Examination of Social Participation in Older Adults Undergoing Frailty Health Checkups Using Deep Learning Models
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Measurement Items
2.3. Analysis Method
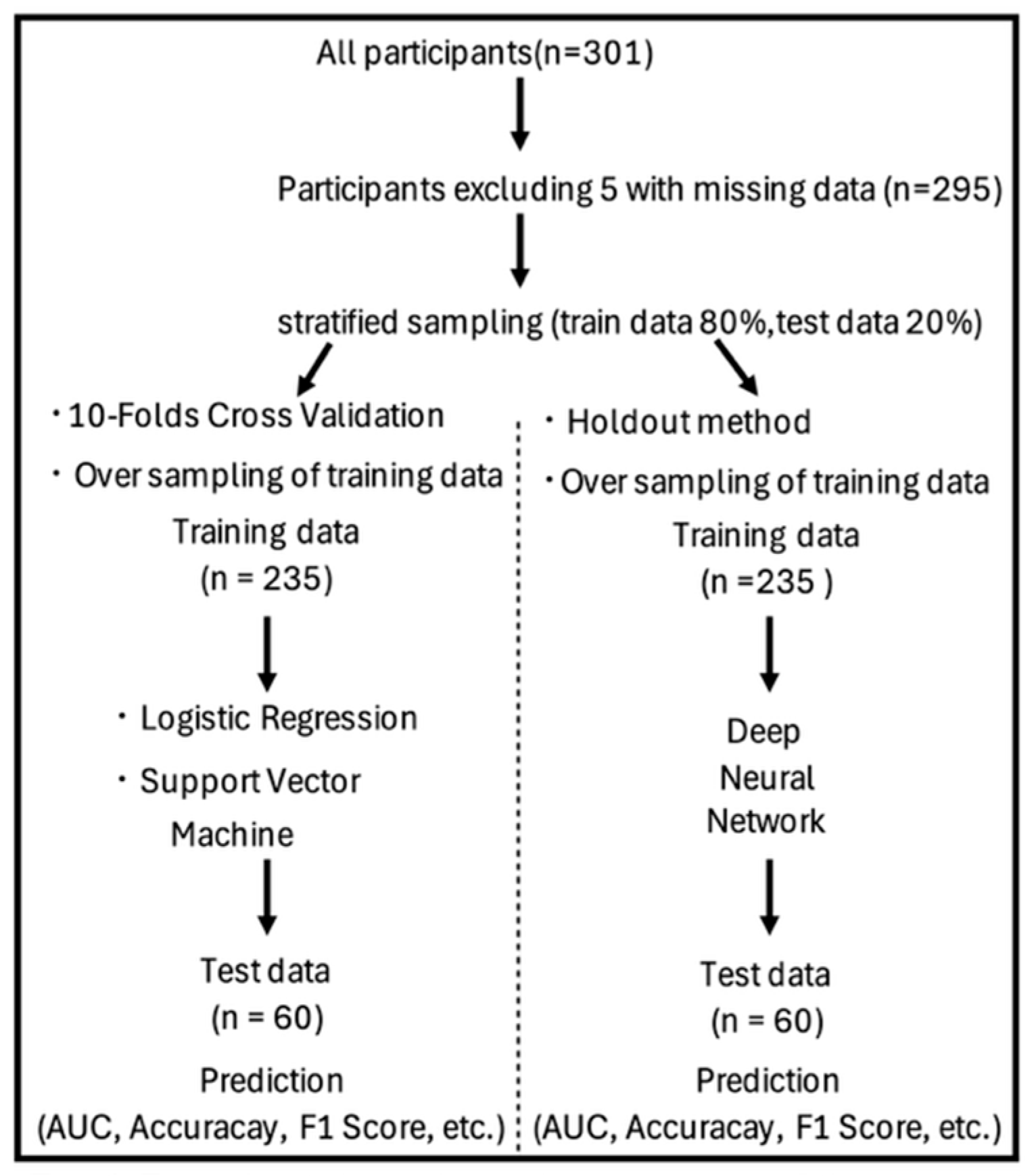
2.3.1. Data Splitting and Handling of Class Imbalance
2.3.2. Logistic Regression (LR) and Nonlinear Support Vector Machine (NLSVM)
2.3.3. Deep Neural Network (DNN)
2.4. Ethical Considerations
3. Results
3.1. Basic Information of Participants
3.2. Characteristics of Social Participation and Analysis of ML Models
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| NLSVM | Nonlinear support vector machine |
| DNN | Deep neural network |
| ML | Machine learning |
| LR | Logistic regression |
| AUC | Area under the curve |
| IADL | Instrumental activities of daily living |
| 10-FCV | 10-fold cross-validation |
| SVM | Support vector machine |
| RMSE | Root mean square error |
References
- Dawson-Townsend, K. Social participation patterns and their associations with health and well-being for older adults. SSM Popul. Health 2019, 8, 100424. [Google Scholar] [CrossRef]
- Tomioka, K.; Kurumatani, N.; Hosoi, H. Association between social participation and 3-year change in Instrumental Activities of Daily Living in community-dwelling elderly adults. J. Am. Geriatr. Soc. 2017, 65, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, Y.; Yoshida, H.; Amano, H.; Fukaya, T.; Liang, J.; Uchida, H.; Shinkai, S. Predictors of improvement or decline in Instrumental Activities of Daily Living among community-dwelling older Japanese. Gerontology 2008, 65, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, Y.; Kondo, N.; Kondo, K.; Saito, T.; Hayashi, H.; Kawachi, I.; JAGES group. Social participation and mortality: Does social position in civic groups matter? BMC Public Health 2016, 16, 394. [Google Scholar] [CrossRef] [PubMed]
- von Humboldt, S.; Costa, A.; Ilyas, N.; Leal, I. Older adults, perceived ageism, civic participation and mental health: A qualitative study. Aging Ment. Health 2024, 28, 1489–1501. [Google Scholar] [CrossRef]
- Hossen, M.S.; Salleh, S.F.B. Social influences on the psychological well-being of elderly individuals. J. Humanit. Appl. Soc. Sci. 2025, 7, 315–332. [Google Scholar] [CrossRef]
- Lee, S.H.; Lee, H.; Yu, S. Effectiveness of social support for community-dwelling elderly with depression: A systematic review and meta-analysis. Healthcare 2022, 10, 1598. [Google Scholar] [CrossRef]
- Teo, R.H.; Cheng, W.H.; Cheng, L.J.; Lau, Y.; Lau, S.T. Global prevalence of social isolation among community-dwelling older adults: A systematic review and meta-analysis. Arch. Gerontol. Geriatr. 2023, 107, 104904. [Google Scholar] [CrossRef]
