Prevalence of Frailty and Associated Sociodemographic, Biomedical, and Biochemical Factors Amongst Participants Residing in Limpopo Province, South Africa
Abstract
1. Introduction
2. Materials and Methods
2.1. Design, Setting, Sampling, and Participants
2.2. Data Collection
2.3. Frailty Measurement
2.4. Statistical Analysis
3. Results
3.1. Characteristics of Participants
3.2. Comparison of Means and Proportions of Sociodemographic and Biochemical Profiles Across the Frailty Groups (Non-Frail, Pre-Frail, and Frail)
3.3. Factors Associated with Frailty
4. Discussion
Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DIMAMO | Dikgale Mamabolo Mothiba |
References
- Proietti, M.; Cesari, M. Frailty: What Is It? In Frailty and Cardiovascular Diseases: Research into an Elderly Population; Veronese, N., Ed.; Springer International Publishing: Cham, Germany, 2020; pp. 1–7. [Google Scholar] [CrossRef]
- British Geriatrics Society. Introduction to Frailty. Available online: https://www.bgs.org.uk/resources/introduction-to-frailty (accessed on 24 April 2024).
- Database.earth. Life Expectancy of South Africa 1950–2024 & Future Projections. Available online: https://database.earth/population/South-Africagrowth-rate (accessed on 18 March 2024).
- De Wet-Billings, N. Preventable deaths among youth in South Africa: Measuring life expectancy in the absence of non-communicable diseases and its implications for the healthcare system. S. Afr. Med. J. 2021, 111, 361–364. [Google Scholar] [CrossRef] [PubMed]
- Maredza, M.; Bertram, M.Y.; Gómez-Olivé, X.F.; Tollman, S.M. Burden of stroke attributable to selected lifestyle risk factors in rural South Africa. BMC Public Health 2016, 16, 143. [Google Scholar] [CrossRef] [PubMed]
- Lai, H.Y.; Huang, S.T.; Anker, S.D.; von Haehling, S.; Akishita, M.; Arai, H.; Chen, L.; Hsiao, F. The burden of frailty in heart failure: Prevalence, impacts on clinical outcomes and the role of heart failure medications. J. Cachexia Sarcopenia Muscle 2024, 15, 660–670. [Google Scholar] [CrossRef] [PubMed]
- George, N.L.; Nethathe, G.D. Frailty: What the South African surgeon needs to know. S. Afr. J. Surg. 2019, 57, 24–29. [Google Scholar] [CrossRef]
- Biritwum, R.B.; Minicuci, N.; Yawson, A.E.; Theou, O.; Mensah, G.P.; Naidoo, N.; Wu, F.; Guo, Y.; Zheng, Y.; Jiang, Y.; et al. Prevalence of and factors associated with frailty and disability in older adults from China, Ghana, India, Mexico, Russia and South Africa. Maturitas 2016, 91, 8–18. [Google Scholar] [CrossRef]
