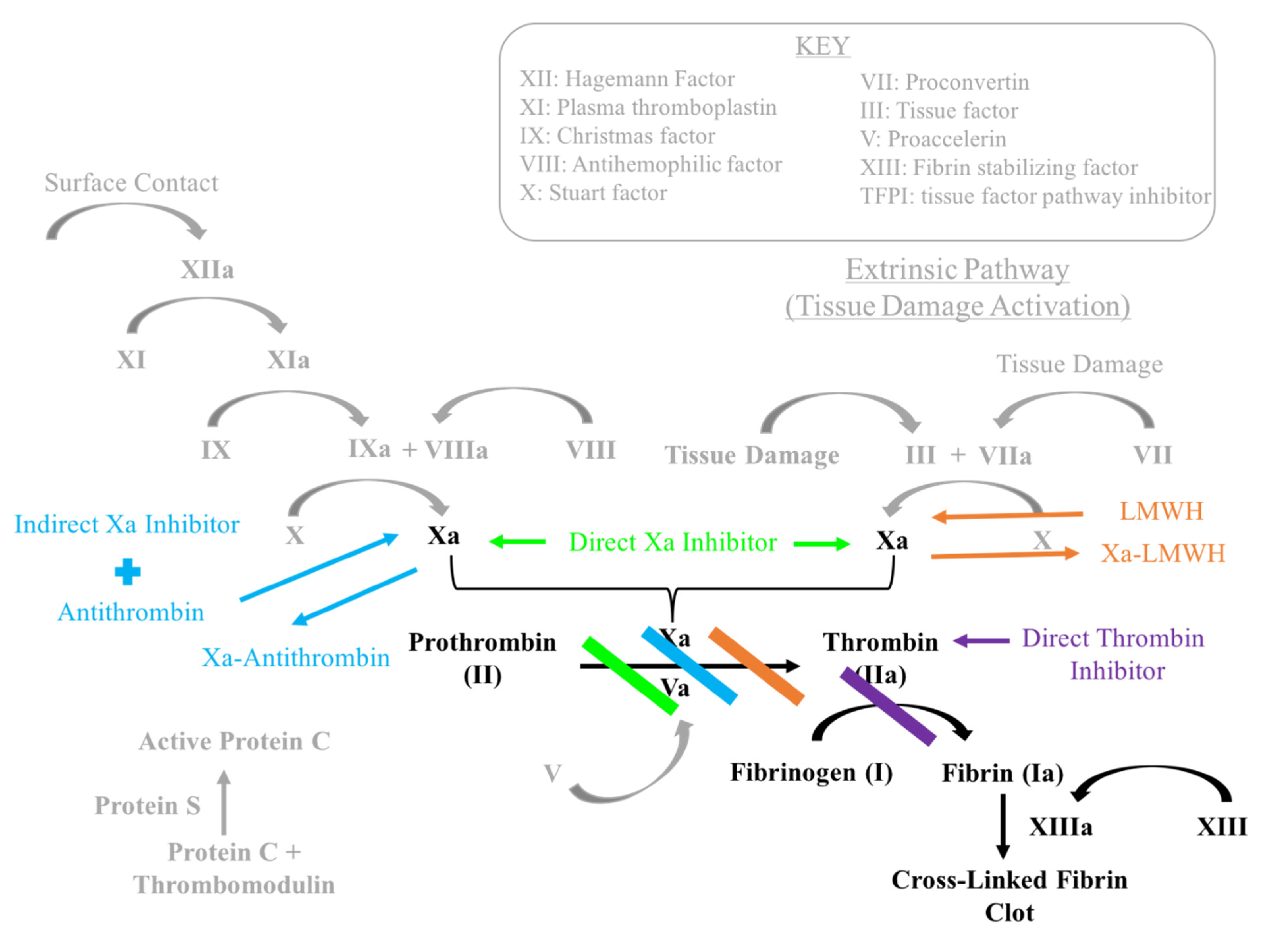
1.3. Factor Xa Inhibitors
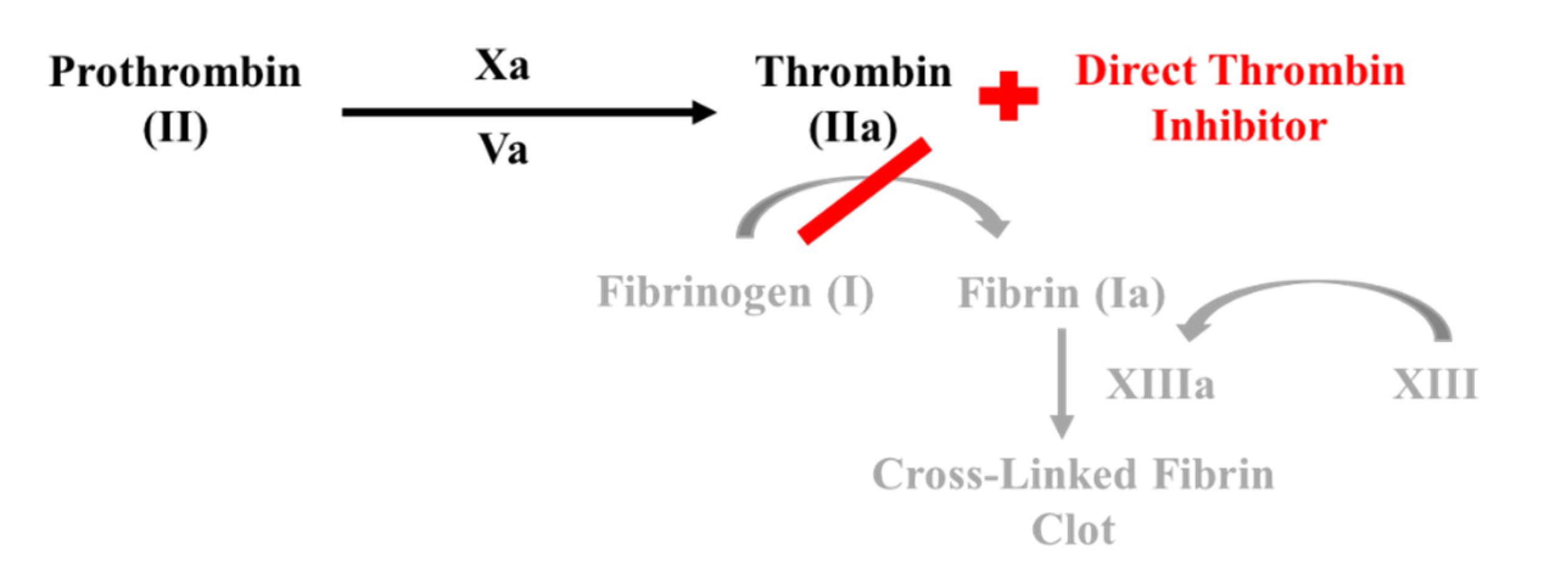
1.4. Direct Thrombin Inhibitors
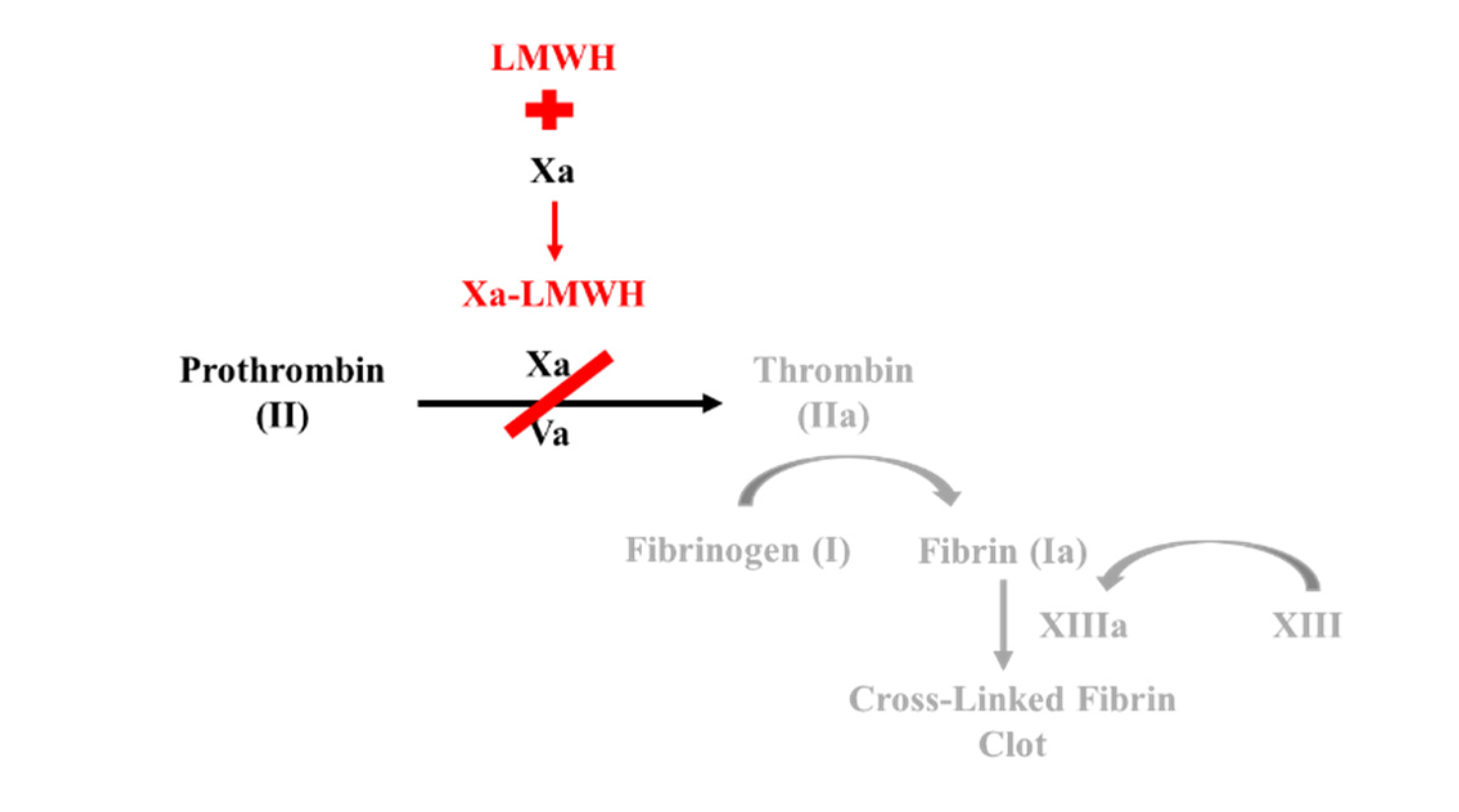
1.5. Low Molecular Weight Heparin
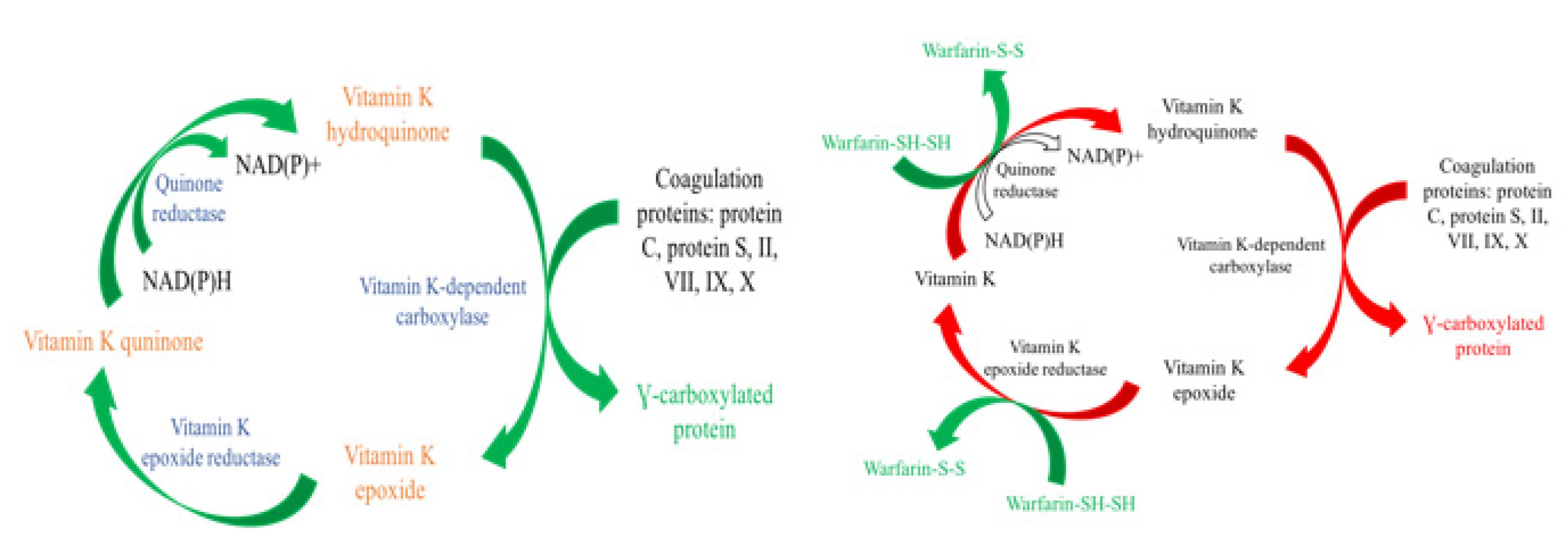
1.6. Vitamin K Antagonist
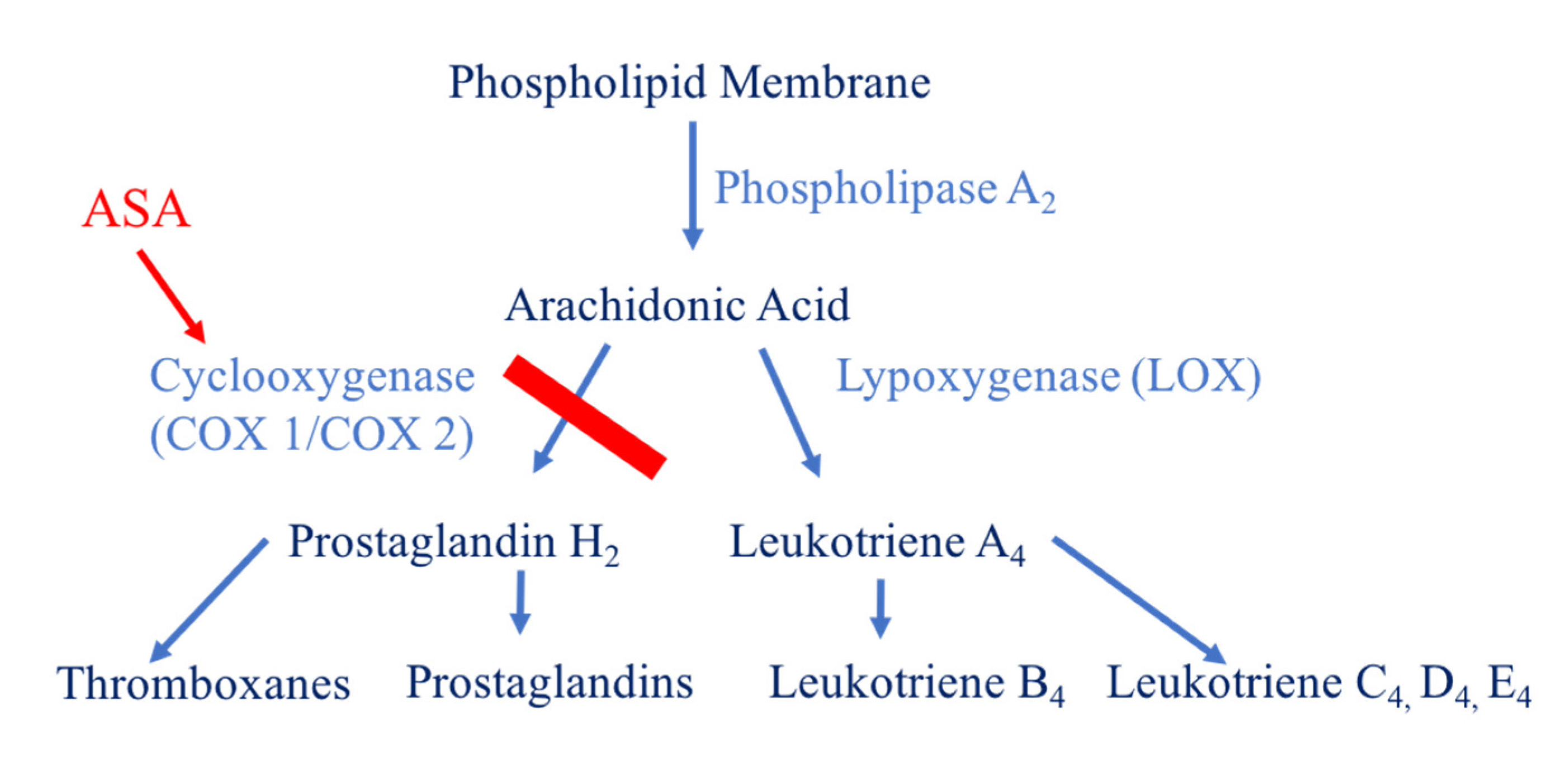
1.7. Aspirin
1.8. Summary of Mechanisms
- Low Molecular Weight Heparin acts similar to indirect Factor Xa inhibitors by binding to and inducing a conformational change in anti-thrombin. This complex increases anti-thrombin affinity for Factor Xa, thereby preventing the formation of thrombin from prothrombin.
- Factor Xa inhibitors ultimately prevent Factor Xa driven conversion of prothrombin to thrombin. Direct Factor Xa inhibitors include rivaroxaban (Xarelto) and apixaban (Eliquis). Fondaparinux (Arixtra) is an indirect factor Xa inhibitor.
- Direct thrombin inhibitors bind directly to thrombin, inhibiting fibrinogen conversion into fibrin.
- Vitamin K antagonists exert their effects by interfering with the cyclic interconversion of vitamin K and vitamin K epoxide. Antagonists like warfarin (Coumadin) prevent the regeneration of vitamin K from vitamin K epoxide and hinder the γ-carboxylation of coagulation factors necessary for the cascade.
- Aspirin impedes the formation of thromboxane and platelet aggregation by inhibiting the cyclooxygenase enzyme.