- Elshaikh, U.; Sheik, R.; Saeed, R.K.M.; Chivese, T.; Alsayed Hassan, D. Barriers and facilitators of older adults for professional mental health help-seeking: A systematic review. BMC Geriatr. 2023, 23, 516. [Google Scholar] [CrossRef]
- Abdi, S.; Spann, A.; Borilovic, J.; de Witte, L.J.; Hawley, M. Understanding the care and support needs of older people: A scoping review and categorisation using the WHO international classification of functioning, disability and health framework (ICF). BMC Geriatr. 2019, 19, 195. [Google Scholar] [CrossRef]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in older adults: Evidence for a phenotype. J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, M146–M156. [Google Scholar] [CrossRef]
- Makizako, H.; Shimada, H.; Doi, T.; Tsutsumimoto, K.; Suzuki, T. Impact of physical frailty on disability in community-dwelling older adults: A prospective cohort study. BMJ Open 2015, 5, e008462. [Google Scholar] [CrossRef]
- Kojima, G.; Iliffe, S.; Taniguchi, Y.; Shimada, H.; Rakugi, H.; Walters, K. Prevalence of frailty in Japan: A systematic review and meta-analysis. J. Epidemiol. 2017, 27, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Satake, S.; Shimada, H.; Yamada, M.; Kim, H.; Yoshida, H.; Gondo, Y.; Matsubayashi, K.; Matsushita, E.; Kuzuya, M.; Kozaki, K.; et al. Prevalence of frailty among community-dwellers and outpatients in Japan as defined by the Japanese version of the cardiovascular Health Study criteria. Geriatr. Gerontol. Int. 2017, 17, 2629–2634. [Google Scholar] [CrossRef] [PubMed]
- Hikichi, H.; Tsuboya, T.; Aida, J.; Matsuyama, Y.; Kondo, K.; Subramanian, S.V.; Kawachi, I. Social capital and cognitive decline in the aftermath of a natural disaster: A natural experiment from the 2011 Great East Japan Earthquake and Tsunami. Lancet Planet. Health 2017, 1, e105–e113. [Google Scholar] [CrossRef] [PubMed]
- Ide, K.; Tsuji, T.; Kanamori, S.; Jeong, S.; Nagamine, Y.; Kondo, K. Social participation and functional decline: A Comparative Study of Rural and Urban Older People, Using Japan Gerontological Evaluation Study Longitudinal Data. Int. J. Environ. Res. Public Health 2020, 17, 617. [Google Scholar] [CrossRef]
- Bertini, F.; Bergami, G.; Montesi, D.; Veronese, G.; Marchesini, G.; Pandolfi, P. Predicting frailty condition in elderly using multidimensional socioclinical databases. Proc. IEEE 2018, 106, 723–737. [Google Scholar] [CrossRef]
