Oculometric Assessment of Sensorimotor Impairment Associated with Liver Disease Is as Sensitive as Standard of Care Cognitive Tests
Abstract
1. Introduction
2. Methods
2.1. Participants
2.2. Measures
2.3. Equipment
2.4. Procedures
2.5. Statistical Analyses
3. Results
3.1. Demographic Data
3.2. Oculometric and PHES Between Groups Comparisons
3.3. Oculometric Correlations with PHES
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Nabi, E.; Bajaj, J.S. Useful tests for hepatic encephalopathy in clinical practice. Curr. Gastroenterol. Rep. 2014, 16, 362. [Google Scholar] [CrossRef]
- Bajaj, J.S.; Saeian, K.; Schubert, C.M.; Hafeezullah, M.; Franco, J.; Varma, R.R.; Gibson, D.P.; Hoffmann, R.G.; Stravitz, R.T.; Heuman, D.M.; et al. Minimal hepatic encephalopathy is associated with motor vehicle crashes: The reality beyond the driving test. Hepatology 2009, 50, 1175–1183. [Google Scholar] [CrossRef]
- Wein, C.; Koch, H.; Popp, B.; Oehler, G.; Schauder, P. Minimal hepatic encephalopathy impairs fitness to drive. Hepatology 2004, 39, 739–745. [Google Scholar] [CrossRef]
- Mangas-Losada, A.; García-García, R.; Urios, A.; Escudero-García, D.; Tosca, J.; Giner-Durán, R.; Serra, M.A.; Montoliu, C.; Felipo, V. Minimal hepatic encephalopathy is associated with expansion and activation of CD4+CD28−, Th22 and Tfh and B lymphocytes. Sci. Rep. 2017, 7, 6683. [Google Scholar] [CrossRef]
- Ferenci, P.; Lockwood, A.; Mullen, K.; Tarter, R.; Weissenborn, K.; Blei, A.T. Hepatic encephalopathy—Definition, nomenclature, diagnosis, and quantification: Final report of the working party at the 11th World Congresses of Gastroenterology, Vienna, 1998. Hepatology 2002, 35, 716–721. [Google Scholar] [CrossRef]
- Bajaj, J.S.; Etemadian, A.; Hafeezullah, M.; Saeian, K. Testing for minimal hepatic encephalopathy in the United States: An AASLD survey. Hepatology 2007, 45, 833–834. [Google Scholar] [CrossRef] [PubMed]