- Weiss, C.O. Frailty and chronic diseases in older adults. Clin. Geriatr. Med. 2011, 27, 39–52. [Google Scholar] [CrossRef]
- Onder, G.; Vetrano, D.L.; Marengoni, A.; Bell, J.S.; Johnell, K.; Palmer, K. Accounting for frailty when treating chronic diseases. Eur. J. Intern. Med. 2018, 56, 49–52. [Google Scholar] [CrossRef]
- Ntimana, C.B.; Mashaba, R.G.; Seakamela, K.P.; Netshapapame, T.; Maimela, E. Risky sexual behaviors and associated factors among adult patients on antiretroviral treatment at Mankweng Hospital in Limpopo Province, South Africa. Front. Public Health 2023, 11, 1245178. [Google Scholar] [CrossRef]
- Maimela, E.; Alberts, M.; Modjadji, S.E.; Choma, S.S.; Dikotope, S.A.; Ntuli, T.S.; Van Geertruyden, J.-P.; Oni, T. The prevalence and determinants of chronic non-communicable disease risk factors amongst adults in the Dikgale health demographic and surveillance system (HDSS) site, Limpopo Province of South Africa. PLoS ONE 2016, 11, e0147926. [Google Scholar] [CrossRef]
- Ringane, M.C.; Choma, S.S.R. The optimal WC cut-off points for the prediction of subclinical CVD as measured by carotid intima-media thickness among African adults: A cross-sectional study. BMC Cardiovasc. Disord. 2021, 21, 575. [Google Scholar] [CrossRef]
- Moshidi, M.L.; Malema, R.N.; Muthelo, L.; Mothiba, T.M. Provision of Care to the People with HIV: Voices of Professional Nurses in the Public Hospitals of Limpopo Province, South Africa. Int. J. Environ. Res. Public Health 2021, 18, 3112. [Google Scholar] [CrossRef] [PubMed]
- Conan, N.; Simons, E.; Chihana, M.L.; Ohler, L.; FordKamara, E.; Mbatha, M.; Vancutsem, G.; Huerga, H.; Parekh, B.S. Increase in HIV viral suppression in KwaZulu-Natal, South Africa: Community-based cross sectional surveys 2018 and 2013. What remains to be done? PLoS ONE 2022, 17, e0265488. [Google Scholar] [CrossRef]
- Ntimana, C.B.; Choma, S.S. Modifiable determinants of central obesity among the rural black population in the DIMAMO HDSS, Limpopo, South Africa. Front. Public Health 2023, 11, 1165662. [Google Scholar] [CrossRef]
- Ali, S.A.; Soo, C.; Agongo, G.; Alberts, M.; Amenga-Etego, L.; Boua, R.P.; Choudhury, A.; Crowther, N.J.; Depuur, C.; Gómez-Olivé, F.X.; et al. Genomic and environmental risk factors for cardiometabolic diseases in Africa: Methods used for Phase 1 of the AWI-Gen population cross-sectional study. Glob. Health Action 2018, 11 (Suppl. 2), 1507133. [Google Scholar] [CrossRef]
- Ntimana, C.B.; Mashaba, R.G.; Seakamela, K.P.; Maimela, E.; Masemola-Maphutha, M.L.; Choma, S.S. Comorbidities of Obesity in a Rural African Population Residing in Limpopo Province, South Africa: A Comparison between General and Central Obesity. Obesities 2024, 4, 375–388. [Google Scholar] [CrossRef]