- Yamada, M.; Arai, H.; Nagai, K.; Uemura, K.; Mori, S.; Aoyama, T. Differential determinants of physical daily activities in frail and nonfrail community-dwelling older adults. J. Clin. Gerontol. Geriatr. 2011, 2, 42–46. [Google Scholar] [CrossRef][Green Version]
- Anzai, S.; Ohsugi, H.; Shiba, Y. Factors associated with social participation among community-dwelling frail older adults in Japan: A cross-sectional study. BMC Geriatr. 2024, 24, 235. [Google Scholar] [CrossRef]
- Chase, J.A.D. Methodological challenges in physical activity research with older adults. West. J. Nurs. Res. 2013, 35, 76–97. [Google Scholar] [CrossRef]
- Miotto, R.; Wang, F.; Wang, S.; Jiang, X.; Dudley, J.T. Deep learning for healthcare: Review, opportunities and challenges. Brief. Bioinform. 2018, 19, 1236–1246. [Google Scholar] [CrossRef]
- Waljee, A.K.; Sauder, K.; Patel, A.; Segar, S.; Liu, B.; Zhang, Y.; Zhu, J.; Stidham, R.W.; Balis, U.; Higgins, P.D.R. Machine learning algorithms for objective remission and clinical outcomes with thiopurines. J. Crohn’s Colitis 2017, 11, 801–810. [Google Scholar] [CrossRef]
- Abhadiomhen, S.E.; Nzeakor, E.O.; Oyibo, K. Health risk assessment using machine learning: Systematic review. Electronics 2024, 13, 4405. [Google Scholar] [CrossRef]
- LeCun, Y.; Bengio, Y.; Hinton, G. Deep learning. Nature 2015, 521, 436–444. [Google Scholar] [CrossRef]
- Rajkomar, A.; Dean, J.; Kohane, I. Machine learning in medicine. N. Engl. J. Med. 2019, 380, 1347–1358. [Google Scholar] [CrossRef] [PubMed]
- Christodoulou, E.; Ma, J.; Collins, G.S.; Steyerberg, E.W.; Verbakel, J.Y.; Van Calster, B. A systematic review shows no performance benefit of machine learning over logistic regression for clinical prediction models. J. Clin. Epidemiol. 2019, 110, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Sasaki, T.; Yokokawa, Y.; Yokouchi, S. Health literacy and frailty: The mediating role of Instrumental Activities of Daily Living. Psychogeriatrics 2025, 25, e70010. [Google Scholar] [CrossRef] [PubMed]
- Fujita, K.; Jinnouchi, H.; Fujii, A. Association between locomotive syndrome and cognitive decline in community dwelling older adults. Nihon Koshu Eisei Zasshi 2021, 68, 23–32. [Google Scholar] [CrossRef]
- Ishikawa, H.; Nomura, K.; Sato, M.; Yano, E. Developing a measure of communicative and critical health literacy: A pilot study of Japanese office workers. Health Promot. Int. 2008, 23, 269–274. [Google Scholar] [CrossRef]
- Koyano, W.; Shibata, H.; Nakazato, K.; Haga, H.; Suyama, Y. Measurement of competence: Reliability and validity of the TMIG index of competence. Arch. Gerontol. Geriatr. 1991, 13, 103–116. [Google Scholar] [CrossRef]
