Associations Between Physical Activity Frequency, Handgrip Strength, and Limitations in Activities of Daily Living in Middle-Aged and Older Adults with Widespread Pain: A Cross-Sectional Study Using Data from the SHARE Project
Abstract
1. Introduction
- Comparing the prevalence of limitations in each of the BADLs and IADLs based on MPA and VPA.
- Estimating the odds ratio (OR) of presenting limitations in each of the BADLs and IADLs based on PA: not performing MPA versus performing it very frequently, not performing VPA versus performing it very frequently, and HGS, all adjusted for age, sex, Body Mass Index (BMI), educational level, long-term illness, depressive symptoms, perception of loneliness, and cognitive functioning.
- Limitations in BADLs and IADLs will be associated with MPA and VPA frequency.
- People with WP who perform MPA or VPA with some frequency will have lower ADL limitation prevalences than people who do not perform them or perform them rarely.
- Performing MPA or VPA rarely or never will increase the likelihood of limitations in each of the ADLs, while greater relative HGS will be a protective factor against the onset of such limitations.
2. Materials and Methods
2.1. Study Design and Ethical Considerations
2.2. Share Data Collection
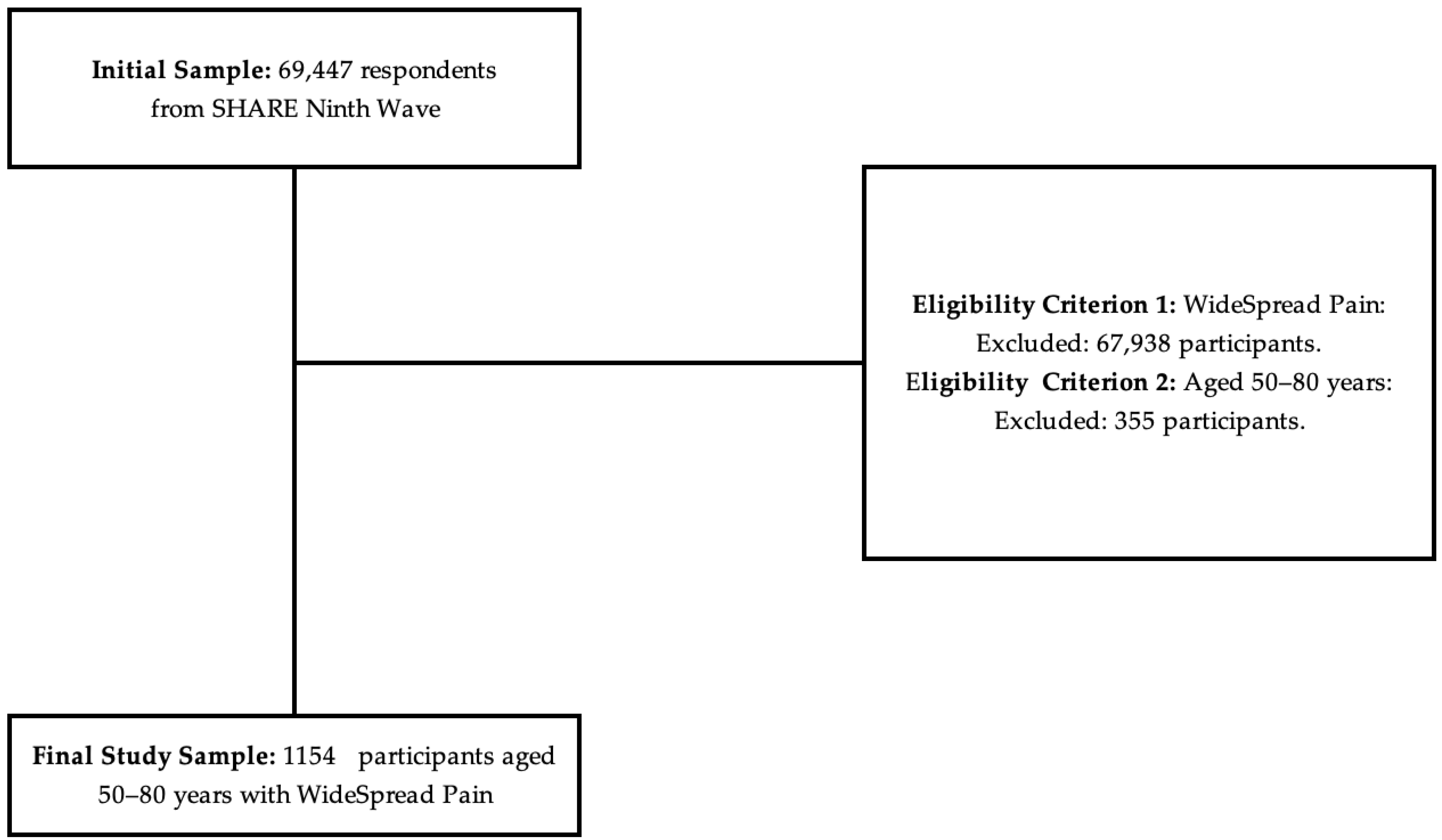
2.3. Sample Selection and Eligibility Criteria
2.4. Variables
2.4.1. Characterization Variables
2.4.2. Dependent Variables
2.5. Statistical Analysis
2.6. GenAI Declaration
3. Results
3.1. Descriptive Results
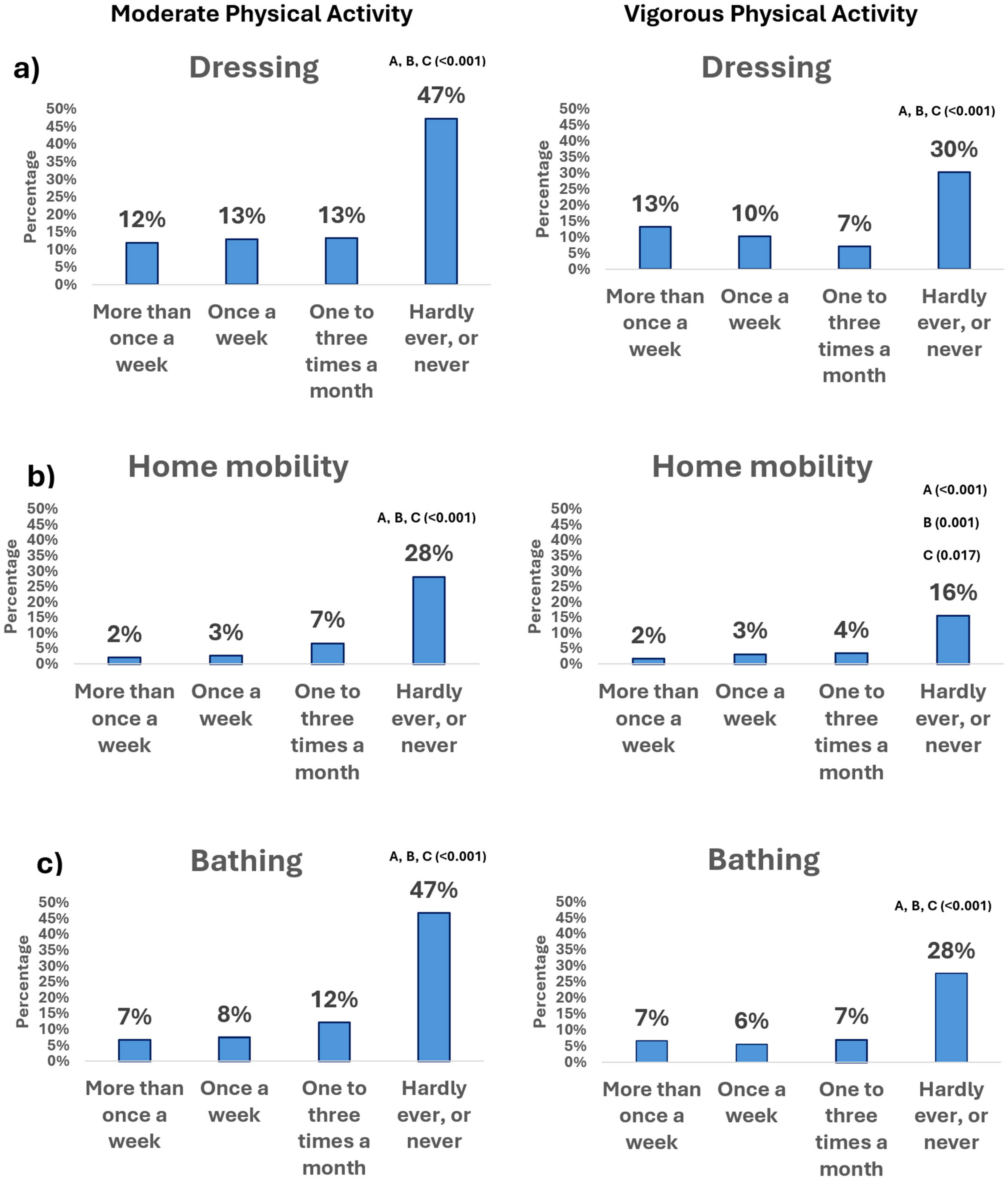
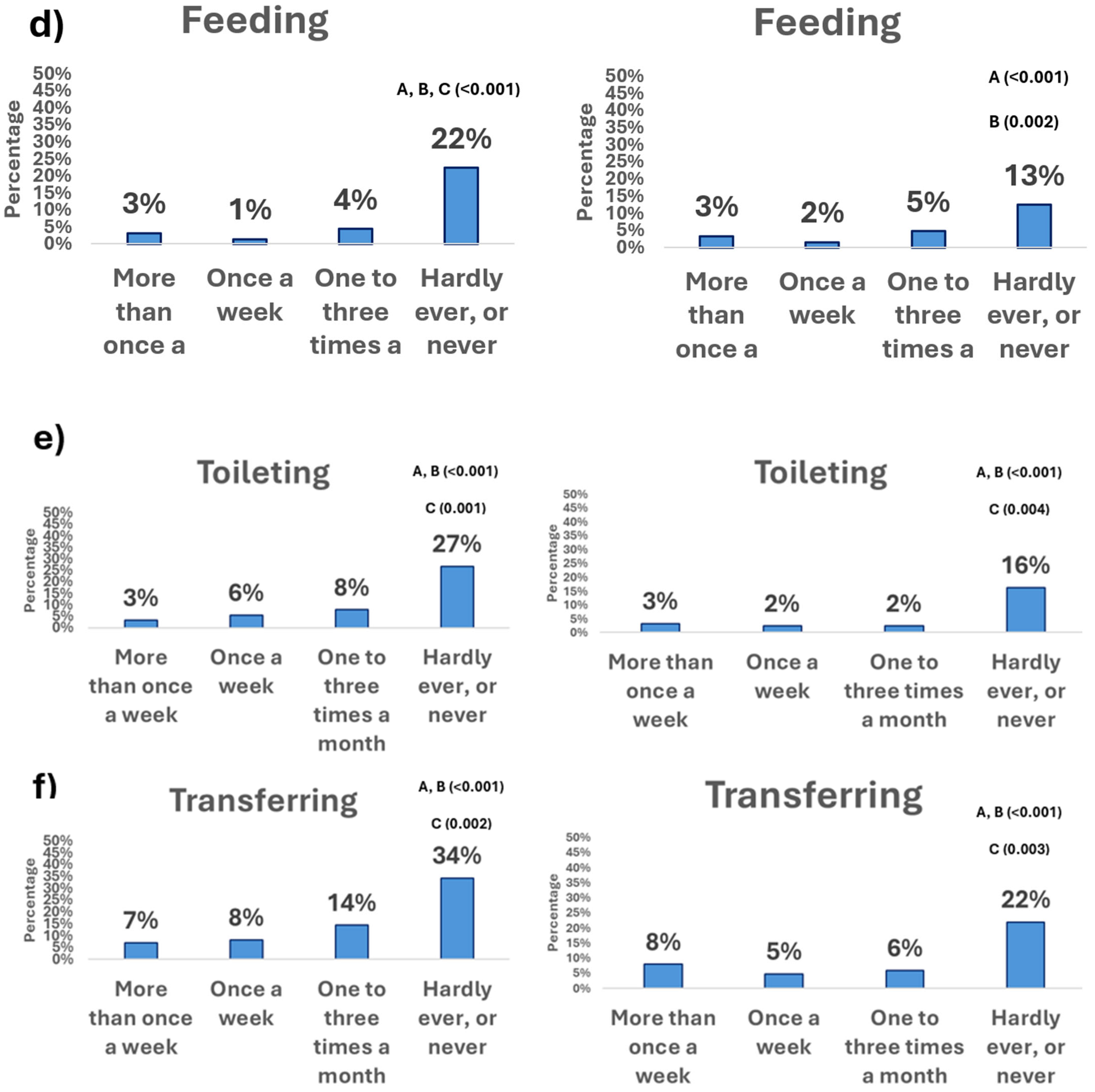
3.2. Physical Activity Frequency and Basic Activities of Daily Living
3.2.1. Moderate Physical Activity
3.2.2. Vigorous Physical Activity
3.3. Physical Activity Frequency and Instrumental Activities of Daily Living
3.4. Multivariate Logistic Binary Adjusted Regression
4. Discussion
4.1. Main Findings
4.2. Practical Applications
4.3. Limitations and Future Lines
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| WP | Widespread Pain |
| ADLs | Activities of Daily Living |
| BADLs | Basic Activities of Daily Living |
| IADLs | Instrumental Activities of Daily Living |
| SHARE | The Survey of Health, Ageing and Retirement in Europe |
| PA | Physical Activity |
| HGS | Handgrip Strength |
| MPA | Moderate Physical Activity |
| VPA | Vigorous Physical Activity |
References
- Singh, A.; Akkala, S.; Nayak, M.; Kotlo, A.; Poondla, N.; Raza, S.; Stankovich, J.; Antony, B. Impact of Pain on Activities of Daily Living in Older Adults: A Cross-Sectional Analysis of Korean Longitudinal Study of Aging (KLoSA). Geriatrics 2024, 9, 65. [Google Scholar] [CrossRef]