- Fried, L.P.; Ferrucci, L.; Darer, J.; Williamson, J.D.; Anderson, G. Untangling the concepts of disability, frailty, and comorbidity: Implications for improved targeting and care. J. Gerontol. A Biol. Sci. Med. Sci. 2004, 59, M255–M263. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, S.D.; Heumann, K.J.; Der Ananian, C.A.; Ainsworth, B.E. Validity and Reliability of the Global Physical Activity Questionnaire (GPAQ). Meas. Phys. Educ. Exerc. Sci. 2013, 17, 221–235. [Google Scholar] [CrossRef]
- Payne, C.F.; Wade, A.; Kabudula, C.W.; Davies, J.I.; Chang, A.Y.; Gomez-Olive, F.X.; Kahn, K.; Berkman, L.F.; Tollman, S.M.; Salomon, J.A.; et al. Prevalence and correlates of frailty in an older rural African population: Findings from the HAALSI cohort study. BMC Geriatr. 2017, 17, 293. [Google Scholar] [CrossRef]
- Kasa, A.S.; Lee, S.C.; Chang, H.C.R. Frailty in older people living in Africa: A systematic review of prevalence and associated factors. Arch. Gerontol. Geriatr. Plus 2024, 1, 100078. [Google Scholar] [CrossRef]
- Soto, M.E.; Pérez-Torres, I.; Rubio-Ruiz, M.E.; Cano-Martínez, A.; Manzano-Pech, L.; Guarner-Lans, V. Frailty and the Interactions between Skeletal Muscle, Bone, and Adipose Tissue-Impact on Cardiovascular Disease and Possible Therapeutic Measures. Int. J. Mol. Sci. 2023, 24, 4534. [Google Scholar] [CrossRef]
- Gielen, E.; Dupont, J.; Dejaeger, M.; Laurent, M.R. Sarcopenia, osteoporosis and frailty. Metabolism 2023, 145, 155638. [Google Scholar] [CrossRef]
- Lee, A.; McArthur, C.; Ioannidis, G.; Duque, G.; Adachi, J.D.; Griffith, L.E.; Thabane, L.; Papaioannou, A. Associations between Osteosarcopenia and Falls, Fractures, and Frailty in Older Adults: Results From the Canadian Longitudinal Study on Aging (CLSA). J. Am. Med. Dir. Assoc. 2024, 25, 167–176.e6. [Google Scholar] [CrossRef]
- Grainger, S.A.; Crawford, J.D.; Riches, J.C.; Kochan, N.A.; Chander, R.J.; Mather, K.A.; Sachdev, P.S.; Henry, J.D.; Krendl, A. Aging Is Associated With Multidirectional Changes in Social Cognition: Findings From an Adult Life-Span Sample Ranging From 18 to 101 Years. J. Gerontol. Ser. B 2023, 78, 62–72. [Google Scholar] [CrossRef]
- Bauman, A.; Merom, D.; Bull, F.C.; Buchner, D.M.; Fiatarone Singh, M.A. Updating the Evidence for Physical Activity: Summative Reviews of the Epidemiological Evidence, Prevalence, and Interventions to Promote “Active Aging”. Gerontologist 2016, 56 (Suppl. 2), S268–S280. [Google Scholar] [CrossRef]