- Iwasa, H.; Masui, Y.; Inagaki, H.; Yoshida, Y.; Shimada, H.; Otsuka, R.; Kikuchi, K.; Nonaka, K.; Yoshida, H.; Yoshida, H.; et al. Assessing competence at a higher level among older adults: Development of the Japan Science and Technology Agency Index of Competence (JST-IC). Aging Clin. Exp. Res. 2018, 30, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Cortes, C.; Vapnik, V. Support-vector networks. Mach. Learn. 1995, 20, 273–297. [Google Scholar] [CrossRef]
- Gevrey, M.; Dimopoulos, I.; Lek, S. Review and comparison of methods to study the contribution of variables in artificial neural network models. Ecol. Modell. 2003, 160, 249–264. [Google Scholar] [CrossRef]
- AIZOTH Inc. Home Page. Available online: https://aizoth.com/service/multi-sigma/ (accessed on 24 April 2025).
- Kim, T.; Choi, J.Y.; Ko, M.J.; Kim, K.I. Development and validation of a machine learning method using vocal biomarkers for identifying frailty in community-dwelling older adults: Cross-sectional study. JMIR Med. Inform. 2025, 13, e57298. [Google Scholar] [CrossRef]
- Yamashita, M.; Kamiya, K.; Hotta, K.; Kubota, A.; Sato, K.; Maekawa, E.; Miyata, H.; Ako, J. Artificial intelligence (AI)-driven frailty prediction using electronic health records in hospitalized patients with cardiovascular disease. Circ. Rep. 2024, 6, 495–504. [Google Scholar] [CrossRef]
- Huang, L.; Chen, H.; Liang, Z. Enhancing the convenience of frailty index assessment for elderly Chinese people with machine learning methods. Sci. Rep. 2024, 14, 23227. [Google Scholar] [CrossRef]
- Isaradech, N.; Sirikul, W.; Buawangpong, N.; Siviroj, P.; Kitro, A. Machine learning models for frailty classification of older adults in Northern Thailand: Model development and validation study. JMIR Aging 2025, 8, e62942. [Google Scholar] [CrossRef]
- Cunha Leme, D.E.D.; Oliveira, C.D. Machine learning models to predict future frailty in community-dwelling middle-aged and older adults: The ELSA cohort study. J. Gerontol. Ser. A 2023, 78, 2176–2184. [Google Scholar] [CrossRef]
- Körlof, L.; Nyman, A.; Isaksson, G.; Larsson, E. Older adults’experiences of using strategies to maintain and foster social participation: A systematic review with metasynthesis of qualitative studies. Health Soc. Care Community 2024, 2024, 7877128. [Google Scholar] [CrossRef]
- Abe, T.; Kitamura, A.; Taniguchi, Y.; Amano, H.; Seino, S.; Yokoyama, Y.; Nishi, M.; Narita, M.; Ikeuchi, T.; Fujiwara, Y.; et al. Pathway from gait speed to incidence of disability and mortality in older adults: A mediating role of physical activity. Maturitas 2019, 123, 32–36. [Google Scholar] [CrossRef]