- Xi, J.-Y.; Liang, B.-H.; Zhang, W.-J.; Yan, B.; Dong, H.; Chen, Y.-Y.; Lin, X.; Gu, J.; Hao, Y.-T. Effects of Population Aging on Quality of Life and Disease Burden: A Population-Based Study. Glob. Health Res. Policy 2025, 10, 2. [Google Scholar] [CrossRef] [PubMed]
- WHO TEAM. Ageing: Global Population. Available online: https://www.who.int/news-room/questions-and-answers/item/population-ageing (accessed on 11 June 2025).
- O’Gorman, C.; Willis, A. Oral Medicine Considerations for the Older Patient. Br. Dent. J. 2024, 236, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Hacker, K. The Burden of Chronic Disease. Mayo Clin. Proc. Innov. Qual. Outcomes 2024, 8, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Kataria, S.; Inggas, M.A.M.; Patel, U.; Wijaya, J.H.; Yabut, K.; Ayub, M.A.; Maniyar, P.; Upadhyay, N.; Davitashvili, B.; Patel, J.; et al. A Systematic Review and Meta-Analysis of Stem Cell Therapies for Pain in Diabetic Neuropathy, Osteoarthritis, and Spinal Cord Injuries. Curr. Pain Headache Rep. 2025, 29, 29. [Google Scholar] [CrossRef]
- Wettstein, M.; Ghisletta, P.; Gerstorf, D. Feeling Older, Feeling Pain? Reciprocal between-Person and within-Person Associations of Pain and Subjective Age in the Second Half of Life. Psychol. Aging 2024, 39, 510–525. [Google Scholar] [CrossRef]
- Presto, P.; Sehar, U.; Kopel, J.; Reddy, P.H. Mechanisms of Pain in Aging and Age-Related Conditions: Focus on Caregivers. Ageing Res. Rev. 2024, 95, 102249. [Google Scholar] [CrossRef]
- Cedraschi, C.; Ludwig, C.; Allaz, A.F.; Herrmann, F.R.; Luthy, C. Pain and Health-Related Quality of Life (HRQoL): A National Observational Study in Community-Dwelling Older Adults. Eur. Geriatr. Med. 2018, 9, 881–889. [Google Scholar] [CrossRef]
- Dong, H.-J.; Yang, J.; Johansson, M.M.; Peolsson, A.; Barbero, M.; Nord, M. Association between Frailty and Pain in Older People at High Risk of Future Hospitalization. Front. Pain Res. 2025, 6, 1576691. [Google Scholar] [CrossRef]
- Shega, J.W.; Weiner, D.K.; Paice, J.A.; Bilir, S.P.; Rockwood, K.; Herr, K.; Ersek, M.; Emanuel, L.; Dale, W. The Association Between Noncancer Pain, Cognitive Impairment, and Functional Disability: An Analysis of the Canadian Study of Health and Aging. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2010, 65A, 880–886. [Google Scholar] [CrossRef]
- American Occupational Therapy Association. Occupational Therapy Practice Framework: Domain and Process—Fourth Edition. Am. J. Occup. Ther. 2020, 74, 7412410010p1–7412410010p87. [Google Scholar] [CrossRef] [PubMed]
- Van Dalen-Kok, A.H.; Pieper, M.J.C.; De Waal, M.W.M.; Van Der Steen, J.T.; Scherder, E.J.A.; Achterberg, W.P. The Impact of Pain on the Course of ADL Functioning in Patients with Dementia. Age Ageing 2021, 50, 906–913. [Google Scholar] [CrossRef] [PubMed]
