Efficacy and Safety of IncobotulinumtoxinA in Older Patients with Upper Limb Spasticity: A Pooled Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Overall Analyses
2.2. Subgroup Analysis: Patients with Moderate-to-Severe ULS
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AESI | adverse events of special interest |
| AS | Ashworth Scale |
| mAS | modified Ashworth Scale |
| SD | standard deviation |
| ULS | upper-limb spasticity |
Appendix A
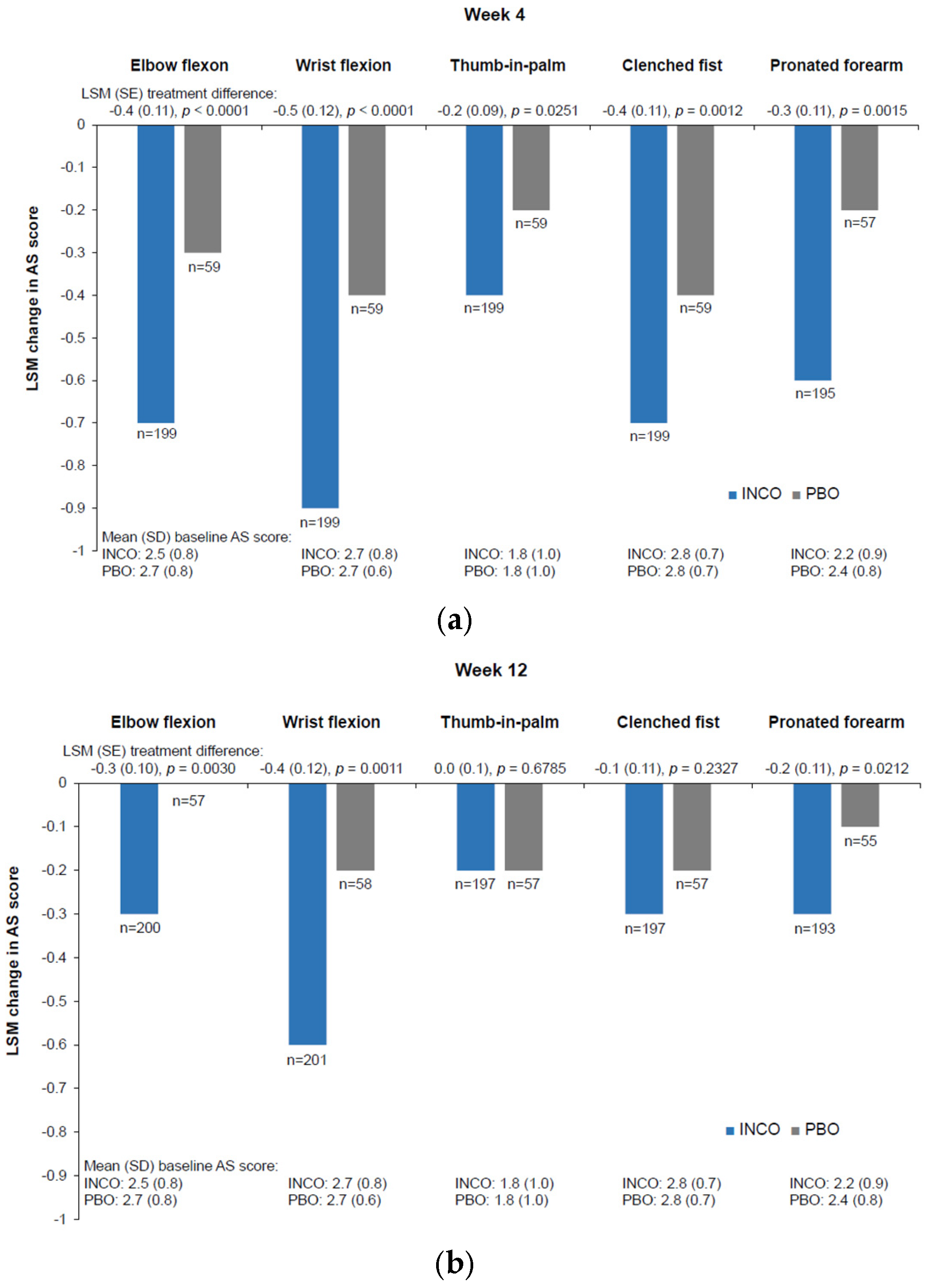
References
- World Stroke Organization. Global Stroke Fact Sheet 2022 [Internet]. 2022 [Cited 2024 Apr 20]. Available online: https://www.world-stroke.org/assets/downloads/WSO_Global_Stroke_Fact_Sheet.pdf (accessed on 18 December 2024).