- Rantakokko, M.; Mänty, M.; Rantanen, T. Mobility decline in old age. Exerc. Sport Sci. Rev. 2013, 41, 19–25. [Google Scholar] [CrossRef]
- Piche, E.; Chorin, F.; Gerus, P.; Jaafar, A.; Guerin, O.; Zory, R. Effects of age, sex, frailty and falls on cognitive and motor performance during dual-task walking in older adults. Exp. Gerontol. 2023, 171, 112022. [Google Scholar] [CrossRef]
- Mitnitski, A.; Collerton, J.; Martin-Ruiz, C.; Jagger, C.; von Zglinicki, T.; Rockwood, K.; Kirkwood, T.B.L. Age-related frailty and its association with biological markers of ageing. BMC Med. 2015, 13, 161. [Google Scholar] [CrossRef]
- Isernia, S.; Cazzoli, M.; Baglio, G.; Cabinio, M.; Rossetto, F.; Giunco, F.; Baglio, F.; Blasi, V. Differential roles of neural integrity, physical activity and depression in frailty: Sex-related differences. Brain Sci. 2023, 13, 950. [Google Scholar] [CrossRef]
- Gordon, E.H.; Hubbard, R.E. Differences in frailty in older men and women. Med. J. Aust. 2020, 212, 183–188. [Google Scholar] [CrossRef]
- Cohen, A.A.; Legault, V.; Li, Q.; Fried, L.P.; Ferrucci, L. Men sustain higher dysregulation levels than women without becoming frail. J. Gerontol. Ser. A 2018, 73, 175–184. [Google Scholar] [CrossRef]
- Lu, Y.W.; Chang, C.C.; Chou, R.H.; Lee, W.J.; Chen, L.K.; Huang, P.H.; Lin, S.-J. Sex differences in the frailty phenotype and mortality in the I-Lan longitudinal aging study cohort. BMC Geriatr. 2024, 24, 182. [Google Scholar] [CrossRef]
- O’Caoimh, R.; Sezgin, D.; O’Donovan, M.R.; Molloy, D.W.; Clegg, A.; Rockwood, K.; Liew, A. Prevalence of frailty in 62 countries across the world: A systematic review and meta-analysis of population-level studies. Age Ageing 2021, 50, 96–104. [Google Scholar] [CrossRef]
- Palomo, I.; García, F.; Albala, C.; Wehinger, S.; Fuentes, M.; Alarcón, M.; Arauna, D.; Montecino, H.; Mendez, D.; Sepúlveda, M.; et al. Characterization by Gender of Frailty Syndrome in Elderly People according to Frail Trait Scale and Fried Frailty Phenotype. J. Pers. Med. 2022, 12, 712. [Google Scholar] [CrossRef]
- Brown, P.J.; Roose, S.P.; O’Boyle, K.R.; Ciarleglio, A.; Maas, B.; Igwe, K.C.; Chung, S.; Gomez, S.; Naqvi, M.; Brickman, A.M.; et al. Frailty and Its Correlates in Adults With Late Life Depression. Am. J. Geriatr. Psychiatr. 2020, 28, 145–154. [Google Scholar] [CrossRef]
- Santamaría-Ulloa, C.; Lehning, A.J.; Cortés-Ortiz, M.V.; Méndez-Chacón, E. Frailty as a predictor of mortality: A comparative cohort study of older adults in Costa Rica and the United States. BMC Public Health 2023, 23, 1960. [Google Scholar] [CrossRef]