- Giebel, C.M.; Sutcliffe, C.; Stolt, M.; Karlsson, S.; Renom-Guiteras, A.; Soto, M.; Verbeek, H.; Zabalegui, A.; Challis, D. Deterioration of Basic Activities of Daily Living and Their Impact on Quality of Life across Different Cognitive Stages of Dementia: A European Study. Int. Psychogeriatr. 2014, 26, 1283–1293. [Google Scholar] [CrossRef] [PubMed]
- Denche-Zamorano, A.; Collado-Mateo, D.; Franco-Garcia, J.M.; Adsuar, J.C.; Salas-Gómez, D. Quality of Life in European Adults and Older with All-Over Pain: Relationship with Frequency of Moderate and Vigorous Physical Activity and Decision Prediction Models with Cross-Sectional Data. Healthcare 2025, 13, 1171. [Google Scholar] [CrossRef]
- Mansfield, K.E.; Sim, J.; Jordan, J.L.; Jordan, K.P. A Systematic Review and Meta-Analysis of the Prevalence of Chronic Widespread Pain in the General Population. Pain 2016, 157, 55–64. [Google Scholar] [CrossRef]
- Kou, M.; Li, X.; Ma, H.; Wang, X.; Heianza, Y.; Qi, L. Abstract P351: The Associations of Short-Term, Chronic Localized and Chronic Widespread Pain With Life Expectancy Free of Major Chronic Diseases. Circulation 2024, 149, AP351. [Google Scholar] [CrossRef]
- Morales-Espinoza, E.M.; Kostov, B.; Salami, D.C.; Perez, Z.H.; Rosalen, A.P.; Molina, J.O.; Paz, L.G.; Momblona, J.M.S.; Àreu, J.B.; Brito-Zerón, P.; et al. Complexity, Comorbidity, and Health Care Costs Associated with Chronic Widespread Pain in Primary Care. Pain 2016, 157, 818–826. [Google Scholar] [CrossRef]
- Dizner-Golab, A.; Lisowska, B.; Kosson, D. Fibromyalgia—Etiology, Diagnosis and Treatment Including Perioperative Management in Patients with Fibromyalgia. Reumatologia 2023, 61, 137–148. [Google Scholar] [CrossRef]
- Beasley, M.; Freidin, M.B.; Basu, N.; Williams, F.M.K.; Macfarlane, G.J. What Is the Effect of Alcohol Consumption on the Risk of Chronic Widespread Pain? A Mendelian Randomisation Study Using UK Biobank. Pain 2019, 160, 501–507. [Google Scholar] [CrossRef]
- Dueñas, M.; Salazar, A.; De Sola, H.; Failde, I. Limitations in Activities of Daily Living in People With Chronic Pain: Identification of Groups Using Clusters Analysis. Pain Pract. 2020, 20, 179–187. [Google Scholar] [CrossRef]
- Bergman, S.; Thorstensson, C.; Andersson, M.L.E. Chronic Widespread Pain and Its Associations with Quality of Life and Function at a 20- Year Follow-up of Individuals with Chronic Knee Pain at Inclusion. BMC Musculoskelet. Disord. 2019, 20, 592. [Google Scholar] [CrossRef]