- Feigin, V.L.; Forouzanfar, M.H.; Krishnamurthi, R.; A Mensah, G.; Connor, M.; A Bennett, D.; E Moran, A.; Sacco, R.L.; Anderson, L.; Truelsen, T.; et al. Global and regional burden of stroke during 1990–2010: Findings from the Global Burden of Disease Study 2010. Lancet 2014, 383, 245–255. [Google Scholar] [CrossRef]
- Rajati, F.; Rajati, M.; Rasulehvandi, R.; Kazeminia, M. Prevalence of stroke in the elderly: A systematic review and meta-analysis. Interdiscip. Neurosurg. 2023, 32, 101746. [Google Scholar] [CrossRef]
- Gupta, A.D.; Eyre, R. Role of botulinum toxin in the management of hand ulceration due to post-stroke spasticity among aged care residents. Aust. J. Gen. Pr. 2022, 51, 484–489. [Google Scholar] [CrossRef] [PubMed]
- Shiner, C.T.; Vratsistas-Curto, A.; Bramah, V.; Faux, S.G.; Watanabe, Y. Prevalence of upper-limb spasticity and its impact on care among nursing home residents with prior stroke. Disabil. Rehabil. 2019, 42, 2170–2177. [Google Scholar] [CrossRef] [PubMed]
- Gill, C.E.; Hacker, M.L.; Meystedt, J.; Turchan, M.; Schnelle, J.F.; Simmons, S.F.; Habermann, R.; Phibbs, F.T.; Charles, D. Prevalence of Spasticity in Nursing Home Residents. J. Am. Med Dir. Assoc. 2020, 21, 1157–1160. [Google Scholar] [CrossRef]
- Edwards, L.; Ellis, B.; Donnellan, C.; Osman, H.; Haboubi, N.; Jones, M.; Sunman, W.; Pinnington, L.; Phillips, M.F. Prevalence of unmet needs for spasticity management in care home residents in the East Midlands, United Kingdom: A cross-sectional observational study. Clin. Rehabil. 2019, 33, 1819–1830. [Google Scholar] [CrossRef]
- Wissel, J.; Müller, J.; Dressnandt, J.; Heinen, F.; Naumann, M.; Topka, H.; Poewe, W. Management of Spasticity Associated Pain with Botulinum Toxin A. J. Pain Symptom Manag. 2000, 20, 44–49. [Google Scholar] [CrossRef]
- Jaul, E.; Factor, H.; Karni, S.; Schiffmiller, T.; Meiron, O. Spasticity and dementia increase the risk of pressure ulcers. Int. Wound J. 2019, 16, 847–851. [Google Scholar] [CrossRef]
- van Kuijk, A.A.; Geurts, A.C.H.; Bevaart, B.J.W.; van Limbeek, J. Treatment of upper extremity spasticity in stroke patients by focal neuronal or neuromuscular blockade: A systematic review of the literature. J. Rehabil. Med. 2002, 34, 51–61. [Google Scholar] [CrossRef]
- Picelli, A.; Santamato, A.; Cosma, M.; Baricich, A.; Chisari, C.; Millevolte, M.; Del Prete, C.; Mazzù, I.; Girardi, P.; Smania, N. Early Botulinum Toxin Type A Injection for Post-Stroke Spasticity: A Longitudinal Cohort Study. Toxins 2021, 13, 374. [Google Scholar] [CrossRef]
- Francisco, G.; Balbert, A.; Bavikatte, G.; Bensmail, D.; Carda, S.; Deltombe, T.; Draulans, N.; Escaldi, S.; Gross, R.; Jacinto, J.; et al. A practical guide to optimizing the benefits of post-stroke spasticity interventions with botulinum toxin A: An international group consensus. J. Rehabil. Med. 2021, 53, jrm00134. [Google Scholar] [CrossRef]
- Ashford, S.A.; Turner-Stokes, L.F. Spasticity in Adults: Management Using Botulinum Toxin: National Guidelines 2018. 2nd Edition. Royal College of Physicians of London [Internet]. [Cited 2024 Sep 10]. Available online: https://www.acpin.net/pdfs/NG_Spasticity_in_adults_final.pdf (accessed on 18 December 2024).
- Therapie des Spastischen Syndroms, S2k-Leitlinie, in: Deutsche Gesellschaft für Neurologie (Hrsg.), Leitlinien für Diagnostik und Therapie in der Neurologie (in German). 2018. Available online: https://dgn.org/leitlinien (accessed on 21 April 2024).