- Allampati, S.; Duarte-Rojo, A.; Thacker, L.R.; Patidar, K.R.; White, M.B.; Klair, J.S.; John, B.; Heuman, D.M.; Wade, J.B.; Flud, C.; et al. Diagnosis of Minimal Hepatic Encephalopathy Using Stroop EncephalApp: A Multicenter US-Based, Norm-Based Study. Am. J. Gastroenterol. 2016, 111, 78–86. [Google Scholar] [CrossRef]
- Ferenci, P. Diagnosis of minimal hepatic encephalopathy: Still a challenge. Gut 2013, 62, 1394. [Google Scholar] [CrossRef] [PubMed]
- Montagnese, S.; Amodio, P.; Morgan, M.Y. Methods for diagnosing hepatic encephalopathy in patients with cirrhosis: A multidimensional approach. Metab. Brain Dis. 2004, 19, 281–312. [Google Scholar] [CrossRef]
- Weissenborn, K.; Ennen, J.C.; Schomerus, H.; Rückert, N.; Hecker, H. Neuropsychological characterization of hepatic encephalopathy. J. Hepatol. 2001, 34, 768–773. [Google Scholar] [CrossRef]
- Liston, D.B.; Wong, L.R.; Stone, L.S. Oculometric Assessment of Sensorimotor Impairment Associated with TBI. Optom. Vis. Sci. 2017, 94, 51–59. [Google Scholar] [CrossRef]
- Kowler, E.; McKee, S.P. Sensitivity of smooth eye movement to small differences in target velocity. Vision Res. 1987, 27, 993–1015. [Google Scholar] [CrossRef]
- Liston, D.; Krauzlis, R.J. Shared decision signal explains performance and timing of pursuit and saccadic eye movements. J. Vis. 2005, 5, 678–689. [Google Scholar] [CrossRef]
- Krukowski, A.E.; Stone, L.S. Expansion of direction space around the cardinal axes revealed by smooth pursuit eye movements. Neuron 2005, 45, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Gegenfurtner, K.R.; Xing, D.; Scott, B.H.; Hawken, M.J. A comparison of pursuit eye movement and perceptual performance in speed discrimination. J. Vis. 2003, 3, 865–876. [Google Scholar] [CrossRef] [PubMed]