- Izquierdo, M.; Duque, G.; Morley, J.E. Physical Activity Guidelines for Older People: Knowledge Gaps and Future Directions. Lancet Healthy Longev. 2021, 2, e380–e383. [Google Scholar] [CrossRef]
- Izquierdo, M.; Merchant, R.A.; Morley, J.E.; Anker, S.D.; Aprahamian, I.; Arai, H.; Aubertin-Leheudre, M.; Bernabei, R.; Cadore, E.L.; Cesari, M.; et al. International Exercise Recommendations in Older Adults (ICFSR): Expert Consensus Guidelines. J. Nutr. Health Aging 2021, 25, 824–853. [Google Scholar] [CrossRef]
- Wong, M.Y.C.; Ou, K.; Chung, P.K.; Chui, K.Y.K.; Zhang, C. The Relationship between Physical Activity, Physical Health, and Mental Health among Older Chinese Adults: A Scoping Review. Front. Public Health 2023, 10, 914548. [Google Scholar] [CrossRef] [PubMed]
- Musich, S.; Wang, S.S.; Hawkins, K.; Greame, C. The Frequency and Health Benefits of Physical Activity for Older Adults. Popul. Health Manag. 2017, 20, 199–207. [Google Scholar] [CrossRef]
- Izquierdo, M.; de Souto Barreto, P.; Arai, H.; Bischoff-Ferrari, H.A.; Cadore, E.L.; Cesari, M.; Chen, L.-K.; Coen, P.M.; Courneya, K.S.; Duque, G.; et al. Global Consensus on Optimal Exercise Recommendations for Enhancing Healthy Longevity in Older Adults (ICFSR). J. Nutr. Health Aging 2025, 29, 100401. [Google Scholar] [CrossRef] [PubMed]
- Zasadzka, E.; Trzmiel, T.; Kasior, I.; Hojan, K. Does Hand Grip Strength (HGS) Predict Functional Independence Differently in Patients Post Hip Replacement Due to Osteoarthritis versus Patients Status Post Hip Replacement Due to a Fracture? CIA 2023, 18, 1145–1154. [Google Scholar] [CrossRef]
- López-Bueno, R.; Andersen, L.L.; Calatayud, J.; Casaña, J.; Smith, L.; Jacob, L.; Koyanagi, A.; López-Gil, J.F.; Del Pozo Cruz, B. Longitudinal Association of Handgrip Strength with All-Cause and Cardiovascular Mortality in Older Adults Using a Causal Framework. Exp. Gerontol. 2022, 168, 111951. [Google Scholar] [CrossRef]
- Pham, T.; McNeil, J.J.; Barker, A.L.; Orchard, S.G.; Newman, A.B.; Robb, C.; Ernst, M.E.; Espinoza, S.; Woods, R.L.; Nelson, M.R.; et al. Longitudinal Association between Handgrip Strength, Gait Speed and Risk of Serious Falls in a Community-Dwelling Older Population. PLoS ONE 2023, 18, e0285530. [Google Scholar] [CrossRef] [PubMed]
- McGrath, R.; Erlandson, K.M.; Vincent, B.M.; Hackney, K.J.; Herrmann, S.D.; Clark, B.C. Decreased Handgrip Strength Is Associated With Impairments in Each Autonomous Living Task for Aging Adults in the United States. J. Frailty Aging 2019, 8, 141–145. [Google Scholar] [CrossRef]
- Börsch-Supan, A. Survey of Health, Ageing and Retirement in Europe (SHARE) Wave 6. 2022. Available online: https://share-eric.eu/data/data-documentation/waves-overview/wave-6 (accessed on 6 March 2025).