- Feng, Z.; Lugtenberg, M.; Franse, C.; Fang, X.; Hu, S.; Jin, C.; Raat, H.; Ginsberg, S.D. Risk factors and protective factors associated with incident or increase of frailty among community-dwelling older adults: A systematic review of longitudinal studies. PLoS ONE 2017, 12, e0178383. [Google Scholar] [CrossRef]
- Ntimana, C.B.; Seakamela, K.P.; Mashaba, R.G.; Maimela, E. Determinants of central obesity in children and adolescents and associated complications in South Africa: A systematic review. Front. Public Health 2024, 12, 1324855. [Google Scholar] [CrossRef]
- Mashala, D.G.; Ntimana, C.B.; Seakamela, K.P.; Mashaba, R.G.; Maimela, E. Sociodemographic Disparities in the Prevalence of Metabolic Syndrome in Rural South Africa: An Analysis of Gender, Age, and Marital, Employment, and Educational Status. Obesities 2024, 4, 480–490. [Google Scholar] [CrossRef]
- DeClercq, V.; Duhamel, T.A.; Theou, O.; Kehler, S. Association between lifestyle behaviors and frailty in Atlantic Canadian males and females. Arch. Gerontol. Geriatr. 2020, 91, 104207. [Google Scholar] [CrossRef]
- Trevisan, C.; Grande, G.; Vetrano, D.L.; Maggi, S.; Sergi, G.; Welmer, A.K.; Rizzuto, D. Gender Differences in the Relationship Between Marital Status and the Development of Frailty: A Swedish Longitudinal Population-Based Study. J. Womens Health 2020, 29, 927–936. [Google Scholar] [CrossRef]
- Kojima, G.; Walters, K.; Iliffe, S.; Taniguchi, Y.; Tamiya, N. Marital Status and Risk of Physical Frailty: A Systematic Review and Meta-analysis. J. Am. Med. Dir. Assoc. 2020, 21, 322–330. [Google Scholar] [CrossRef]
- Boucham, M.; Salhi, A.; El Hajji, N.; Gbenonsi, G.Y.; Belyamani, L.; Khalis, M. Factors associated with frailty in older people: An umbrella review. BMC Geriatr. 2024, 24, 737. [Google Scholar] [CrossRef]
- Crow, R.S.; Lohman, M.C.; Titus, A.J.; Cook, S.B.; Bruce, M.L.; Mackenzie, T.A.; Bartels, S.J.; Batsis, J.A. Association of Obesity and Frailty in Older Adults: NHANES 1999–2004. J. Nutr. Health Aging 2019, 23, 138–144. [Google Scholar] [CrossRef]
- Newman, A.B.; Enright, P.L.; Manolio, T.A.; Haponik, E.F.; Wahl, P.W.; On behalf of the Cardiovascular Health Study Research Group. Sleep Disturbance, Psychosocial Correlates, and Cardiovascular Disease in 5201 Older Adults: The Cardiovascular Health Study. J. Am. Geriatr. Soc. 1997, 45, 1–7. [Google Scholar] [CrossRef]
- Shakya, S.; Bajracharya, R.; Ledbetter, L.; Cary, M.P., Jr. The association between cardiometabolic risk factors and frailty in older adults: A systematic review. Innov. Aging 2022, 6, igac032. [Google Scholar] [CrossRef]