- Liston, D.; Krauzlis, R.J. Shared response preparation for pursuit and saccadic eye movements. J. Neurosci. 2003, 23, 11305–11314. [Google Scholar] [CrossRef]
- Liston, D.B.; Stone, L.S. Effects of prior information and reward on oculomotor and perceptual choices. J. Neurosci. 2008, 28, 13866–13875. [Google Scholar] [CrossRef]
- Stone, L.S.; Beutter, B.R.; Eckstein, M.P.; Liston, D.B. Perception and Eye Movements, in Encyclopedia of Neuroscience; Squire, L.R., Ed.; Academic Press: Oxford, UK, 2009; pp. 503–511. [Google Scholar]
- Antoniades, C.A.; Ettinger, U.; Gaymard, B.; Gilchrist, I.; Kristjánsson, A.; Kennard, C.; Leigh, R.J.; Noorani, I.; Pouget, P.; Smyrnis, N.; et al. An internationally standardised antisaccade protocol. Vision Res. 2013, 84, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Liston, D.B.; Simpson, S.; Wong, L.R.; Rich, M.; Stone, L.S. Design and Validation of a Simple Eye-Tracking System. In Proceedings of the Ninth Biennial ACM Symposium on Eye Tracking Research and Applications, Charleston, SC, USA, 14–17 March 2016; Association for Computing Machinery: New York, NY, USA, 2016. [Google Scholar]
- Bahill, A.T.; Brockenbrough, A.; Troost, B.T. Variability and development of a normative data base for saccadic eye movements. Investig. Ophthalmol. Vis. Sci. 1981, 21 Pt 1, 116–125. [Google Scholar]
- Liston, D.B.; Stone, L.S. Oculometric assessment of dynamic visual processing. J. Vis. 2014, 14, 12. [Google Scholar] [CrossRef]
- Leigh, R.J.; Zee, D.S. The Neurology of Eye Movements; Oxford University Press: Oxford, UK, 2006. [Google Scholar]
- Crutcher, M.D.; Calhoun-Haney, R.; Manzanares, C.M.; Lah, J.J.; Levey, A.I.; Zola, S.M. Eye tracking during a visual paired comparison task as a predictor of early dementia. Am. J. Alzheimer’s Dis. Other Demen. 2009, 24, 258–266. [Google Scholar] [CrossRef]