- Börsch-Supan, A.; Czaplicki, C.; Friedel, S.; Herold, I.; Korbmacher, J.; Mika, T. SHARE-RV: Linked Data to Study Aging in Germany. Jahrb. Natl. Stat. 2020, 240, 121–132. [Google Scholar] [CrossRef]
- Börsch-Supan, A.; Brandt, M.; Hunkler, C.; Kneip, T.; Korbmacher, J.; Malter, F.; Schaan, B.; Stuck, S.; Zuber, S. Data Resource Profile: The Survey of Health, Ageing and Retirement in Europe (SHARE). Int. J. Epidemiol. 2013, 42, 992–1001. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, M.; Wagner, M.; Börsch-Supan, A. SHARE Wave 9 Methodology: From the Second SHARE Corona Survey to the Regular SHARE Interview; SHARE-ERIC: Munich, Germany, 2024; 180p. [Google Scholar]
- Ogliari, G.; Ryg, J.; Andersen-Ranberg, K.; Scheel-Hincke, L.L.; Masud, T. Association between Body Mass Index and Falls in Community-Dwelling Men and Women: A Prospective, Multinational Study in the Survey of Health, Ageing and Retirement in Europe (SHARE). Eur. Geriatr. Med. 2021, 12, 837–849. [Google Scholar] [CrossRef]
- Organisation for Economic Co-Operation and Development. Classifying Educational Programmes—Manual for ISCED-97 Implementation in OECD Countries—1999 Edition; Organisation for Economic Co-operation and Development: Paris, France, 1999. [Google Scholar]
- Ogliari, G.; Ryg, J.; Andersen-Ranberg, K.; Scheel-Hincke, L.L.; Masud, T. Perceived Neighbourhood Environment and Falls among Community-Dwelling Adults: Cross-Sectional and Prospective Findings from the Survey of Health, Ageing and Retirement in Europe (SHARE). Eur. J. Ageing 2022, 19, 1121–1134. [Google Scholar] [CrossRef]
- Mohd Hairi, F.; Mackenbach, J.P.; Andersen-Ranberg, K.; Avendano, M. Does Socio-Economic Status Predict Grip Strength in Older Europeans? Results from the SHARE Study in Non-Institutionalised Men and Women Aged 50+. J. Epidemiol. Community Health 2010, 64, 829–837. [Google Scholar] [CrossRef]
- Maskileyson, D.; Seddig, D.; Davidov, E. The EURO-D Measure of Depressive Symptoms in the Aging Population: Comparability Across European Countries and Israel. Front. Political Sci. 2021, 3, 665004. [Google Scholar] [CrossRef]
- Castro-Costa, E.; Dewey, M.; Stewart, R.; Banerjee, S.; Huppert, F.; Mendonca-Lima, C.; Bula, C.; Reisches, F.; Wancata, J.; Ritchie, K.; et al. Prevalence of Depressive Symptoms and Syndromes in Later Life in Ten European Countries: The SHARE Study. Br. J. Psychiatry 2007, 191, 393–401. [Google Scholar] [CrossRef]
- Trucharte, A.; Calderón, L.; Cerezo, E.; Contreras, A.; Peinado, V.; Valiente, C. Three-Item Loneliness Scale: Psychometric Properties and Normative Data of the Spanish Version. Curr. Psychol. 2023, 42, 7466–7474. [Google Scholar] [CrossRef] [PubMed]
- Bosch-Farré, C.; Garre-Olmo, J.; Bonmatí-Tomàs, A.; Malagón-Aguilera, M.C.; Gelabert-Vilella, S.; Fuentes-Pumarola, C.; Juvinyà-Canal, D. Prevalence and Related Factors of Active and Healthy Ageing in Europe According to Two Models: Results from the Survey of Health, Ageing and Retirement in Europe (SHARE). PLoS ONE 2018, 13, e0206353. [Google Scholar] [CrossRef] [PubMed]
- Katz, S. Studies of Illness in the Aged: The Index of ADL: A Standardized Measure of Biological and Psychosocial Function. JAMA 1963, 185, 914. [Google Scholar] [CrossRef]
- Lawton, M.P.; Brody, E.M. Assessment of Older People: Self-Maintaining and Instrumental Activities of Daily Living. Gerontologist 1969, 9, 179–186. [Google Scholar] [CrossRef]
- Crombie, K.M.; Leitzelar, B.N.; Almassi, N.E.; Mahoney, J.E.; Koltyn, K.F. The Feasibility and Effectiveness of a Community-Based Intervention to Reduce Sedentary Behavior in Older Adults. J. Appl. Gerontol. 2022, 41, 92–102. [Google Scholar] [CrossRef]
- Bull, F.C.; Al-Ansari, S.S.; Biddle, S.; Borodulin, K.; Buman, M.P.; Cardon, G.; Carty, C.; Chaput, J.-P.; Chastin, S.; Chou, R.; et al. World Health Organization 2020 Guidelines on Physical Activity and Sedentary Behaviour. Br. J. Sports Med. 2020, 54, 1451–1462. [Google Scholar] [CrossRef]
- Fowler-Brown, A.; Wee, C.C.; Marcantonio, E.; Ngo, L.; Leveille, S. The Mediating Effect of Chronic Pain on the Relationship Between Obesity and Physical Function and Disability in Older Adults. J. Am. Geriatr. Soc. 2013, 61, 2079–2086. [Google Scholar] [CrossRef]
- Qian, M.; Shi, Y.; Yu, M. The Association between Obesity and Chronic Pain among Community-Dwelling Older Adults: A Systematic Review and Meta-Analysis. Geriatr. Nurs. 2021, 42, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.-J.; Larsson, B.; Levin, L.-Å.; Bernfort, L.; Gerdle, B. Is Excess Weight a Burden for Older Adults Who Suffer Chronic Pain? BMC Geriatr. 2018, 18, 270. [Google Scholar] [CrossRef] [PubMed]
- Bloomberg, M.; Bu, F.; Fancourt, D.; Steptoe, A. Trajectories of Loneliness, Social Isolation, and Depressive Symptoms before and after Onset of Pain in Middle-Aged and Older Adults. eClinicalMedicine 2025, 84, 103209. [Google Scholar] [CrossRef]