- Ma, L.L.; Chen, N.; Zhang, Y.; Feng, X.M.; Gong, M.; Yan, Y.X. Association of phenotypic frailty and frailty index with type 2 diabetes and dyslipidemia in middle-aged and elderly Chinese: A longitudinal cohort study. Arch. Gerontol. Geriatr. 2024, 119, 105311. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, J.; Shen, S.; Hong, X.; Zeng, X.; Yang, Y.; Liu, Z.; Chen, L.; Chen, X. Association Between Body Composition and Frailty in Elder Inpatients. Clin. Interv. Aging 2020, 15, 313–320. [Google Scholar] [CrossRef]
- Chandrasekaran, P.; Weiskirchen, R. The Role of Obesity in Type 2 Diabetes Mellitus—An Overview. Int. J. Mol. Sci. 2024, 25, 1882. [Google Scholar] [CrossRef] [PubMed]
- Jung, U.J.; Choi, M.S. Obesity and its metabolic complications: The role of adipokines and the relationship between obesity, inflammation, insulin resistance, dyslipidemia and nonalcoholic fatty liver disease. Int. J. Mol. Sci. 2014, 15, 6184–6223. [Google Scholar] [CrossRef]
- Wu, H.; Ballantyne, C.M. Metabolic Inflammation and Insulin Resistance in Obesity. Circ. Res. 2020, 126, 1549–1564. [Google Scholar] [CrossRef]
- Inglés, M.; Gambini, J.; Carnicero, J.A.; García-García, F.J.; Rodríguez-Mañas, L.; Olaso-González, G.; Dromant, M.; Borrás, C.; Viña, J. Oxidative Stress Is Related to Frailty, Not to Age or Sex, in a Geriatric Population: Lipid and Protein Oxidation as Biomarkers of Frailty. J. Am. Geriatr. Soc. 2014, 62, 1324–1328. [Google Scholar] [CrossRef]
| Variables | N (%)/Mean ± SD | |
|---|---|---|
| Age | 66.78 ± 5.72 | |
| Biological sex | Males | 264 (48.40) |
| Females | 282 (51.6) | |
| Marital status | Single | 92 (16.9) |
| Married | 310 (56.9) | |
| Divorced | 52 (9.50) | |
| Widowed | 91 (16.7) | |
| Educational status | No formal education | 57 (10.4) |
| Primary | 291 (53.3) | |
| Secondary | 184 (33.7) | |
| Tertiary | 14 (2.60) | |
| Current smoker | 125 (22.9) | |
| Current alcohol consumption | 168 (30.8) | |
| Hypertension | 225 (41.3) | |
| Diabetes | 72 (13.6) | |
| Obesity | 176 (32.5) | |
| Central obesity | 336 (62.1) | |
| HIV | 55 (10.7) | |
| Frailty | 144 (26.4) | |
| Non-Frail | Pre-Frail | Frail | p-Value | ||
|---|---|---|---|---|---|
| Age (mean ± SD) | 65.6 ± 3.76 | 66.02 ± 5.09 | 68.94 ± 6.88 | <0.001 bc | |
| Biological sex | Male N (%) | 4 (21.1) | 168 (43.9) | 92 (63.9) | <0.001 |
| Female N (%) | 15 (78.9) | 215 (56.1) | 52 (36.1) | ||
| Marital status | Single N (%) | 7 (36.8) | 62 (16.2) | 23 (16.1) | 0.146 |