- Simpson, D.M.; Hallett, M.; Ashman, E.J.; Comella, C.L.; Green, M.W.; Gronseth, G.S.; Armstrong, M.J.; Gloss, D.; Potrebic, S.; Jankovic, J.; et al. Practice guideline update summary: Botulinum neurotoxin for the treatment of blepharospasm, cervical dystonia, adult spasticity, and headache [RETIRED]. Neurology 2016, 86, 1818–1826. [Google Scholar] [CrossRef] [PubMed]
- Shenoy, P.; Harugeri, A. Elderly patients’ participation in clinical trials. Perspect. Clin. Res. 2015, 6, 184-9. [Google Scholar] [CrossRef] [PubMed]
- Pitkala, K.H.; E Strandberg, T. Clinical trials in older people. Age Ageing 2022, 51, afab282. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.D.; Addison, S. Healing hand ulcers caused by focal spasticity. Int. Wound J. 2020, 17, 774–780. [Google Scholar] [CrossRef]
- European Medicines Agency. ICH Topic E 7. Studies in Support of Special Populations: Geriatrics. Note for Guidance on Studies in Support of Special Populations: Geriatrics (CPMP/ICH/379/95) [Internet]. 1994 [Cited 2024 May 29]. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/ich-e-7-studies-support-special-populations-geriatrics-step-5_en.pdf (accessed on 18 December 2024).
- Kaňovský, P.; Slawek, J.; Denes, Z.; Platz, T.; Sassin, I.; Comes, G.; Grafe, S. Efficacy and Safety of Botulinum Neurotoxin NT 201 in Poststroke Upper Limb Spasticity. Clin. Neuropharmacol. 2009, 32, 259–265. [Google Scholar] [CrossRef]
- Elovic, E.P.; Munin, M.C.; Kaňovský, P.; Hanschmann, A.; Hiersemenzel, R.; Marciniak, C. Randomized, placebo--controlled trial of incobotulinumtoxina for upper--limb post--stroke spasticity. Muscle Nerve 2015, 53, 415–421. [Google Scholar] [CrossRef]
- Barnes, M.; Schnitzler, A.; Medeiros, L.; Aguilar, M.; Lehnert-Batar, A.; Minnasch, P. Efficacy and safety of NT 201 for upper limb spasticity of various etiologies—A randomized parallel-group study. Acta Neurol. Scand. 2010, 122, 295–302. [Google Scholar] [CrossRef]