- Powell, V.D.; Kumar, N.; Galecki, A.T.; Kabeto, M.; Clauw, D.J.; Williams, D.A.; Hassett, A.; Silveira, M.J. Bad Company: Loneliness Longitudinally Predicts the Symptom Cluster of Pain, Fatigue, and Depression in Older Adults. J. Am. Geriatr. Soc. 2022, 70, 2225–2234. [Google Scholar] [CrossRef] [PubMed]
- Bell, T.R.; Sprague, B.N.; Ross, L.A. Longitudinal Associations of Pain and Cognitive Decline in Community-Dwelling Older Adults. Psychol. Aging 2022, 37, 715–730. [Google Scholar] [CrossRef]
- Bell, T.; Franz, C.E.; Kremen, W.S. Persistence of Pain and Cognitive Impairment in Older Adults. J. Am. Geriatr. Soc. 2022, 70, 449–458. [Google Scholar] [CrossRef]
- Whitlock, E.L.; Diaz-Ramirez, L.G.; Glymour, M.M.; Boscardin, W.J.; Covinsky, K.E.; Smith, A.K. Association Between Persistent Pain and Memory Decline and Dementia in a Longitudinal Cohort of Elders. JAMA Intern. Med. 2017, 177, 1146. [Google Scholar] [CrossRef]
- Huang, C.-C.; Lee, L.-H.; Lin, W.-S.; Hsiao, T.-H.; Chen, I.-C.; Lin, C.-H. The Association between Bodily Pain and Cognitive Impairment in Community-Dwelling Older Adults. J. Pers. Med. 2022, 12, 350. [Google Scholar] [CrossRef]
- Higgins, D.M.; Martin, A.M.; Baker, D.G.; Vasterling, J.J.; Risbrough, V. The Relationship Between Chronic Pain and Neurocognitive Function: A Systematic Review. Clin. J. Pain 2018, 34, 262–275. [Google Scholar] [CrossRef] [PubMed]
- Dagnino, A.P.A.; Campos, M.M. Chronic Pain in the Elderly: Mechanisms and Perspectives. Front. Hum. Neurosci. 2022, 16, 736688. [Google Scholar] [CrossRef] [PubMed]
- Otones Reyes, P.; García Perea, E.; Pedraz Marcos, A. Chronic Pain and Frailty in Community-Dwelling Older Adults: A Systematic Review. Pain Manag. Nurs. 2019, 20, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Mundal, I.; Gråwe, R.W.; Bjørngaard, J.H.; Linaker, O.M.; Fors, E.A. Prevalence and Long-Term Predictors of Persistent Chronic Widespread Pain in the General Population in an 11-Year Prospective Study: The HUNT Study. BMC Musculoskelet. Disord. 2014, 15, 213. [Google Scholar] [CrossRef]
- Domenichiello, A.F.; Ramsden, C.E. The Silent Epidemic of Chronic Pain in Older Adults. Prog. Neuropsychopharmacol. Biol. Psychiatry 2019, 93, 284–290. [Google Scholar] [CrossRef]
- Staudt, M.D. The Multidisciplinary Team in Pain Management. Neurosurg. Clin. N. Am. 2022, 33, 241–249. [Google Scholar] [CrossRef]
- Wang, L.-Y.; Chen, H.-X.; Zhu, H.; Hu, Z.-Y.; Zhou, C.-F.; Hu, X.-Y. Physical Activity as a Predictor of Activities of Daily Living in Older Adults: A Longitudinal Study in China. Front. Public Health 2024, 12, 1444119. [Google Scholar] [CrossRef]
- Amaral Gomes, E.S.; Ramsey, K.A.; Rojer, A.G.; Reijnierse, E.M.; Maier, A.B. The Association of Objectively Measured Physical Activity and Sedentary Behavior with (Instrumental) Activities of Daily Living in Community-Dwelling Older Adults: A Systematic Review. CIA 2021, 16, 1877–1915. [Google Scholar] [CrossRef]
- Wilhelm, L.O.; Pauly, T.; Ashe, M.C.; Hoppmann, C.A. Daily Physical Activity in Older Age: Associations with Affective Barriers. GeroPsych 2021, 34, 125–135. [Google Scholar] [CrossRef]
- Chen, Z.; Tse, M.M.Y.; Wong, B.Y.M. Exercise Habits and Preferences of Community-Dwelling Older Adults with Chronic Pain: An Exploratory Study. Healthcare 2025, 13, 384. [Google Scholar] [CrossRef] [PubMed]
- Stubbs, B.; Binnekade, T.T.; Soundy, A.; Schofield, P.; Huijnen, I.P.J.; Eggermont, L.H.P. Are Older Adults with Chronic Musculoskeletal Pain Less Active than Older Adults Without Pain? A Systematic Review and Meta-Analysis. Pain Med. 2013, 14, 1316–1331. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, T.; Rashid, M.; Zanwar, P.P. Examining the Association of Pain and Pain Frequency With Self-Reported Difficulty in Activities of Daily Living and Instrumental Activities of Daily Living Among Community-Dwelling Older Adults: Findings From the Longitudinal Aging Study in India. J. Gerontol. Ser. B 2023, 78, 1545–1554. [Google Scholar] [CrossRef]
- Stamm, T.A.; Pieber, K.; Crevenna, R.; Dorner, T.E. Impairment in the Activities of Daily Living in Older Adults with and without Osteoporosis, Osteoarthritis and Chronic Back Pain: A Secondary Analysis of Population-Based Health Survey Data. BMC Musculoskelet. Disord. 2016, 17, 139. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Zeng, Z.; Wen, Y.; Chen, C.; Cheng, D.; Li, Y.; Huang, N.; Ruan, J.; Zhao, D.; Xue, Q. Cumulative Handgrip Strength and Longitudinal Changes in Cognitive Function and Daily Functioning among People Aged 50 Years and Older: Evidence from Two Longitudinal Cohort Studies. Arch. Public Health 2025, 83, 150. [Google Scholar] [CrossRef]
- Sosowska, N.; Guligowska, A.; Sołtysik, B.; Borowiak, E.; Kostka, T.; Kostka, J. Better Handgrip Strength Is Related to the Lower Prevalence of Pain and Anxiety in Community-Dwelling Older Adults. J. Clin. Med. 2023, 12, 3846. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, H.; Zhang, T.J.; Wu, N. Association of Handgrip Strength Weakness and Asymmetry with Later Life Pain Risk in Middle-aged and Older Individuals: Results from Four Prospective Cohorts. Aging Med. 2024, 7, 596–605. [Google Scholar] [CrossRef]