2. Materials and Methods
2.1. Search Strategy
2.2. Selection Criteria
2.3. Analysis
3. Results
3.1. LMWH Compared to Factor Xa Inhibitors
3.1.1. Enoxaparin and Apixaban
3.1.2. Enoxaparin and Rivaroxaban
3.2. LMWH Compared to Direct Thrombin Inhibitors
Enoxaparin and Dabigatran
3.3. LMWH Compared to Warfarin
3.3.1. Enoxaparin and Warfarin
3.3.2. Dalteparin and Warfarin
3.4. Aspirin Compared to Low Molecular Weight Heparin (LMWH)
3.5. Aspirin Compared to Factor Xa Inhibitors (Rivaroxaban as an Example)
3.6. Aspirin Compared to Direct Thrombin Inhibitors (Dabigatran as an Example)
3.7. Aspirin Compared to Warfarin
3.8. Aspirin High Dose Compared to Aspirin Low Dose
3.9. Emerging Trends
4. Discussion
4.1. Reconciling Current Guidelines
4.2. Authors Recommendations
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
| Level of Evidence for References Included in this Review |
| Level I–6 Articles |
| Evidence from a systematic review or meta-analysis of all relevant RCTs (randomized controlled trial) or evidence –based clinical practice guidelines based on systematic reviews of RCTs or three or more RCTs of good quality that have similar results |
| Hirsh et al., 2001; Ansell et al., 2008; Ansell et al., 2004; Eriksson et al., 2009; Suen et al., 2017; Salazar et al., 2014 |
| Level II–7 Articles |
| Evidence obtained from at least one well-designed RCT (e.g., large multi-site RCT) |
| Raskob et al., 2012; Ginsberg et al., 2009; Fitzgerald et al., 2001; Wilson et al., 2016; Anderson et al., 2013; Weitz et al., 2017; An et al., 2016 |
| Level III–2 Articles |
| Evidence obtained from well-designed controlled trials without randomization (i.e., quasi-experimental) |
| Parvizi et al., 2017; Pollock et al., 2016 |
| Level IV–9 Articles |
| Evidence from well-designed case-control or cohort studies |
| Gillette et al., 2013; Nielen et al., 2016; Bloch et al., 2014; IJRCWC, 2012; Huang et al., 2016; Deirmengian et al., 2016; Cafri et al., 2017; Gutowski et al., 2015; Heller et al., 2016 |
| Level V–12 Articles |
| Evidence from systematic reviews of descriptive and qualitative studies (meta-synthesis) |
| Cionac-Florescu et al., 2013; Leme et al., 2012; Cushman et al., 2007; Parvizi et al., 2017; Lu et al., 2010; Solayar et al., 2014; Mekaj et al., 2015; Turpie et al., 2007; Samama et al., 2011; Di Nisio et al., 2005; Lachiewicz et al., 2009; Stewart et al., 2013 |
| Level VI–0 Articles |
| Evidence from a single descriptive or qualitative study |
| Level VII–9 Articles |
| Evidence from the opinion or authorities and/or reports of expert committees |
| Parvizi et al., 2007; Dalury et al., 2015; Kearon et al., 2012; Eikelboom et al., 2009; Johanson et al., 2009; Mont et al., 2011; Mont et al., 2011 Liberman et al., 2011; Markel et al., 2010 |
References
- Cionac Florescu, S.; Anastase, D.M.; Munteanu, A.M.; Stoica, I.C.; Antonescu, D. Venous thromboembolism following major orthopedic surgery. Maedica (Buchar.) 2013, 8, 189–194. [Google Scholar] [PubMed]
- Leme, L.E.; Sguizzatto, G.T. Prophylaxis of venous thromboembolism in orthopaedic surgery. Rev. Bras. Ortop. 2012, 47, 685–693. [Google Scholar] [CrossRef]
- Parvizi, J.; Ceylan, H.H.; Kucukdurmaz, F.; Merli, G.; Tuncay, I.; Beverland, D. Venous Thromboembolism Following Hip and Knee Arthroplasty: The Role of Aspirin. J. Bone Jt. Surg. Am. 2017, 99, 961–972. [Google Scholar] [CrossRef] [PubMed]
- Cushman, M. Epidemiology and risk factors for venous thrombosis. Sem. Hematol. 2007, 44, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Lu, N.; Salvati, E.A. Multimodal prophylaxis for venous thromboembolic disease after total hip and knee arthroplasty: Current perspectives. Chin. J. Traumatol. 2010, 13, 362–369. [Google Scholar] [PubMed]
- Solayar, G.N.; Shannon, F.J. Thromboprophylaxis and orthopaedic surgery: Options and current guidelines. Malays. J. Med. Sci. 2014, 21, 71–77. [Google Scholar] [PubMed]