- The J-PURE Study Group; Masakado, Y.; Abo, M.; Kondo, K.; Saeki, S.; Saitoh, E.; Dekundy, A.; Hanschmann, A.; Kaji, R. Efficacy and safety of incobotulinumtoxinA in post-stroke upper-limb spasticity in Japanese subjects: Results from a randomized, double-blind, placebo-controlled study (J-PURE). J. Neurol. 2020, 267, 2029–2041. [Google Scholar] [CrossRef]
- Merz Therapeutics GmbH, Frankfurt/Main, Germany. EudraCT No. 2006-000073-29, 2009 [Cited 2024 Sep 10]. Available online: https://www.clinicaltrialsregister.eu/ctr-search/search?query=2006-000073-29 (accessed on 18 December 2024).
- Wissel, J.; Bensmail, D.; Ferreira, J.J.; Molteni, F.; Satkunam, L.; Moraleda, S.; Rekand, T.; McGuire, J.; Scheschonka, A.; Flatau-Baqué, B.; et al. Safety and efficacy of incobotulinumtoxinA doses up to 800 U in limb spasticity. Neurology 2017, 88, 1321–1328. [Google Scholar] [CrossRef]
- Kleiner-Fisman, G.; Khoo, E.; Moncrieffe, N.; Forbell, T.; Gryfe, P.; Fisman, D. A Randomized, Placebo Controlled Pilot Trial of Botulinum Toxin for Paratonic Rigidity in People with Advanced Cognitive Impairment. PLoS ONE 2014, 9, e114733. [Google Scholar] [CrossRef]
- Chol, C.; Blanchon, M.-A.; Le Quang, B.; Celarier, T.; Gonthier, R. Botulinum toxin in the elderly to the care of limbs spastic hypertonia and toes or fingers dystonias. Geriatr. Psychol. Neuropsychiatr. Vieil. 2012, 10, 17–26. [Google Scholar] [CrossRef]
- Levy, J.; Karam, P.; Forestier, A.; Loze, J.-Y.; Bensmail, D. Botulinum toxin use in patients with post-stroke spasticity: A nationwide retrospective study from France. Front. Neurol. 2023, 14, 1245228. [Google Scholar] [CrossRef]
- Doan, Q.V.; Brashear, A.; Gillard, P.J.; Varon, S.F.; Vandenburgh, A.M.; Turkel, C.C.; Elovic, E.P. Relationship Between Disability and Health--Related Quality of Life and Caregiver Burden in Patients with Upper Limb Poststroke Spasticity. PM&R 2011, 4, 4–10. [Google Scholar] [CrossRef]
- Gillard, P.J.; Sucharew, H.; Kleindorfer, D.; Belagaje, S.; Varon, S.; Alwell, K.; Moomaw, C.J.; Woo, D.; Khatri, P.; Flaherty, M.L.; et al. The negative impact of spasticity on the health-related quality of life of stroke survivors: A longitudinal cohort study. Health Qual. Life Outcomes 2015, 13, 1–9. [Google Scholar] [CrossRef]