- Kaczorowska, A.; Kozieł, S.; Ignasiak, Z. Hand Grip Strength and Quality of Life among Adults Aged 50–90 Years from South West Poland. Sci. Rep. 2025, 15, 882. [Google Scholar] [CrossRef]
- Labott, B.K.; Bucht, H.; Morat, M.; Morat, T.; Donath, L. Effects of Exercise Training on Handgrip Strength in Older Adults: A Meta-Analytical Review. Gerontology 2019, 65, 686–698. [Google Scholar] [CrossRef]
- United Nations. Proceedings of the United Nations Conference on Sustainable Development (UNCSD or “Rio+20”), Rio de Janeiro, Brazil, 20–22 June 2012; United Nations: New York, NY, USA, 2012. [Google Scholar]
| Total n (1154) | Female n (819) 71% | Male n (335) 29% | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Mdn | IQR | Mdn | IQR | Mdn | IQR | UM | df | p-Value | |
| Age (years) | 68 | 12 | 68 | 12 | 70 | 12 | 124,866 | 0.016 * | |
| Perception of loneliness | 4 | 3 | 4 | 3 | 4 | 3 | 103,751 | 0.139 | |
| Hand Grip Ratio (kg/Weight) | 0.35 | 0.17 | 0.33 | 0.15 | 0.44 | 0.18 | 41,414 | <0.001 *** | |
| Cognitive Functioning | 17 | 7 | 17 | 7 | 16 | 7 | 91,449 | <0.001 *** | |
| n | (%) | n | (%) | n | (%) | ||||
| Body Mass Index | |||||||||
| Underweight | 33 | 3% | 22 | 3% | 11 | 4% | 2.14 | 3 | 0.544 |
| Normal | 333 | 31% | 145 | 32% | 88 | 28% | |||
| Overweight | 410 | 28% | 283 | 37% | 127 | 40% | |||
| Obese | 306 | 28% | 217 | 28% | 89 | 28% | |||
| Depression | |||||||||
| No | 40 | 40% | 283 | 37% | 126 | 46% | 6.60 | 1 | 0.012 |
| Yes | 627 | 61% | 479 | 63% | 148 | 54% | |||
| Long-term illness | |||||||||
| No | 49 | 13% | 110 | 13% | 39 | 12% | 0.63 | 1 | 0.426 |
| Yes | 1002 | 87% | 708 | 87% | 294 | 88% | |||
| Education level | |||||||||
| None | 41 | 4% | 32 | 4% | 9 | 3% | 4.39 | 7 | 0.613 |
| Pre-primary | 215 | 1% | 149 | 18% | 66 | 20% | |||
| Primary | 230 | 20% | 168 | 21% | 62 | 19% | |||
| Lower Secondary | 447 | 29% | 310 | 38% | 137 | 41% | |||
| Upper secondary | 41 | 4% | 28 | 3% | 13 | 4% | |||
| Post-secondary non-tertiary | 169 | 15% | 124 | 15% | 45 | 13% | |||
| First stage of tertiary education | 7 | 1% | 5 | 1% | 2 | 1% | |||
| Still in school | 1 | 1% | 0 | 0% | 1 | 0% | |||
| Moderate Physical Activity | |||||||||
| More than once a week | 568 | 49% | 410 | 50% | 158 | 47% | 10.65 | 3 | 0.014 |
| Once a week | 147 | 13% | 114 | 14% | 33 | 10% | |||
| One to three times a month | 91 | 8% | 69 | 8% | 22 | 7% | |||
| Hardly ever, or never | 345 | 30% | 224 | 27% | 121 | 36% | |||
| Vigorous Physical Activity | |||||||||
| More than once a week | 242 | 21% | 175 | 21% | 67 | 20% | 3.64 | 3 | 0.303 |
| Once a week | 126 | 11% | 97 | 12% | 29 | 9% | |||
| One to three times a month | 86 | 8% | 63 | 8% | 23 | 7% | |||
| Hardly ever, or never | 696 | 61% | 482 | 59% | 214 | 64% | |||
| Variable | Moderate Physical Activity Frequency | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| More Than Once a Week | Once a Week | 1–3 Times a Month | Hardly Ever, or Never | χ2 | df | p | V | |||||
| n | (%) | n | (%) | n | (%) | n | (%) | |||||
| Meal preparation (hot meal) | 18a | 3% | 4a | 3% | 4a | 4% | 132b | 38% | 250.768 | 3 | <0.001 *** | 0.467 |
| Ability to use the phone | 3a | 1% | 2a | 1% | 1a | 1% | 66b | 19% | 139.518 | 3 | <0.001 *** | 0.349 |
| Responsibility for medication | 8a | 1% | 3 | 2% | 1 | 1% | 87 | 25% | 173.234 | 3 | <0.001 *** | 0.388 |
| Housekeeping (house, garden) | 107a | 19% | 42a | 29% | 22a | 24% | 207b | 60% | 170.768 | 3 | <0.001 *** | 0.386 |
| Financial management | 21a | 4% | 10a | 7% | 7a | 8% | 117b | 34% | 178.715 | 3 | <0.001 *** | 0.395 |
| Community mobility | 33a | 6% | 16a,b | 11% | 16b | 18% | 181c | 53% | 291.246 | 3 | <0.001 *** | 0.504 |
| Doing laundry | 15a | 3% | 5a | 3% | 6a | 7% | 134b | 39% | 257.323 | 3 | <0.001 *** | 0.473 |
| Shopping | 42a | 7% | 17a | 12% | 11a | 12% | 164b | 48% | 227.493 | 3 | <0.001 *** | 0.445 |
| Variable | Vigorous Physical Activity Frequency | |||||||||||
| More Than Once a Week | Once a Week | 1–3 Times a Month | Hardly Ever, or Never | χ2 | df | p | V | |||||
| n | (%) | n | (%) | n | (%) | n | (%) | |||||
| Meal preparation (hot meal) | 5a | 2% | 1a | 1% | 0a | 0% | 152b | 22% | 97.581 | 3 | <0.001 *** | 0.292 |
| Ability to use the phone | 3a | 1% | 1 | 1% | 0a | 0% | 68b | 10% | 32.036 | 3 | <0.001 *** | 0.18 |
| Responsibility for medication | 3a | 1% | 0a | 0% | 1a | 1% | 95b | 14% | 56.932 | 3 | <0.001 *** | 0.223 |
| Housekeeping (house, garden) | 41a | 17% | 19a | 15% | 14a | 17% | 304b | 44% | 92.994 | 3 | <0.001 *** | 0.285 |
| Financial management | 6a | 3% | 3a | 2% | 4a | 5% | 142b | 20% | 72.523 | 3 | <0.001 *** | 0.251 |
| Community mobility | 11a | 5% | 3a | 2% | 7a | 8% | 225b | 32% | 126.022 | 3 | <0.001 *** | 0.331 |