| Married N (%) | 9 (47.4) | 213 (55.6) | 88 (61.5) | ||
| Divorced N (%) | 1 (5.30) | 36 (9.40) | 13 (10.5) | ||
| Widowed N (%) | 2 (10.5) | 72(18.8) | 17 (11.9) | ||
| Highest level of education | No-formal education N (%) | 0 (0.0) | 36 (9.40) | 21 (14.6) | 0.011 |
| Primary N (%) | 14 (73.7) | 36 (9.40) | 81 (56.3) | ||
| Secondary N (%) | 3 (15.8) | 141 (36.8) | 40 (27.8) | ||
| Tertiary N (%) | 2 (10.5) | 10 (2.60) | 2 (1.40) | ||
| Current smoker N (%) | 2 (10.5) | 79 (20.6) | 44 (30.6) | 0.023 | |
| Current alcohol consumption N (%) | 5 (26.3) | 108 (28.2) | 55 (38.2) | 0.078 | |
| BMI (mean ± SD) | 28.85 ± 5.86 | 28.40 ± 6.72 | 25.14 ± 9.00 | <0.001 c | |
| WC (mean ± SD) | 91.07 ± 9.39 | 93.91 ± 12.48 | 88.35 ± 15.61 | <0.001 c | |
| SBP (mean ± SD) | 138.79 ± 16.41 | 135.98 ± 22.51 | 136.22 ± 26.69 | 0.878 | |
| DBP (mean ± SD) | 82.89 ± 7.81 | 79.01 ± 11.89 | 78.22 ± 12.44 | 0.270 | |
| VAT (mean ± SD) | 5.36 ± 1.55 | 5.76 ± 2.36 | 5.31 ± 2.51 | 0.156 | |
| SAT (mean ± SD) | 2.07 ± 0.82 | 1.84 ± 1.07 | 1.60 ± 1.05 | 0.049 | |
| Glucose (mean ± SD) | 5.52 ± 0.75 | 5.99 ± 2.21 | 5.89 ± 2.29 | 0.622 | |
| HDL-c (mean ± SD) | 1.29 ± 0.24 | 1.26 ± 0.35 | 1.34 ± 0.44 | 0.067 | |
| LDL-c (mean ± SD) | 3.01 ± 0.83 | 2.84 ± 0.93 | 2.67 ± 0.82 | 0.109 | |
| TC (mean ± SD) | 4.93 ± 0.96 | 4.72 ± 1.10 | 4.60 ± 1.01 | 0.358 | |
| TG (mean ± SD) | 1.38 ± 0.58 | 1.34 ± 0.58 | 1.29 ± 0.68 | 0.721 | |
| LDL/HDL-c (mean ± SD) | 2.39 ± 0.74 | 2.36 ± 0.83 | 2.16 ± 0.84 | 0.043 c | |
| TC/HDL-c (mean ± SD) | 3.89 ± 0.86 | 3.91 ± 0.98 | 3.65 ± 1.04 | 0.040 c | |
| TG/HDL-c (mean ± SD) | 1.11 ± 0.54 | 1.16 ± 0.65 | 1.10 ± 0.75 | 0.593 | |
| Obesity N (%) | 8 (42.1) | 137 (36.2) | 31 (21.5) | 0.004 | |
| Diabetes N (%) | 1 (5.3) | 54 (14.5) | 17(12.6) | 0.451 | |
| Hypertension N (%) | 9 (47.4) | 156 (40.8) | 60 (41.7) | 0.848 | |
| HIV N (%) | 0 (0.0) | 43 (11.8) | 12 (9.0) | 0.223 | |
| Central obesity N (%) | 14 (73.3) | 256 (67.7) | 66 (45.8) | <0.001 | |
| Variables | Unadjusted Model | Fully Adjusted Model | |||
|---|---|---|---|---|---|
| Exp B (95% CI) | p Value | Exp B (95% CI) | p Value | ||
| Biological sex | Male | ref | |||
| Female | 0.71 (0.38–1.33) | 0.282 | |||
| Age groups | 60 to 65 | ref | |||
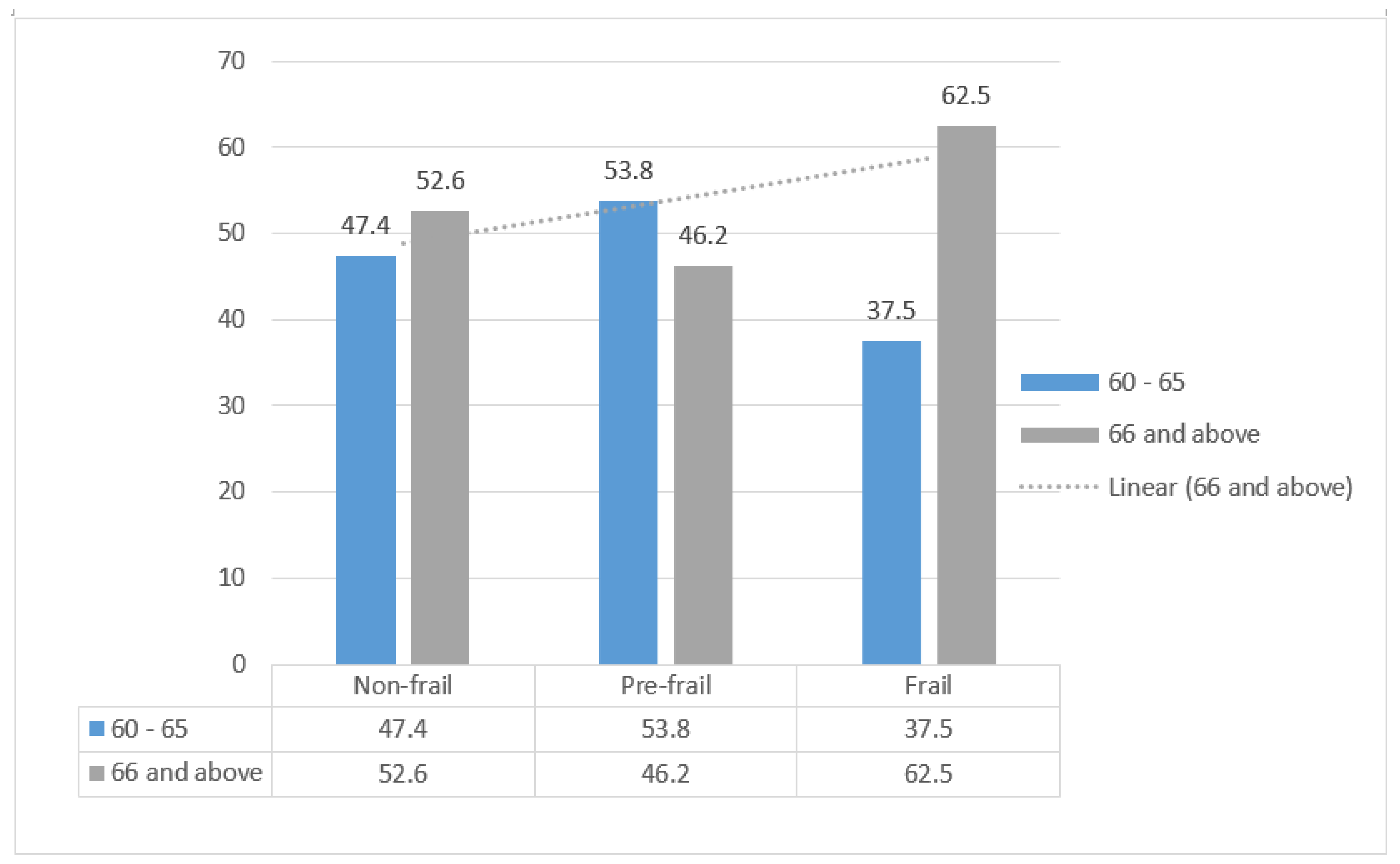
| 66 and above | 1.61 (1.00–2.60) | 0.049 | 1.75 (1.4–2.70) | 0.011 | |
| Marital status | Married | 1.53 (0.79–2.78) | 0.206 | ||
| Single | 1.68 (0.75–3.75) | 0.205 | |||
| Divorced | 1.60 (0.66–3.86) | 0.292 | |||
| Widowed | ref | ||||
| Highest level of education | No formal education | ref | |||