- Schinwelski, M.J.; Sitek, E.J.; Wąż, P.; Sławek, J.W. Prevalence and predictors of post-stroke spasticity and its impact on daily living and quality of life. Neurol. Neurochir. Polska 2019, 53, 449–457. [Google Scholar] [CrossRef]
- Zorowitz, R.D.; Gillard, P.J.; Brainin, M. Poststroke spasticity. Neurology 2013, 80, S45–S52. [Google Scholar] [CrossRef] [PubMed]
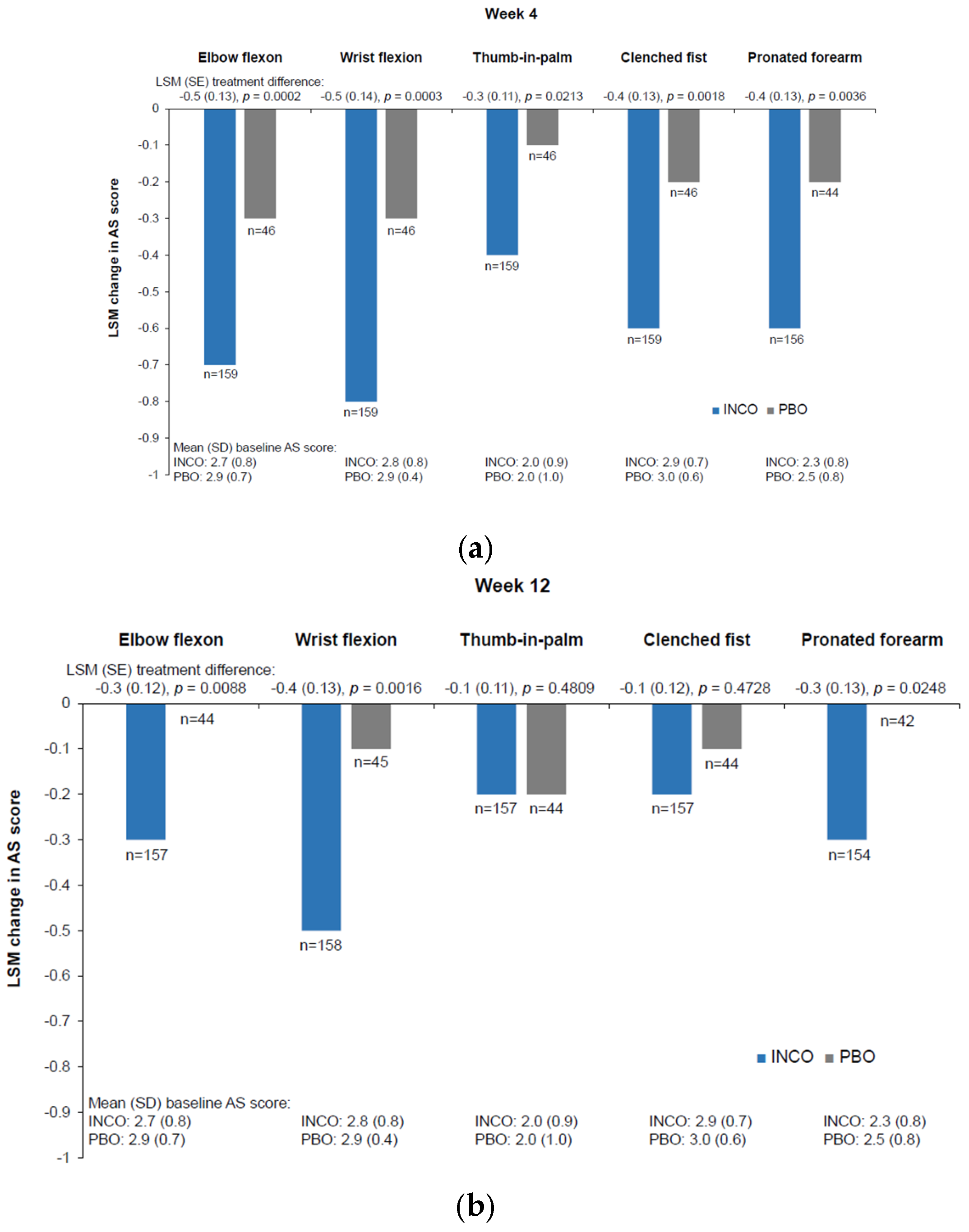
| Reference | Study Design | Treatments in First Injection Cycle a | Older Patients b, n | Baseline Patient b Characteristics (Study Location) | Duration c |
|---|---|---|---|---|---|
| MRZ_60201_0307 (unpublished) d | Double blind, randomized, placebo-controlled, parallel group, multicenter, phase II pilot | INCO ≤ 400 U Placebo | 4 2 | 33.3% female; mean age 70.2 years; 100.0% white; time since spasticity diagnosis 1.2 years (Germany) | 12 weeks |
| NCT00432666 [20] | Double blind, randomized, placebo-controlled, multicenter, phase III | INCO ≤ 400 U (intended) Placebo | 22 16 | 39.5% female; mean age 69.5 years; 100.0% white; time since spasticity diagnosis 5.6 years (Czech Republic, Hungary, Poland) | ≤20 weeks |
| NCT01392300 [21] | Double blind, randomized, placebo-controlled, multicenter | INCO 400 U Placebo | 48 32 | 43.8% female; mean age 69.6 years; 88.8% white, 7.5% Asian, 2.5% Black; time since spasticity diagnosis 3.3 years (Czech Republic, Germany, Hungary, India, Poland, Russian Federation, USA) | 12 weeks |
| NCT00465738 [22] | Observer blind, randomized, parallel group, multicenter | INCO (20 or 50 U/mL) ≤400 U (median 300 U) | 58 | 44.8% female; mean age 71.4 years; 84.5% White; time since spasticity diagnosis 5.7 years (Austria, France, Germany, Italy, Portugal, Spain, Switzerland, UK) | 20 weeks |
| NCT01603459 [25] | Open label, non-randomized, single arm, multicenter | INCO 400 U | 40 | 20.0% female; mean age 69.5 years; 85.0% White, 2.5% Asian; time since spasticity diagnosis 5.6 years (Canada, France, Germany, Italy, Norway, Portugal, Spain, USA) | 36–48 weeks |