- Zola, S.M.; Manzanares, C.M.; Clopton, P.; Lah, J.J.; Levey, A.I. A behavioral task predicts conversion to mild cognitive impairment and Alzheimer’s disease. Am. J. Alzheimer’s Dis. Other Demen. 2013, 28, 179–184. [Google Scholar] [CrossRef]
- Weissenborn, K.; Lockwood, A.H. Toxic and Metabolic Encephalopathies, in Bradley’s Neurology in Clinical Practice; Butterworth-Heinemann: Philadelphia, PA, USA, 2015; pp. 1209–1225. [Google Scholar]
- Gimenez-Garzo, C.; Garcés, J.J.; Urios, A.; Mangas-Losada, A.; García-García, R.; González-López, O.; Giner-Durán, R.; Escudero-García, D.; Serra, M.A.; Soria, E.; et al. The PHES battery does not detect all cirrhotic patients with early neurological deficits, which are different in different patients. PLoS ONE 2017, 12, e0171211. [Google Scholar] [CrossRef]
- Krismer, F.; Roos, J.C.P.; Schranz, M.; Graziadei, I.W.; Mechtcheriakov, S.; Vogel, W.; Carpenter, R.H.S.; Zoller, H. Saccadic latency in hepatic encephalopathy: A pilot study. Metab. Brain Dis. 2010, 25, 285–295. [Google Scholar] [CrossRef] [PubMed]
- Kamath, P.S.; Kim, W.R. Advanced Liver Disease Study, The model for end-stage liver disease (MELD). Hepatology 2007, 45, 797–805. [Google Scholar] [CrossRef]
- Tsoris, A.; Marlar, C.A. Use of The Child Pugh Score in Liver Disease; StatPearls: Treasure Island, FL, USA, 2025. [Google Scholar]
- Dhiman, R.K.; Kurmi, R.; Thumburu, K.K.; Venkataramarao, S.H.; Agarwal, R.; Duseja, A.; Chawla, Y. Diagnosis and prognostic significance of minimal hepatic encephalopathy in patients with cirrhosis of liver. Dig. Dis. Sci. 2010, 55, 2381–2390. [Google Scholar] [CrossRef]
- Duarte-Rojo, A.; Estradas, J.; Hernández-Ramos, R.; Ponce-De-León, S.; Córdoba, J.; Torre, A. Validation of the psychometric hepatic encephalopathy score (PHES) for identifying patients with minimal hepatic encephalopathy. Dig. Dis. Sci. 2011, 56, 3014–3023. [Google Scholar] [CrossRef] [PubMed]