| Doing laundry | 5a | 2% | 1a | 1% | 2a | 2% | 152b | 22% | 92.329 | 3 | <0.001 *** | 0.284 |
| Shopping | 15a | 6% | 5a | 4% | 7a | 8% | 207b | 29% | 96.208 | 3 | <0.001 *** | 0.29 |
| Basic Activities of Daily Living Limitations | |||||
|---|---|---|---|---|---|
| OR | (95% CI) | p | |||
| Dressing | Never vs. Very Frequently MPA | 2.55 | 1.44 | 4.50 | 0.001 ** |
| Never vs. Very Frequently VPA | 0.78 | 0.45 | 1.35 | 0.372 | |
| Relative Handgrip Strength | 0.03 | 0.00 | 0.22 | <0.001 *** | |
| Home Mobility | Never vs. Very Frequently MPA | 9.08 | 2.99 | 27.55 | <0.001 *** |
| Never vs. Very Frequently VPA | 1.47 | 0.38 | 5.65 | 0.579 | |
| Relative Handgrip Strength | 0.01 | 0.00 | 0.22 | 0.006 ** | |
| Bathing | Never vs. Very Frequently MPA | 4.52 | 2.34 | 8.73 | <0.001 *** |
| Never vs. Very Frequently VPA | 0.88 | 0.43 | 1.80 | 0.728 | |
| Relative Handgrip Strength | 0.01 | 0.00 | 0.11 | <0.001 * | |
| Feeding | Never vs. Very Frequently MPA | 5.00 | 1.61 | 15.49 | 0.005 ** |
| Never vs. Very Frequently VPA | 0.66 | 0.22 | 2.02 | 0.471 | |
| Relative Handgrip Strength | 0.00 | 0.00 | 0.00 | <0.001 *** | |
| Transferring | Never vs. Very Frequently MPA | 2.28 | 1.13 | 4.62 | 0.022 * |
| Never vs. Very Frequently VPA | 0.87 | 0.44 | 1.75 | 0.703 | |
| Relative Handgrip Strength | 0.03 | 0.00 | 0.40 | 0.008 ** | |
| Toileting | Never vs. Very Frequently MPA | 2.69 | 1.04 | 6.97 | 0.042 * |
| Never vs. Very Frequently VPA | 1.15 | 0.41 | 3.22 | 0.785 | |
| Relative Handgrip Strength | 0.00 | 0.00 | 0.10 | <0.001 *** | |
| Instrumental Activities of Daily Living Limitations | |||||
|---|---|---|---|---|---|
| OR | (95% CI) | p | |||
| Preparing meal | Never vs. Very Frequently MPA | 4.05 | 1.57 | 10.48 | 0.004 ** |
| Never vs. Very Frequently VPA | 2.67 | 0.73 | 9.80 | 0.139 | |
| Relative Handgrip Strength | 0.02 | 0.00 | 0.63 | 0.027 * | |
| Shopping | Never vs. Very Frequently MPA | 3.88 | 2.03 | 7.40 | <0.001 *** |
| Never vs. Very Frequently VPA | 1.61 | 0.77 | 3.38 | 0.207 | |
| Relative Handgrip Strength | 0.02 | 0.00 | 0.20 | 0.001 ** | |
| Ability using the telephone | Never vs. Very Frequently MPA | 12.10 | 1.76 | 83.29 | 0.011 * |
| Never vs. Very Frequently VPA | 0.35 | 0.05 | 2.55 | 0.298 | |
| Relative Handgrip Strength | 0.02 | 0.00 | 1.02 | 0.051 | |
| Housekeeping | Never vs. Very Frequently MPA | 2.97 | 1.82 | 4.86 | <0.001 *** |
| Never vs. Very Frequently VPA | 1.60 | 0.99 | 2.57 | 0.055 | |
| Relative Handgrip Strength | 0.02 | 0.00 | 0.11 | <0.001 *** | |
| Financial management | Never vs. Very Frequently MPA | 3.14 | 1.21 | 8.18 | 0.019 * |
| Never vs. Very Frequently VPA | 0.99 | 0.32 | 3.11 | 0.990 | |
| Relative Handgrip Strength | 0.02 | 0.00 | 0.80 | 0.038 * | |
| Responsibility for medication | Never vs. Very Frequently MPA | 9.20 | 2.20 | 38.47 | 0.002 ** |
| Never vs. Very Frequently VPA | 1.33 | 0.24 | 7.34 | 0.744 | |
| Relative Handgrip Strength | 0.03 | 0.00 | 2.94 | 0.129 | |
| Community mobility | Never vs. Very Frequently MPA | 4.97 | 2.62 | 9.42 | <0.001 *** |
| Never vs. Very Frequently VPA | 1.98 | 0.89 | 4.40 | 0.094 | |
| Relative Handgrip Strength | 0.01 | 0.00 | 0.17 | <0.001 *** | |
| Doing laundry | Never vs. Very Frequently MPA | 7.53 | 2.97 | 19.06 | <0.001 *** |
| Never vs. Very Frequently VPA | 1.61 | 0.50 | 5.25 | 0.426 | |
| Relative Handgrip Strength | 0.00 | 0.00 | 0.10 | <0.001 *** | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Denche-Zamorano, Á.; Adsuar, J.C.; Barrios-Fernandez, S.; Salas-Gómez, D. Associations Between Physical Activity Frequency, Handgrip Strength, and Limitations in Activities of Daily Living in Middle-Aged and Older Adults with Widespread Pain: A Cross-Sectional Study Using Data from the SHARE Project. Geriatrics 2025, 10, 125. https://doi.org/10.3390/geriatrics10050125
Denche-Zamorano Á, Adsuar JC, Barrios-Fernandez S, Salas-Gómez D. Associations Between Physical Activity Frequency, Handgrip Strength, and Limitations in Activities of Daily Living in Middle-Aged and Older Adults with Widespread Pain: A Cross-Sectional Study Using Data from the SHARE Project. Geriatrics. 2025; 10(5):125. https://doi.org/10.3390/geriatrics10050125
Chicago/Turabian StyleDenche-Zamorano, Ángel, José Carmelo Adsuar, Sabina Barrios-Fernandez, and Diana Salas-Gómez. 2025. "Associations Between Physical Activity Frequency, Handgrip Strength, and Limitations in Activities of Daily Living in Middle-Aged and Older Adults with Widespread Pain: A Cross-Sectional Study Using Data from the SHARE Project" Geriatrics 10, no. 5: 125. https://doi.org/10.3390/geriatrics10050125
APA StyleDenche-Zamorano, Á., Adsuar, J. C., Barrios-Fernandez, S., & Salas-Gómez, D. (2025). Associations Between Physical Activity Frequency, Handgrip Strength, and Limitations in Activities of Daily Living in Middle-Aged and Older Adults with Widespread Pain: A Cross-Sectional Study Using Data from the SHARE Project. Geriatrics, 10(5), 125. https://doi.org/10.3390/geriatrics10050125