- Mekaj, Y.H.; Mekaj, A.Y.; Duci, S.B.; Miftari, E.I. New oral anticoagulants: Their advantages and disadvantages compared with vitamin K antagonists in the prevention and treatment of patients with thromboembolic events. Ther. Clin. Risk Manag. 2015, 11, 967–977. [Google Scholar] [CrossRef]
- Turpie, A.G. Oral, direct factor Xa inhibitors in development for the prevention and treatment of thromboembolic diseases. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 1238–1247. [Google Scholar] [CrossRef]
- Samama, M.M. The mechanism of action of rivaroxaban--an oral, direct Factor Xa inhibitor—Compared with other anticoagulants. Thromb. Res. 2011, 127, 497–504. [Google Scholar] [CrossRef]
- Di Nisio, M.; Middeldorp, S.; Buller, H.R. Direct thrombin inhibitors. N. Engl. J. Med. 2005, 353, 1028–1040. [Google Scholar] [CrossRef]
- Hirsh, J.; Dalen, J.E.; Anderson, D.R.; Poller, L.; Bussey, H.; Ansell, J.; Deykin, D.; Brandt, J.T. Oral anticoagulants: Mechanism of action, clinical effectiveness, and optimal therapeutic range. Chest 1998, 114 (Suppl. S5), 445s–469s. [Google Scholar] [CrossRef] [PubMed]
- Hirsh, J.; Warkentin, T.E.; Shaughnessy, S.G.; Anand, S.S.; Halperin, J.L.; Raschke, R.; Granger, C.; Ohman, E.M.; Dalen, J.E. Heparin and low-molecular-weight heparin: Mechanisms of action, pharmacokinetics, dosing, monitoring, efficacy, and safety. Chest 2001, 119 (Suppl. S1), 64s–94s. [Google Scholar] [CrossRef] [PubMed]
- Ansell, J.; Hirsh, J.; Hylek, E.; Jacobson, A.; Crowther, M.; Palareti, G. Pharmacology and management of the vitamin K antagonists: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest 2008, 133 (Suppl. S6), 160s–198s. [Google Scholar] [CrossRef]
- Ansell, J.; Hirsh, J.; Poller, L.; Bussey, H.; Jacobson, A.; Hylek, E. The pharmacology and management of the vitamin K antagonists: The Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest 2004, 126 (Suppl. S3), 204s–233s. [Google Scholar] [CrossRef]
- Raskob, G.E.; Gallus, A.S.; Pineo, G.F.; Chen, D.; Ramirez, L.M.; Lassen, M.R. Apixaban versus enoxaparin for thromboprophylaxis after hip or knee replacement: Pooled analysis of major venous thromboembolism and bleeding in 8464 patients from the ADVANCE-2 and ADVANCE-3 trials. J. Bone Jt. Surg. Br. Vol. 2012, 94, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, B.O.; Kakkar, A.K.; Turpie, A.G.G.; Gent, M.; Bandel, T.-J.; Homering, M.; Misselwitz, F.; Lassen, M.R. Oral rivaroxaban for the prevention of symptomatic venous thromboembolism after elective hip and knee replacement. J. Bone Jt. Surg. Br. Vol. 2009, 91, 636–644. [Google Scholar] [CrossRef]
- Suen, K.; Westh, R.N.; Churilov, L.; Hardidge, A.J. Low-Molecular-Weight Heparin and the Relative Risk of Surgical Site Bleeding Complications: Results of a Systematic Review and Meta-Analysis of Randomized Controlled Trials of Venous Thromboprophylaxis in Patients After Total Joint Arthroplasty. J. Arthroplast. 2017, 32, 2911–2919. [Google Scholar] [CrossRef]
- Ginsberg, J.S.; Davidson, B.L.; Comp, P.C. Oral thrombin inhibitor dabigatran etexilate vs. North American enoxaparin regimen for prevention of venous thromboembolism after knee arthroplasty surgery. J. Arthroplast. 2009, 24, 1–9. [Google Scholar]
- Fitzgerald, R.H.; Spiro, T.E.; Trowbridge, A.A.; Gardiner, G.A.; Whitsett, T.L.; O’Connell, M.B.; Ohar, J.A.; Young, T.R.; Enoxaparin Clinical Trial Group. Prevention of venous thromboembolic disease following primary total knee arthroplasty. A randomized, multicenter, open-label, parallel-group comparison of enoxaparin and warfarin. J. Bone Jt. Surg. Br. Vol. 2001, 83, 900–906. [Google Scholar] [CrossRef]
- Salazar, C.A.; Malaga, G.; Malasquez, G. Direct thrombin inhibitors versus vitamin K antagonists or low molecular weight heparins for prevention of venous thromboembolism following total hip or knee replacement. Cochrane Database Syst. Rev. 2010. [Google Scholar] [CrossRef]
- Gillette, B.P.; DeSimone, L.J.; Trousdale, R.T.; Pagnano, M.W.; Sierra, R.J. Low risk of thromboembolic complications with tranexamic acid after primary total hip and knee arthroplasty. Clin. Orthop. Relat. Res. 2013, 471, 150–154. [Google Scholar] [CrossRef] [PubMed]