- Weissenborn, K. Diagnosis of minimal hepatic encephalopathy. J. Clin. Exp. Hepatol. 2015, 5, S54–S59. [Google Scholar] [CrossRef]
- Rashbass, C. The relationship between saccadic and smooth tracking eye movements. J. Physiol. 1961, 159, 326–338. [Google Scholar] [CrossRef] [PubMed]
- Ettenhofer, M.L.; Hershaw, J.N.; Barry, D.M. Multimodal assessment of visual attention using the Bethesda Eye & Attention Measure (BEAM). J. Clin. Exp. Neuropsychol. 2016, 38, 96–110. [Google Scholar]
- Gordon, C.C.; Blackwell, C.L.; Bradtmiller, B.; Parham, J.L.; Barrientos, P.; Paquette, S.; Corner, B.D.; Carson, J.; Venezia, J.; Rockwell, B.M.; et al. Anthropometric Survey of US Army Personnel: Methods and Summary Statistics; Army Natick Soldier Research Development and Engineering Center: Natick, MA, USA, 2014. [Google Scholar]
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Cournot, M.; Marquié, J.C.; Ansiau, D.; Martinaud, C.; Fonds, H.; FerrièrEs, J.; Ruidavets, J.B. Relation between body mass index and cognitive function in healthy middle-aged men and women. Neurology 2006, 67, 1208–1214. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, J.S.; Heuman, D.M.; Sterling, R.K.; Sanyal, A.J.; Siddiqui, M.; Matherly, S.; Luketic, V.; Stravitz, R.T.; Fuchs, M.; Thacker, L.R.; et al. Validation of EncephalApp, Smartphone-Based Stroop Test, for the Diagnosis of Covert Hepatic Encephalopathy. Clin. Gastroenterol. Hepatol. 2015, 13, 1828–1835 e1. [Google Scholar] [CrossRef] [PubMed]
- Rehnberg, J.; Huisman, M.; Fors, S.; Marseglia, A.; Kok, A. The Association between Education and Cognitive Performance Varies at Different Levels of Cognitive Performance: A Quantile Regression Approach. Gerontology 2024, 70, 318–326. [Google Scholar] [CrossRef]
- Bass, N.M.; Mullen, K.D.; Sanyal, A.; Poordad, F.; Neff, G.; Leevy, C.B.; Sigal, S.; Sheikh, M.Y.; Beavers, K.; Frederick, T.; et al. Rifaximin treatment in hepatic encephalopathy. N. Engl. J. Med. 2010, 362, 1071–1081. [Google Scholar] [CrossRef]