| JapicCTI-153029 [23] | Double blind, randomized, placebo-controlled, multicenter, phase III | INCO 250 U INCO 400 U Placebo | 28 11 | 30.8% female; mean age 70.8 years; 100.0% Asian; time since spasticity diagnosis 7.8 years (Japan) | 12 weeks |
| MRZ_60201_0528 [24] (unpublished) d | Double blind, randomized, placebo-controlled, multicenter, phase II | INCO 100–200 U Placebo | 3 3 | 33.3% female; mean age 75.0 years; 100.0% white; time since spasticity diagnosis 0.0 years (Germany) | 12 weeks |
| Parameter | IncobotulinumtoxinA (n = 203) | Placebo (n = 64) | Total (n = 267) |
|---|---|---|---|
| Mean (SD) age, years | 70.3 (4.2) | 70.2 (4.2) | 70.3 (4.2) |
| Age ≥ 75 years, n (%) | 31 (15.3) | 9 (14.1) | 40 (15.0) |
| Female, n (%) | 70 (34.5) | 30 (46.9) | 100 (37.5) |
| Race, n (%) | |||
| White | 154 (75.9) | 50 (78.1) | 204 (76.4) |
| Black or African American | 1 (0.5) | 1 (1.6) | 2 (0.8) |
| Asian | 33 (16.3) | 13 (20.3) | 46 (17.2) |
| Other/missing | 15 (7.4) | 0 | 15 (5.6) |
| Mean (SD) weight, kg | 74.2 (13.6) | 71.7 (13.1) | 73.6 (13.5) |
| Baseline disease severity, n (%) | |||
| Mild | 43 (21.2) | 17 (26.6) | 60 (22.5) |
| Moderate | 138 (68.0) | 39 (60.9) | 177 (66.3) |
| Severe | 22 (10.8) | 8 (12.5) | 30 (11.2) |
| Mean (SD) time since spasticity diagnosis, years | 5.2 (5.5) | 4.3 (4.8) | 5.0 (5.4) |
| Etiology of spasticity, n (%) | |||
| Stroke—ischemic | 111 (54.7) | 46 (71.9) | 157 (58.8) |
| Stroke—hemorrhagic | 27 (13.3) | 16 (25.0) | 43 (16.1) |
| Stroke—not known | 63 (31.0) | 2 (3.1) | 65 (24.3) |
| Other | 2 (1.0) | 0 | 2 (0.8) |
| Any concomitant medication, n (%) | 202 (99.5) | 64 (100.0) | 266 (99.6) |
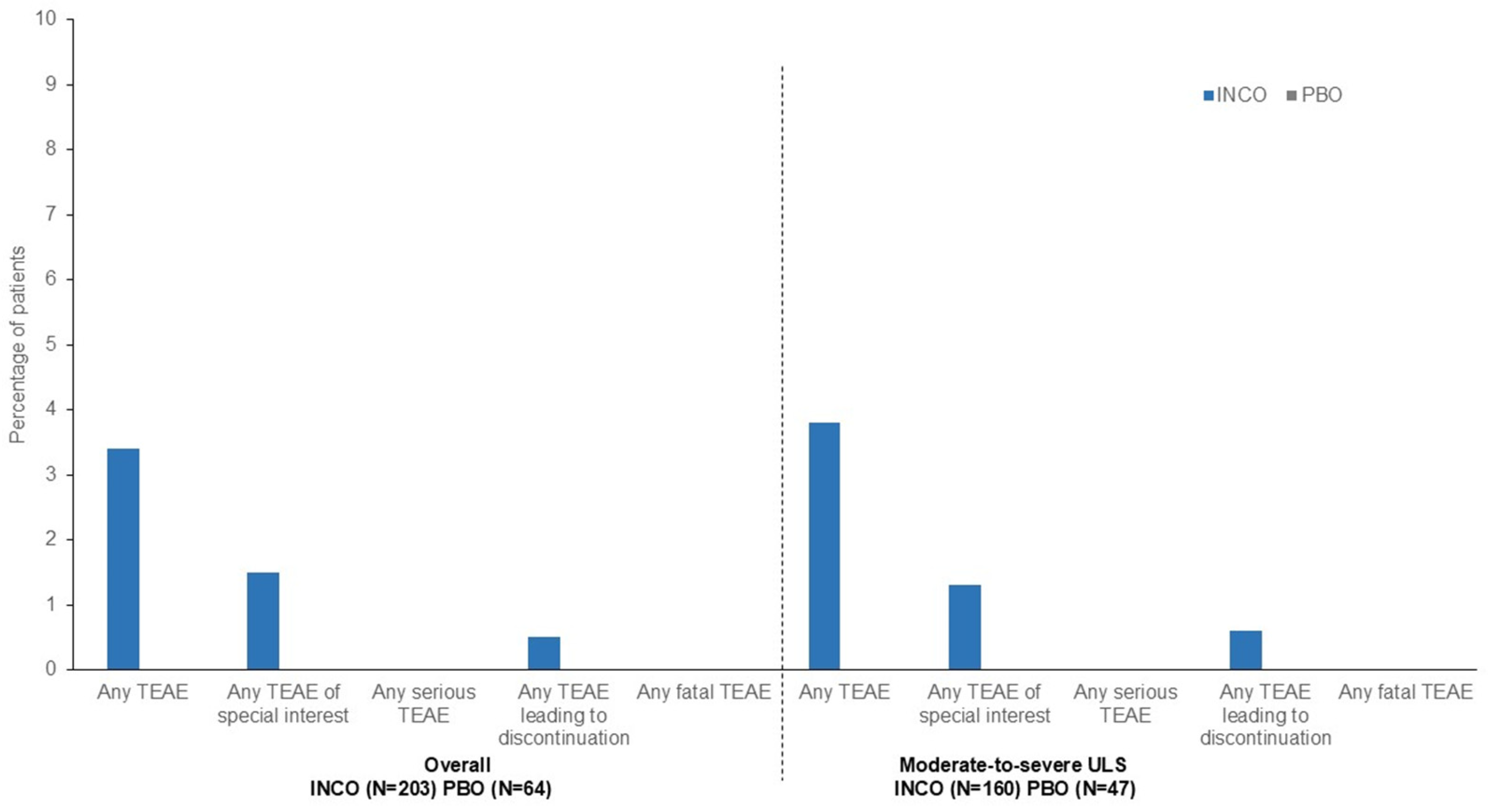
| Number (%) of Patients with: | Overall | Moderate-to-Severe ULS | ||||
|---|---|---|---|---|---|---|
| INCO (n = 203) | PBO (n = 64) | Total (n = 267) | INCO (n = 160) | PBO (n = 47) | Total (n = 207) | |
| Any TEAE | 72 (35.5) | 21 (32.8) | 93 (34.8) | 61 (38.1) | 16 (34.0) | 77 (37.2) |
| Any TEAE related to treatment | 7 (3.4) | 0 | 7 (2.6) | 6 (3.8) | 0 | 6 (2.9) |
| Any TEAE of special interest a | 7 (3.4) | 1 (1.6) | 8 (3.0) | 6 (3.8) | 1 (2.1) | 7 (3.4) |