| Primary | 0.79 (0.39–1.60) | 0.509 | |||
| Secondary | 0.61(0.28–1.30) | 0.198 | |||
| Tertiary | 0.31(0.03–2.94) | 0.309 | |||
| Current alcohol consumption | No | ref | |||
| Yes | 1.03(0.58–1.82) | 0.917 | |||
| Current smoker | No | ref | |||
| Yes | 1.05 (0.55–2.01) | 0.884 | |||
| Hypertension | No | ref | |||
| Yes | 0.89 (0.57–1.40) | 0.609 | |||
| Diabetes | No | ref | |||
| Yes | 1.09 (0.53–2.24) | 0.809 | |||
| Obesity | No | ref | |||
| Yes | 1.02 (0.55–1.87) | 0.959 | |||
| Central obesity | No | ref | |||
| Yes | 0.48 (0.26–0.86) | 0.040 | 0.37 (0.24–0.57) | <0.001 | |
| HIV | Negative | ref | |||
| Positive | 0.93 (0.45–1.94) | 0.848 | |||
| High Trig | No | ref | |||
| Yes | 0.69 (0.36–1.33) | 0.268 | |||
| High TC | No | Ref | |||
| Yes | 2.25 (1.04–4.88) | 0.040 | 2.05 (1.03–4.01) | 0.041 | |
| High LDL-c | No | ref | |||
| Yes | 0.41 (0.18–0.91) | 0.029 | |||
| Low HDL-c | No | ref | |||
| Yes | 0.83 (0.47–1.48) | 0.526 | |||
| High TC/HDL-c | No | ref | |||
| Yes | 1.85 (0.76–4.52) | 0.179 | |||
| High TG/HDL-c | No | ref | |||
| Yes | 4.55 (0.27–76.3) | 0.292 | |||
| High LDL/HDL-c | No | ref | |||
| Yes | 1.35 (0.77–2.35) | 0.296 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mashaba, R.G.; Seakamela, K.P.; Choma, S.S.R.; Maimela, E.; Tlouyamma, J.; Ntimana, C.B. Prevalence of Frailty and Associated Sociodemographic, Biomedical, and Biochemical Factors Amongst Participants Residing in Limpopo Province, South Africa. Geriatrics 2025, 10, 134. https://doi.org/10.3390/geriatrics10050134
Mashaba RG, Seakamela KP, Choma SSR, Maimela E, Tlouyamma J, Ntimana CB. Prevalence of Frailty and Associated Sociodemographic, Biomedical, and Biochemical Factors Amongst Participants Residing in Limpopo Province, South Africa. Geriatrics. 2025; 10(5):134. https://doi.org/10.3390/geriatrics10050134
Chicago/Turabian StyleMashaba, Reneilwe Given, Kagiso P. Seakamela, Solomon S. R. Choma, Eric Maimela, Joseph Tlouyamma, and Cairo Bruce Ntimana. 2025. "Prevalence of Frailty and Associated Sociodemographic, Biomedical, and Biochemical Factors Amongst Participants Residing in Limpopo Province, South Africa" Geriatrics 10, no. 5: 134. https://doi.org/10.3390/geriatrics10050134
APA StyleMashaba, R. G., Seakamela, K. P., Choma, S. S. R., Maimela, E., Tlouyamma, J., & Ntimana, C. B. (2025). Prevalence of Frailty and Associated Sociodemographic, Biomedical, and Biochemical Factors Amongst Participants Residing in Limpopo Province, South Africa. Geriatrics, 10(5), 134. https://doi.org/10.3390/geriatrics10050134