- Wahrendorf, M.; von dem Knesebeck, O.V.D.; Siegrist, J. Social productivity and well-being of older people: Baseline results from the SHARE study. Eur. J. Ageing 2006, 3, 67–73. [Google Scholar] [CrossRef]
- Fernandez, D.M.; Larson, J.L.; Brian, J.; Zikmund-Fisher, B.J. Associations between health literacy and preventive health behaviors among older adults: Findings from the health and retirement study. BMC Public Health 2016, 16, 596. [Google Scholar] [CrossRef]
- Abe, T.; Okuyama, K.; Kamada, M.; Yano, S.; Toyama, Y.; Isomura, M.; Nabika, T.; Sakane, N.; Ando, H.; Miyazaki, R. Social participation and physical prefrailty in older Japanese adults: The Shimane CoHRE study. PLoS ONE 2020, 15, e0243548. [Google Scholar] [CrossRef]
- Takeuchi, H.; Ide, K.; Hayashi, T.; Abe, N.; Nakagomi, A.; Kondo, K. Association between social participation and frailty among older adults: A longitudinal study from Japan Gerontological Evaluation Study. Nihon Koshu Eisei Zasshi 2023, 70, 529–543. [Google Scholar] [CrossRef]
| Social Participation | |||
|---|---|---|---|
| Characteristic | No (N = 59) | Yes (N = 236) | p-Value |
| Sex (female) | 50 (85%) | 191 (81%) | 0.5 |
| Age | 81 (78, 85) | 79 (73, 83) | 0.042 |
| Walk speed (m/s) | 1.09 (0.96, 1.31) | 1.22 (1.04, 1.38) | 0.012 |
| Grip strength (kg) | 23 (18, 26) | 23 (20, 26) | 0.083 |
| Frailty category classification | 0.051 | ||
| Robust | 34 (58%) | 149 (63%) | |
| Prefrail | 18 (31%) | 55 (23%) | |
| Frail | 7 (12%) | 32 (14%) | |
| Number of diseases (n = 0) | 45 (76%) | 186 (79%) | 0.6 |
| Year of education | 0.6 | ||
| 9 year | 11 (19%) | 37 (16%) | |
| 12 year | 37 (63%) | 135 (57%) | |
| 15 year | 9 (15%) | 53 (22%) | |
| others | 2 (3.4%) | 11 (4.7%) | |
| Number of cohabitants | 0.7 | ||
| Couple | 14 (24%) | 74 (31%) | |
| Alone | 16 (27%) | 65 (28%) | |
| Couple and child | 8 (14%) | 28 (12%) | |
| Three generation household | 6 (10%) | 18 (7.6%) | |
| Child | 12 (20%) | 33 (14%) | |
| Others | 3 (5.1%) | 18 (7.6%) | |
| Subjective economic status | 0.8 | ||
| Can afford to live | 4 (6.8%) | 14 (5.9%) | |
| Can afford to live a little | 10 (17%) | 35 (15%) | |
| Neither | 37 (63%) | 163 (69%) | |
| Can not afford to live a little | 6 (10%) | 18 (7.6%) | |
| Can not afford to live | 2 (3.4%) | 6 (2.5%) | |
| Employment status (yes) | 6 (10%) | 32 (14%) | 0.5 |
| Health checkups (yes) | 48 (81%) | 195 (83%) | 0.8 |
| Sleeping duration(h) | 7.00 (5.25, 8.00) | 7.00 (6.00, 8.00) | 0.6 |
| History of falls (yes) | 10 (17%) | 49 (21%) | 0.5 |
| Cognitive decline (yes) | 55(93.2%) | 223(94.5%) | 0.3 |