- Wilson, D.G.; Poole, W.E.; Chauhan, S.K.; Rogers, B.A. Systematic review of aspirin for thromboprophylaxis in modern elective total hip and knee arthroplasty. Bone Jt. J. 2016, 98, 1056–1061. [Google Scholar] [CrossRef]
- Nielen, J.T.; Dagnelie, P.C.; Emans, P.J.; Veldhorst-Janssen, N.; Lalmohamed, A.; Van Staa, T.P.; Boonen, A.E.R.C.H.; Bemt, B.J.F.V.D.; De Vries, F. Safety and efficacy of new oral anticoagulants and low-molecular-weight heparins compared with aspirin in patients undergoing total knee and hip replacements. Pharmacoepidemiol. Drug Saf. 2016, 25, 1245–1252. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.R.; Dunbar, M.; Bohm, E.; Zukor, D.; Fisher, W.; Gofton, W.; Macdonald, S.; Pleasance, S.; Davis, N.; Wells, P.S.; et al. Aspirin versus low-molecular-weight heparin for extended venous thromboembolism prophylaxis after total hip arthroplasty: A randomized trial. Ann. Int. Med. 2013, 158, 800–806. [Google Scholar] [CrossRef] [PubMed]
- Weitz, J.I.; Lensing, A.W.A.; Prins, M.H.; Bauersachs, R.; Beyer-Westendorf, J.; Bounameaux, H.; Brighton, T.A.; Cohen, A.T.; Davidson, B.L.; Decousus, H.; et al. Rivaroxaban or Aspirin for Extended Treatment of Venous Thromboembolism. N. Engl. J. Med. 2017, 376, 1211–1222. [Google Scholar] [CrossRef]
- Bloch, B.V.; Patel, V.; Best, A.J. Thromboprophylaxis with dabigatran leads to an increased incidence of wound leakage and an increased length of stay after total joint replacement. Bone Jt. J. 2014, 96, 122–126. [Google Scholar] [CrossRef] [PubMed]
- Intermountain Joint Replacement Center Writing Committee. A prospective comparison of warfarin to aspirin for thromboprophylaxis in total hip and total knee arthroplasty. J. Arthroplast. 2012, 27, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.C.; Parvizi, J.; Hozack, W.J.; Chen, A.F.; Austin, M.S. Aspirin Is as Effective as and Safer Than Warfarin for Patients at Higher Risk of Venous Thromboembolism Undergoing Total Joint Arthroplasty. J. Arthroplast. 2016, 31 (Suppl. S9), 83–86. [Google Scholar] [CrossRef]
- Parvizi, J.; Huang, R.; Restrepo, C.; Chen, A.F.; Austin, M.S.; Hozack, W.J.; Lonner, J.H. Low-Dose Aspirin Is Effective Chemoprophylaxis Against Clinically Important Venous Thromboembolism Following Total Joint Arthroplasty: A Preliminary Analysis. J. Bone Jt. Surg. Am. Vol. 2017, 99, 91–98. [Google Scholar] [CrossRef]
- Parvizi, J.; Kahl, L.K.; Dalsey, C. Aggressive Anticoagulation after TJA: An evaluation of the ACCP guidelines for Thromboprophylaxis. J. Long-Term Eff. Med. Implants 2007, 17, 359–365. [Google Scholar] [CrossRef]
- An, V.V.; Phan, K.; Levy, Y.D.; Bruce, W.J. Aspirin as Thromboprophylaxis in Hip and Knee Arthroplasty: A Systematic Review and Meta-Analysis. J. Arthroplast. 2016, 31, 2608–2616. [Google Scholar] [CrossRef] [PubMed]
- Deirmengian, G.K.; Heller, S.; Smith, E.B.; Maltenfort, M.; Chen, A.F.; Parvizi, J. Aspirin Can Be Used as Prophylaxis for Prevention of Venous Thromboembolism After Revision Hip and Knee Arthroplasty. J. Arthroplast. 2016, 31, 2237–2240. [Google Scholar] [CrossRef] [PubMed]
- Cafri, G.; Paxton, E.W.; Chen, Y.; Cheetham, C.T.; Gould, M.K.; Sluggett, J.K.; Bini, S.; Khatod, M. Comparative Effectiveness and Safety of Drug Prophylaxis for Prevention of Venous Thromboembolism After Total Knee Arthroplasty. J. Arthroplast. 2017, 32, 3524–3528. [Google Scholar] [CrossRef] [PubMed]
- Dalury, D.; Lonner, J.; Parvizi, J. Prevention of venous thromboembolism after total joint arthroplasty: Aspirin is enough for most patients. Am. J. Orthop. 2015, 44, 59–60. [Google Scholar]
- Gutowski, C.J.; Zmistowski, B.M.; Lonner, J.H.; Purtill, J.J.; Parvizi, J. Direct Costs of Aspirin versus Warfarin for Venous Thromboembolism Prophylaxis after Total Knee or Hip Arthroplasty. J. Arthroplast. 2015, 30 (Suppl. S9), 36–38. [Google Scholar] [CrossRef]