| Any TEAE of special interest a related to treatment | 3 (1.5) | 0 | 3 (1.1) | 2 (1.3) | 0 | 2 (1.0) |
| Any serious TEAE | 13 (6.4) | 6 (9.4) | 19 (7.1) | 11 (6.9) | 4 (8.5) | 15 (7.2) |
| Any serious TEAE related to treatment | 0 | 0 | 0 | 0 | 0 | 0 |
| Any TEAE leading to discontinuation | 2 (1.0) | 5 (7.8) | 7 (2.6) | 2 (1.3) | 3 (6.4) | 5 (2.4) |
| Any TEAE leading to discontinuation related to treatment | 1 (0.5) | 0 | 1 (0.4) | 1 (0.6) | 0 | 1 (0.5) |
| Any fatal TEAE | 0 | 2 (3.1) | 2 (0.7) | 0 | 1 (2.1) | 1 (0.5) |
| Any fatal TEAE related to treatment | 0 | 0 | 0 | 0 | 0 | 0 |
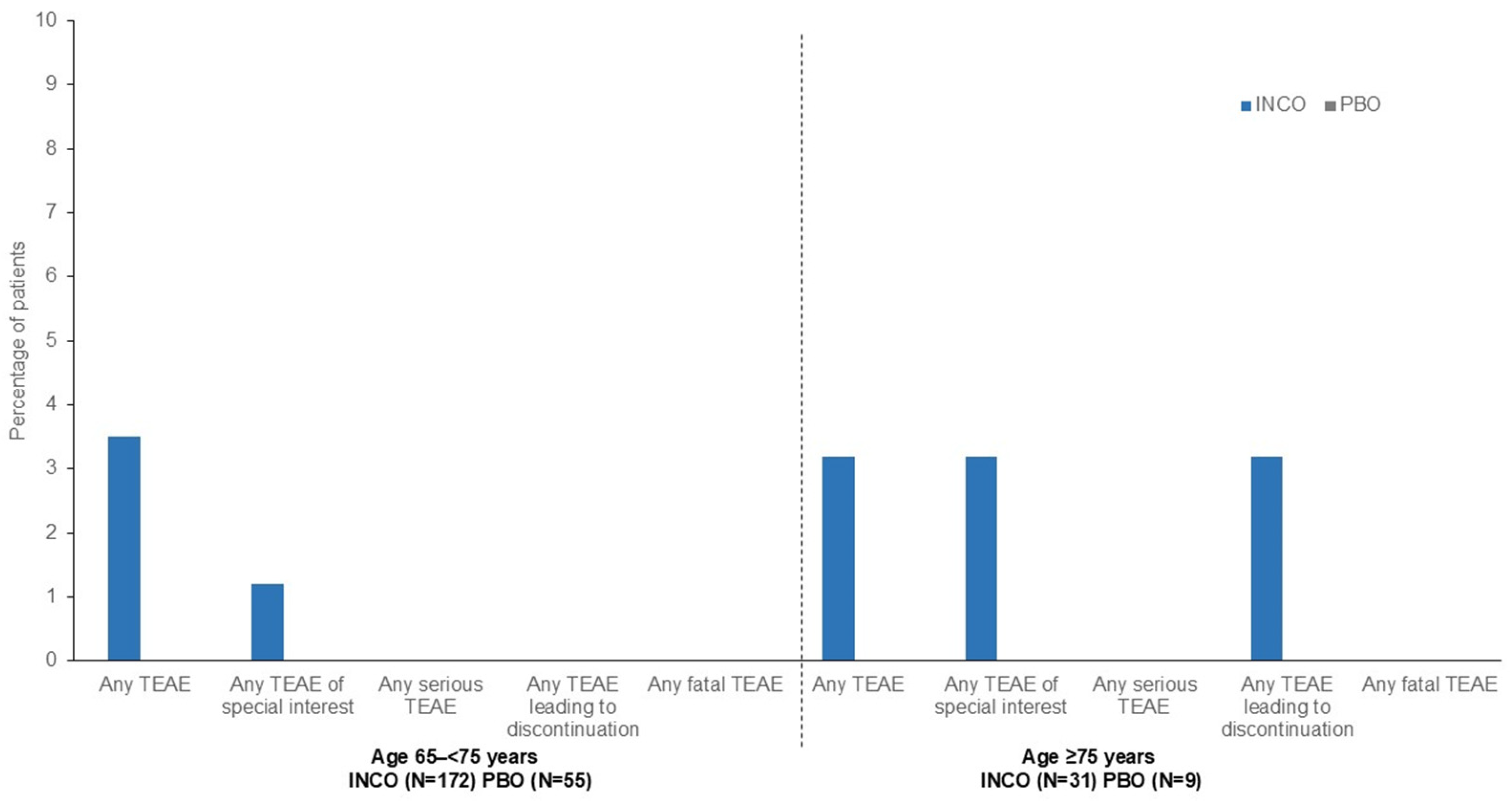
| Number (%) of Patients with: | Age 65 to <75 Years | Age ≥ 75 Years | ||||
|---|---|---|---|---|---|---|
| INCO (n = 172) | PBO (n = 55) | Total (n = 227) | INCO (n = 31) | PBO (n = 9) | Total (n = 40) | |
| Any TEAE | 60 (34.9) | 19 (34.5) | 79 (34.8) | 12 (38.7) | 2 (22.2) | 14 (35.0) |
| Any TEAE related to treatment | 6 (3.5) | 0 | 6 (2.6) | 1 (3.2) | 0 | 1 (2.5) |
| Any TEAE of special interest a | 6 (3.5) | 1 (1.8) | 7 (3.1) | 1 (3.2) | 0 | 1 (2.5) |
| Any TEAE of special interest a related to treatment | 2 (1.2) | 0 | 2 (0.9) | 1 (3.2) | 0 | 1 (2.5) |
| Any serious TEAE | 9 (5.2) | 4 (7.3) | 13 (5.7) | 4 (12.9) | 2 (22.2) | 6 (15.0) |
| Any serious TEAE related to treatment | 0 | 0 | 0 | 0 | 0 | 0 |
| Any TEAE leading to discontinuation | 1 (0.6) | 4 (7.3) | 5 (2.2) | 1 (3.2) | 1 (11.1) | 2 (5.0) |
| Any TEAE leading to discontinuation related to treatment | 0 | 0 | 0 | 1 (3.2) | 0 | 1 (2.5) |
| Any fatal TEAE | 0 | 1 (1.8) | 1 (0.4) | 0 | 1 (11.1) | 1 (2.5) |
| Any fatal TEAE related to treatment | 0 | 0 | 0 | 0 | 0 | 0 |
| Parameter | IncobotulinumtoxinA (n = 160) | Placebo (n = 47) | Total (n = 207) |
|---|---|---|---|
| Mean (SD) age, years | 70.3 (4.2) | 69.3 (3.5) | 70.0 (4.1) |
| Age ≥ 75 years, n (%) | 24 (15.0) | 4 (8.5) | 28 (13.5) |
| Female, n (%) | 52 (32.5) | 24 (51.1) | 76 (36.7) |
| Race, n (%) | |||
| White | 117 (73.1) | 34 (72.3) | 151 (73.0) |