| IADL | 5.00 [4.00, 5.00] | 5.00 [5.00, 5.00] | <0.001 |
| Health Literacy | 18 (16, 20) | 20 (18, 21) | 0.002 |
| Information collection ability | 2.00 [0.00, 3.00] | 4.00 [3.00, 4.00] | <0.001 |
| Medical cost in 2022 | 267,540 (137,235, 433,490) | 236,790 (96,868, 423,120) | 0.3 |
| Median (IQR); n (%) | |||
| Wilcoxon rank sum test; Pearson’s Chi-squared test; Fisher’s exact test | |||
| Precision | Accuracy | Sensitivity | Specificity | F1 Score | AUC | |
|---|---|---|---|---|---|---|
| LR | 0.894 | 0.783 | 0.583 | 0.833 | 0.519 | 0.776 |
| NLSVM | 0.438 | 0.767 | 0.583 | 0.812 | 0.5 | 0.795 |
| DNN | 0.5 | 0.8 | 0.833 | 0.792 | 0.625 | 0.788 |
| Contribution [%] | Contribution_Negative [%] | Contribution_Positive [%] | |
|---|---|---|---|
| Information-collection ability | 12.45 | 0.17 | 12.28 |
| Walk speed | 5.78 | 1.24 | 4.54 |
| Sleeping duration | 5.98 | 1.99 | 3.98 |
| Number of cohabitants | 5.16 | 1.31 | 3.85 |
| IADL | 5.95 | 3.14 | 2.81 |
| Cognitive decline | 5.65 | 2.95 | 2.7 |
| Health Literacy | 8.04 | 5.44 | 2.61 |
| Grip strength | 6.31 | 3.74 | 2.56 |
| History of falls | 3.85 | 1.46 | 2.39 |
| Number of diseases | 4.3 | 2.19 | 2.11 |
| Health checkups | 3.21 | 1.2 | 2.02 |
| Year of education | 4.82 | 2.92 | 1.9 |
| Employment status | 3.75 | 2.02 | 1.73 |
| Age | 5.19 | 3.51 | 1.68 |
| Medical cost in 2022 | 5.33 | 3.97 | 1.35 |
| Sex | 4.28 | 2.95 | 1.33 |
| Subjective economic status | 5.46 | 4.27 | 1.19 |
| Frailty category classification | 4.5 | 3.58 | 0.93 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yokokawa, Y.; Nakamura, K.; Sasaki, T.; Yokouchi, S.; Kimura, F. Examination of Social Participation in Older Adults Undergoing Frailty Health Checkups Using Deep Learning Models. Geriatrics 2025, 10, 124. https://doi.org/10.3390/geriatrics10050124
Yokokawa Y, Nakamura K, Sasaki T, Yokouchi S, Kimura F. Examination of Social Participation in Older Adults Undergoing Frailty Health Checkups Using Deep Learning Models. Geriatrics. 2025; 10(5):124. https://doi.org/10.3390/geriatrics10050124
Chicago/Turabian StyleYokokawa, Yoshiharu, Keisuke Nakamura, Tomohiro Sasaki, Shinobu Yokouchi, and Fumikazu Kimura. 2025. "Examination of Social Participation in Older Adults Undergoing Frailty Health Checkups Using Deep Learning Models" Geriatrics 10, no. 5: 124. https://doi.org/10.3390/geriatrics10050124
APA StyleYokokawa, Y., Nakamura, K., Sasaki, T., Yokouchi, S., & Kimura, F. (2025). Examination of Social Participation in Older Adults Undergoing Frailty Health Checkups Using Deep Learning Models. Geriatrics, 10(5), 124. https://doi.org/10.3390/geriatrics10050124