| Decompensated Cirrhosis (n = 19) | Compensated Cirrhosis (n = 10) | Control (n = 19) | |
|---|---|---|---|
| Gender (% male) * | 74 | 50 | 47 |
| Age (years) *** | 58 (10.16) | 63 (7.42) | 51 (16.65) |
| BMI (median) *** | 29.3 (5.24) | 29.2 (5.91) | 23.4 (5.58) |
| Educational years *** | 14 (3.78) | 16 (2.77) | 16 (1.44) |
| Etiology of cirrhosis (%) | |||
| Hepatitis C Virus | 26.3 | 60.0 | N/A |
| NASH | 26.3 | 20.0 | N/A |
| Alcohol | 21.1 | 0 | N/A |
| Mixed | 10.5 | 0 | N/A |
| Other | 15.8 | 20.0 | N/A |
| History of hepatic encephalopathy (%) | 73.6 | 0 | N/A |
| History of hospitalization for HE (%) | 36.8 | 0 | N/A |
| Median MELD-Na score | 14 (3.88) | 8 (1.9) | N/A |
| Median CTP score | 7 (1.34) | 5 (0.67) | N/A |
| Decompensated Cirrhosis (n = 19) | Compensated Cirrhosis (n = 10) | Control (n = 19) | Kruskal–Wallis Statistic (df = 2) | |
|---|---|---|---|---|
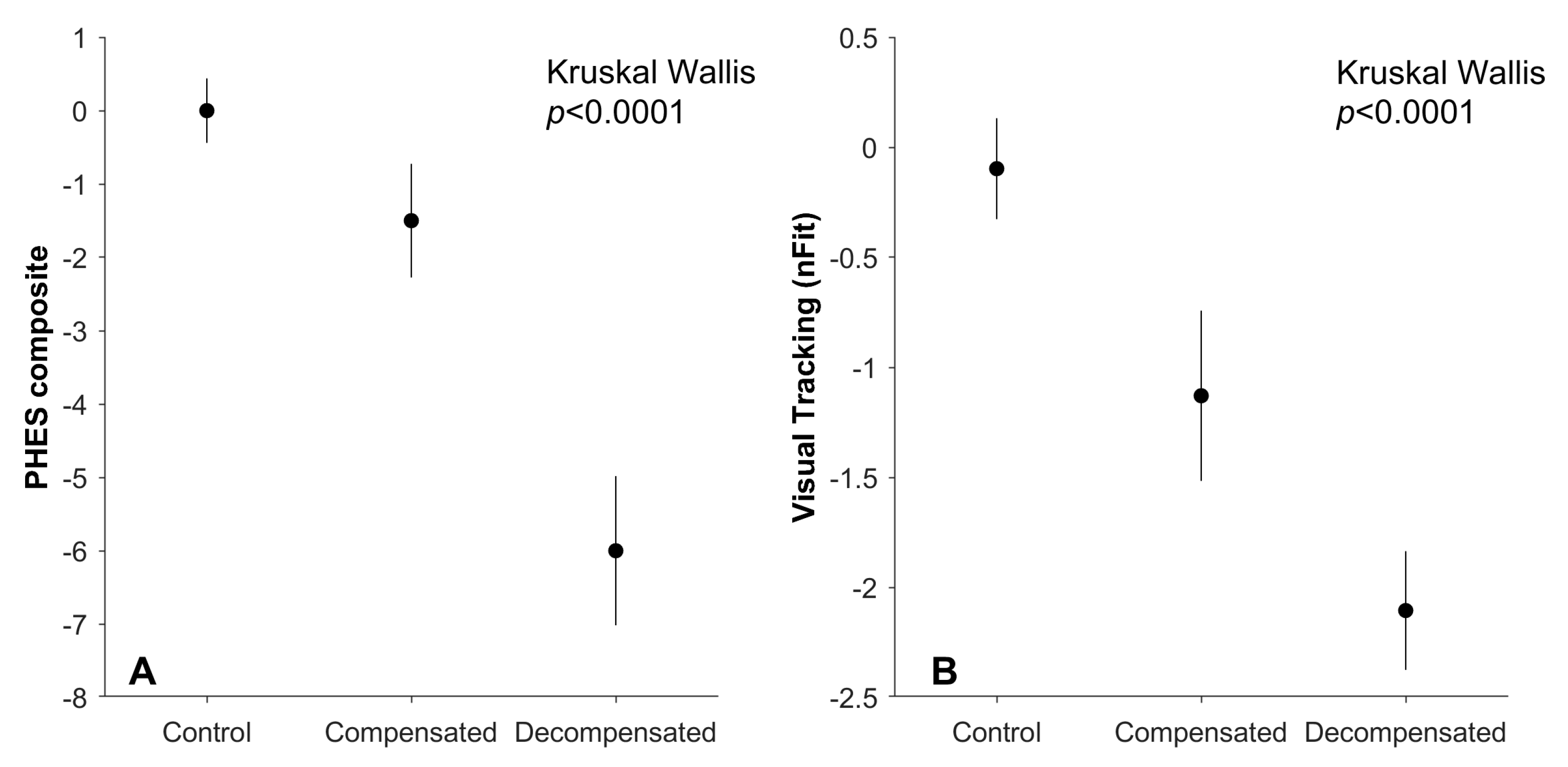
| PHES Composite | −6 (4.32) | −1.5 (2.33) | 0 (1.87) | 15.55, p = 0.0004 |
| DST (# in 120 secs) | 40.0 (11.2) | 57 (13.6) | 63.0 (12.7) | 22.45, p < 0.0001 |
| NCT-A (secs) | 56.9 (46.3) | 39.0 (13.8) | 33.1 (12.4) | 8.79, p < 0.01 |
| NCT-B (secs) | 115.6 (203.6) | 75.1 (17.2) | 62.2 (24.9) | 20.96 p < 0.0001 |
| SDT (secs) | 86.5 (35.1) | 78.7 (16.9) | 57.7 (15.4) | 11.89, p = 0.003 |
| LTT (composite) | 77.6 (38.7) | 76.1 (41.0) | 69.6 (27.1) | 0.58, p > 0.05 |
| MHE Dx (% yes) | 63.2 | 30.0 | 5.0 | 16.05, p = 0.0003 |
| nFit Score | −2.1 (1.2) | −1.1 (1.2) | −0.1 (1.00) | 13.64, p = 0.001 |
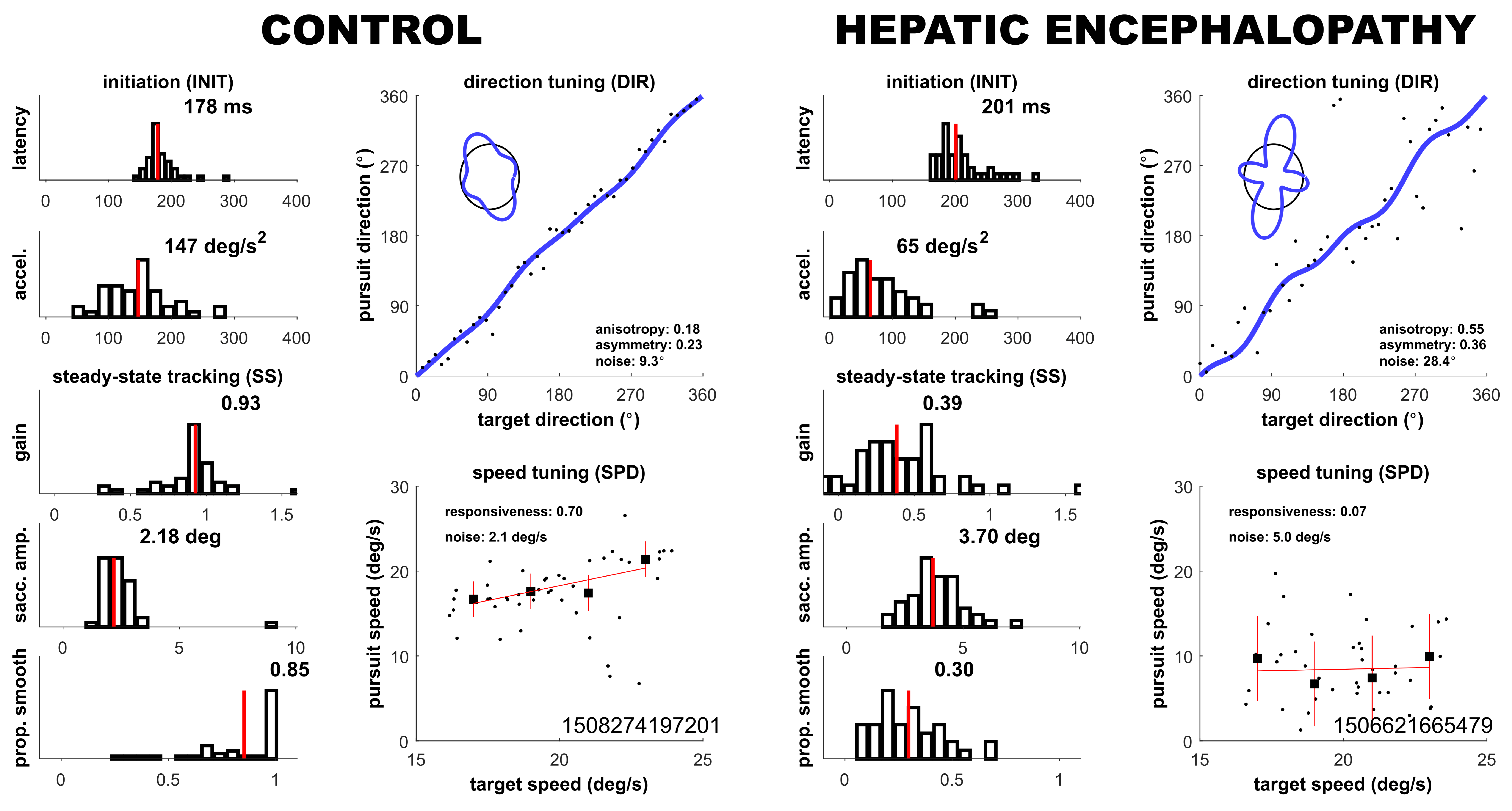
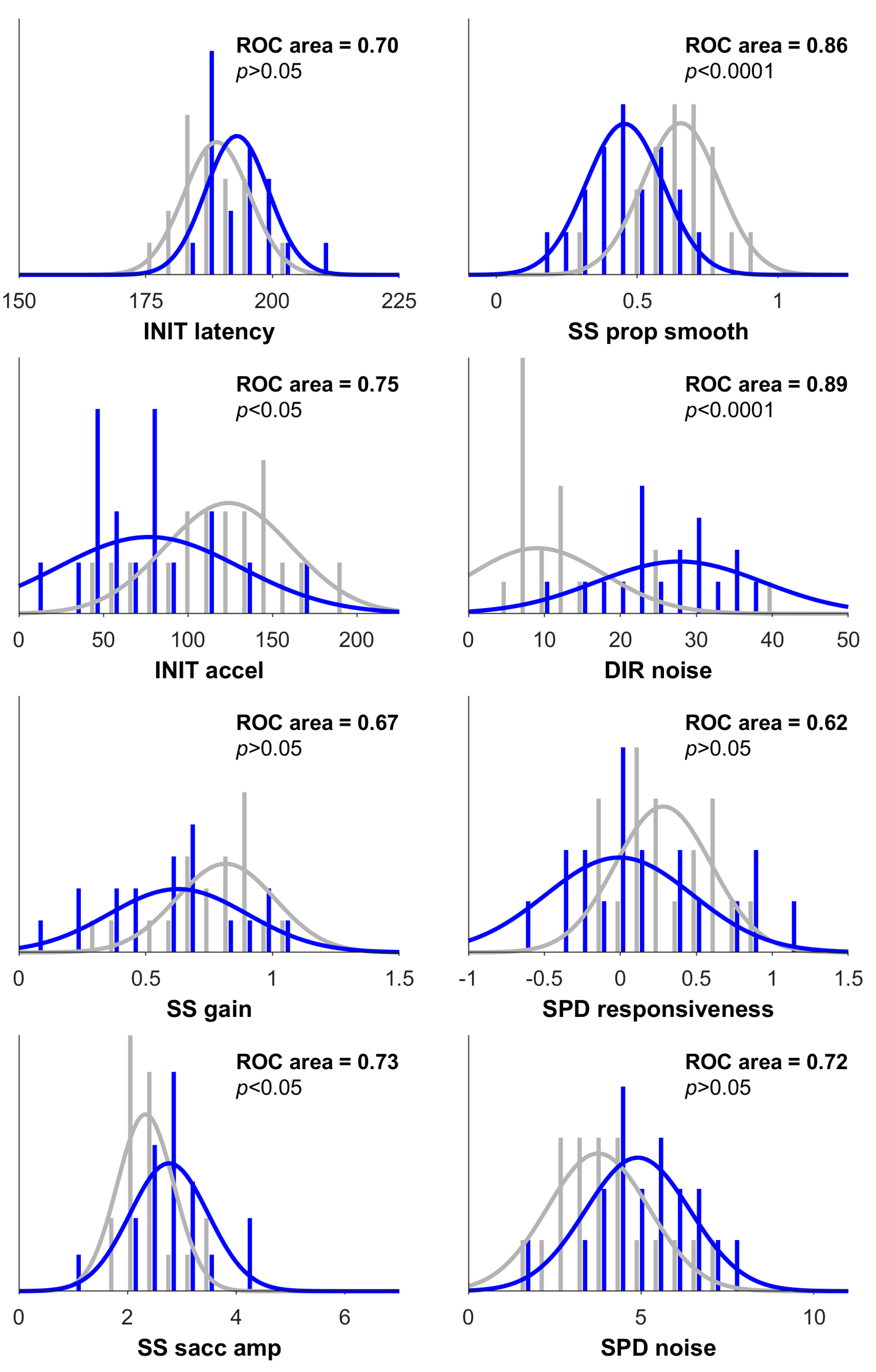
| INIT latency (ms) | 193 (6) | 191 (8) | 189 (6) | 4.21, p > 0.05 |
| INIT acceleration (deg/s2) | 77 (54) | 84 (35) | 124 (37) | 7.25, p = 0.03 |