| Black or African American | 1 (0.6) | 1 (2.1) | 2 (1.0) |
| Asian | 30 (18.8) | 12 (25.5) | 42 (20.3) |
| Other/missing | 12 (7.5) | 0 | 12 (5.8) |
| Mean (SD) weight, kg | 73.7 (13.9) | 71.2 (13.9) | 73.1 (13.9) |
| Baseline disease severity, n (%) | |||
| Moderate | 138 (86.3) | 39 (83.0) | 177 (85.5) |
| Severe | 22 (13.8) | 8 (17.0) | 30 (14.5) |
| Mean (SD) time since spasticity diagnosis, years | 5.5 (5.4) | 4.7 (4.8) | 5.3 (5.3) |
| Etiology of spasticity, n (%) | |||
| Stroke—ischemic | 91 (56.9) | 31 (66.0) | 122 (58.9) |
| Stroke—hemorrhagic | 24 (15.0) | 14 (29.8) | 38 (18.4) |
| Stroke—not known | 43 (26.9) | 2 (4.3) | 45 (21.7) |
| Other | 2 (1.3) | 0 | 2 (1.0) |
| Any concomitant medication, n (%) | 159 (99.4) | 47 (100.0) | 206 (99.5) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Munin, M.C.; Camões-Barbosa, A.; Cordero-García, C.; Baricich, A.; Carda, S.; Althaus, M.; Comes, G.; Vacchelli, M.; Wissel, J. Efficacy and Safety of IncobotulinumtoxinA in Older Patients with Upper Limb Spasticity: A Pooled Analysis. Geriatrics 2025, 10, 155. https://doi.org/10.3390/geriatrics10060155
Munin MC, Camões-Barbosa A, Cordero-García C, Baricich A, Carda S, Althaus M, Comes G, Vacchelli M, Wissel J. Efficacy and Safety of IncobotulinumtoxinA in Older Patients with Upper Limb Spasticity: A Pooled Analysis. Geriatrics. 2025; 10(6):155. https://doi.org/10.3390/geriatrics10060155
Chicago/Turabian StyleMunin, Michael C., Alexandre Camões-Barbosa, Carlos Cordero-García, Alessio Baricich, Stefano Carda, Michael Althaus, Georg Comes, Matteo Vacchelli, and Jörg Wissel. 2025. "Efficacy and Safety of IncobotulinumtoxinA in Older Patients with Upper Limb Spasticity: A Pooled Analysis" Geriatrics 10, no. 6: 155. https://doi.org/10.3390/geriatrics10060155
APA StyleMunin, M. C., Camões-Barbosa, A., Cordero-García, C., Baricich, A., Carda, S., Althaus, M., Comes, G., Vacchelli, M., & Wissel, J. (2025). Efficacy and Safety of IncobotulinumtoxinA in Older Patients with Upper Limb Spasticity: A Pooled Analysis. Geriatrics, 10(6), 155. https://doi.org/10.3390/geriatrics10060155