- Heller, S.; Secrist, E.; Shahi, A.; Chen, A.F.; Parvizi, J. Tranexamic Acid Can Be Administered to Arthroplasty Patients Who Receive Aspirin for Venous Thromboembolic Prophylaxis. J. Arthroplast. 2016, 31, 1437–1441. [Google Scholar] [CrossRef]
- Pollock, M.; Somerville, L.; Firth, A.; Lanting, B. Outpatient Total Hip Arthroplasty, Total Knee Arthroplasty, and Unicompartmental Knee Arthroplasty: A Systematic Review of the Literature. JBJS Rev. 2016, 4, 1. [Google Scholar] [CrossRef]
- Lachiewicz, P.F. Comparison of ACCP and AAOS guidelines for VTE prophylaxis after total hip and total knee arthroplasty. Orthopedics 2009, 32 (Suppl. S12), 74–78. [Google Scholar] [CrossRef]
- Stewart, D.W.; Freshour, J.E. Aspirin for the prophylaxis of venous thromboembolic events in orthopedic surgery patients: A comparison of the AAOS and ACCP guidelines with review of the evidence. Ann. Pharmacother. 2013, 47, 63–74. [Google Scholar] [CrossRef]
- Kearon, C.; Akl, E.A.; Comerota, A.J.; Prandoni, P.; Bounameaux, H.; Goldhaber, S.Z.; Nelson, M.E.; Wells, P.S.; Gould, M.K.; Dentali, F.; et al. Antithrombotic therapy for VTE disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012, 141 (Suppl. S2), e419S–e496S. [Google Scholar] [CrossRef]
- Eikelboom, J.W.; Karthikeyan, G.; Fagel, N.; Hirsh, J. American Association of Orthopedic Surgeons and American College of Chest Physicians guidelines for venous thromboembolism prevention in hip and knee arthroplasty differ: What are the implications for clinicians and patients? Chest 2009, 135, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Johanson, N.A.; Lachiewicz, P.F.; Lieberman, J.R.; Lotke, P.A.; Parvizi, J.; Pellegrini, V.; Stringer, T.A.; Tornetta, P.; Haralson, R.H.; Watters, W.C. American academy of orthopaedic surgeons clinical practice guideline on Prevention of symptomatic pulmonary embolism in patients undergoing total hip or knee arthroplasty. J. Bone Jt. Surg. Am. 2009, 91, 1756–1757. [Google Scholar] [CrossRef] [PubMed]
- Mont, M.A.; Jacobs, J.J. AAOS clinical practice guideline: Preventing venous thromboembolic disease in patients undergoing elective hip and knee arthroplasty. J. Am. Acad. Orthop. Surg. 2011, 19, 777–778. [Google Scholar] [CrossRef] [PubMed]
- Mont, M.A.; Jacobs, J.J.; Boggio, L.N.; Bozic, K.J.; Della Valle, C.J.; Goodman, S.B.; Lewis, C.G.; Yates, A.J.; Watters, W.C.; Turkelson, C.M.; et al. Preventing venous thromboembolic disease in patients undergoing elective hip and knee arthroplasty. J. Am. Acad. Orthop. Surg. 2011, 19, 768–776. [Google Scholar] [CrossRef] [PubMed]
- Lieberman, J.R. The new AAOS clinical practice guidelines on venous thromboembolic prophylaxis: How to adapt them to your practice. J. Am. Acad. Orthop. Surg. 2011, 19, 717–721. [Google Scholar] [CrossRef] [PubMed]
- Markel, D.C.; York, S.; Liston, M.J.; Flynn, J.C.; Barnes, C.L.; Davis, C.M., 3rd. Venous thromboembolism: Management by American Association of Hip and Knee Surgeons. J. Arthroplast. 2010, 25. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Etscheidt, J.; Shahien, A.; Gainey, M.; Kronenfeld, D.; Niu, R.; Freccero, D.; Smith, E. Review of Therapeutic Options for the Prevention of VTE in Total Joint Arthroplasty. Geriatrics 2020, 5, 18. https://doi.org/10.3390/geriatrics5010018
Etscheidt J, Shahien A, Gainey M, Kronenfeld D, Niu R, Freccero D, Smith E. Review of Therapeutic Options for the Prevention of VTE in Total Joint Arthroplasty. Geriatrics. 2020; 5(1):18. https://doi.org/10.3390/geriatrics5010018
Chicago/Turabian StyleEtscheidt, Jordan, Amir Shahien, Monique Gainey, Daniel Kronenfeld, Ruijia Niu, David Freccero, and Eric Smith. 2020. "Review of Therapeutic Options for the Prevention of VTE in Total Joint Arthroplasty" Geriatrics 5, no. 1: 18. https://doi.org/10.3390/geriatrics5010018
APA StyleEtscheidt, J., Shahien, A., Gainey, M., Kronenfeld, D., Niu, R., Freccero, D., & Smith, E. (2020). Review of Therapeutic Options for the Prevention of VTE in Total Joint Arthroplasty. Geriatrics, 5(1), 18. https://doi.org/10.3390/geriatrics5010018