| SS gain | 0.63 (0.27) | 0.58 (0.28) | 0.81 (0.19) | 3.17, p > 0.05 |
| SS saccade amplitude (deg) | 2.8 (0.7) | 2.4 (0.3) | 2.3 (0.5) | 7.68, p = 0.02 |
| SS prop smooth | 0.46 (0.14) | 0.56 (0.17) | 0.66 (0.14) | 14.57, p = 0.0007 |
| DIR noise (deg) | 27.8 (11.0) | 18.0 (11.1) | 9.1 (8.8) | 14.01, p = 0.0009 |
| SPD responsiveness | −0.01 (0.48) | 0.46 (0.43) | 0.28 (0.31) | 2.87, p > 0.05 |
| SPD noise (deg/s) | 4.9 (1.4) | 4.0 (1.4) | 3.8 (1.4) | 6.56, p = 0.04 |
| anisotropy | 2.43 (5.19) | −1.15 (2.41) | 0.02 (1.92) | 7.48, p = 0.02 |
| asymmetry | −0.23 (4.80) | −0.72 (4.00) | −1.02 (2.58) | 0.12, p > 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liston, D.; Wong, K.; Yeoh, A.; Haywood, S.; Goel, A.; Kwo, P.; Kennedy, Q.; Okafor, P.N. Oculometric Assessment of Sensorimotor Impairment Associated with Liver Disease Is as Sensitive as Standard of Care Cognitive Tests. Geriatrics 2025, 10, 112. https://doi.org/10.3390/geriatrics10040112
Liston D, Wong K, Yeoh A, Haywood S, Goel A, Kwo P, Kennedy Q, Okafor PN. Oculometric Assessment of Sensorimotor Impairment Associated with Liver Disease Is as Sensitive as Standard of Care Cognitive Tests. Geriatrics. 2025; 10(4):112. https://doi.org/10.3390/geriatrics10040112
Chicago/Turabian StyleListon, Dorion, Katherine Wong, Aaron Yeoh, Shalonda Haywood, Aparna Goel, Paul Kwo, Quinn Kennedy, and Philip N. Okafor. 2025. "Oculometric Assessment of Sensorimotor Impairment Associated with Liver Disease Is as Sensitive as Standard of Care Cognitive Tests" Geriatrics 10, no. 4: 112. https://doi.org/10.3390/geriatrics10040112
APA StyleListon, D., Wong, K., Yeoh, A., Haywood, S., Goel, A., Kwo, P., Kennedy, Q., & Okafor, P. N. (2025). Oculometric Assessment of Sensorimotor Impairment Associated with Liver Disease Is as Sensitive as Standard of Care Cognitive Tests. Geriatrics, 10(4), 112. https://doi.org/10.3390/geriatrics10040112






