Adverse Childhood Experiences and Sarcopenia in Later Life: Baseline Data from the Canadian Longitudinal Study on Aging
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling and Design
2.2. Primary Outcome (Sarcopenia)
2.3. Independent Variable (ACE)
2.4. Covariates
2.5. Statistical Analyses
3. Results
3.1. Study Population
3.2. Association Between ACE and Sarcopenia
3.3. Sensitivity Analyses
4. Discussion
4.1. Methodological Considerations
4.2. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 601. [Google Scholar] [CrossRef] [PubMed]
- Petermann-Rocha, F.; Balntzi, V.; Gray, S.R.; Lara, J.; Ho, F.K.; Pell, J.P.; Celis-Morales, C. Global prevalence of sarcopenia and severe sarcopenia: A systematic review and meta-analysis. J. Cachexia Sarcopenia Muscle 2022, 13, 86. [Google Scholar] [CrossRef]
- Sayer, A.A.; Cruz-Jentoft, A. Sarcopenia definition, diagnosis and treatment: Consensus is growing. Age Ageing 2022, 51, afac220. [Google Scholar] [CrossRef]
- Dodds, R.; Sayer, A.A. Sarcopenia and frailty: New challenges for clinical practice. Clin. Med. 2016, 16, 455. [Google Scholar] [CrossRef]
- Bellis, M.A.; Hughes, K.; Ford, K.; Ramos Rodriguez, G.; Sethi, D.; Passmore, J. Life course health consequences and associated annual costs of adverse childhood experiences across Europe and North America: A systematic review and meta-analysis. Lancet Public Health 2019, 4, e517. [Google Scholar] [CrossRef]
- Felitti, V.J.; Anda, R.F.; Nordenberg, D.; Williamson, D.F.; Spitz, A.M.; Edwards, V.; Koss, M.P.; Marks, J.S. Relationship of Childhood Abuse and Household Dysfunction to Many of the Leading Causes of Death in Adults The Adverse Childhood Experiences (ACE) Study. Am. J. Prev. Med. 1998, 14, 245–258. [Google Scholar] [CrossRef]
- Mian, O.; Anderson, L.N.; Belsky, D.W.; Gonzalez, A.; Ma, J.; Sloboda, D.M.; Bowdish, D.M.E.; Verschoor, C.P. Associations of Adverse Childhood Experiences with Frailty in Older Adults: A Cross-Sectional Analysis of Data from the Canadian Longitudinal Study on Aging. Gerontology 2021, 68, 1091–1100. [Google Scholar] [CrossRef] [PubMed]
- van der Linden, B.W.A.; Sieber, S.; Cheval, B.; Orsholits, D.; Guessous, I.; Gabriel, R.; Von Arx, M.; Kelly-Irving, M.; Aartsen, M.; Blane, D. Life-course circumstances and frailty in old age within different European welfare regimes: A longitudinal study with SHARE. J. Gerontol. Ser. B 2019, 75, 1326–1335. [Google Scholar] [CrossRef]
- Dimitriadis, M.M.; Jeuring, H.W.; Marijnissen, R.M.; Wieringa, T.H.; Hoogendijk, E.O.; Oude Voshaar, R.C. Adverse Childhood Experiences and frailty in later life: A prospective population-based cohort study. Age Ageing 2023, 52, afad010. [Google Scholar] [CrossRef]
- Cesari, M.; Landi, F.; Vellas, B.; Bernabei, R.; Marzetti, E. Sarcopenia and Physical Frailty: Two Sides of the Same Coin. Front. Aging Neurosci. 2014, 6, 192. [Google Scholar] [CrossRef] [PubMed]
- Landi, F.; Calvani, R.; Cesari, M.; Tosato, M.; Martone, A.M.; Bernabei, R.; Onder, G.; Marzetti, E. Sarcopenia as the Biological Substrate of Physical Frailty. Clin. Geriatr. Med. 2015, 31, 367–374. [Google Scholar] [CrossRef]
- Huang, R.; Li, Y.; Ma, C.; Ren, R.; Yuan, X.; Peng, Y.; Wang, D. Adverse childhood experiences, sarcopenia, and social participation in older adults: A cohort study. BMC Public Health 2024, 24, 711. [Google Scholar] [CrossRef]
- Hailes, H.P.; Yu, R.; Danese, A.; Fazel, S. Long-term outcomes of childhood sexual abuse: An umbrella review. Lancet Psychiatry 2019, 6, 830–839. [Google Scholar] [CrossRef]
- Hughes, K.; Bellis, M.A.; Hardcastle, K.A.; Sethi, D.; Butchart, A.; Mikton, C.; Jones, L.; Dunne, M.P. The effect of multiple adverse childhood experiences on health: A systematic review and meta-analysis. Lancet Public Health 2017, 2, e356–e366. [Google Scholar] [CrossRef]
- Tuttle, C.S.L.; Thang, L.A.N.; Maier, A.B. Markers of inflammation and their association with muscle strength and mass: A systematic review and meta-analysis. Ageing Res. Rev. 2020, 64, 101185. [Google Scholar] [CrossRef]
- Raina, P.; Wolfson, C.; Kirkland, S.; Griffith, L.E.; Tuokko, H.; Penning, M.; Balion, C.; Cossette, B.; Hogan, D.; Wister, A.; et al. Cohort Profile: The Canadian Longitudinal Study on Aging (CLSA). Int. J. Epidemiol. 2019, 48, 1752–1753. [Google Scholar] [CrossRef]
- Raina, P.S.; Wolfson, C.; Kirkland, S.A.; Griffith, L.E.; Oremus, M.; Patterson, C.; Tuokko, H.; Penning, M.; Balion, C.M.; Hogan, D.; et al. The Canadian longitudinal study on aging (CLSA). Can. J. Aging 2009, 28, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Wekerle, C.; Leung, E.; Waechter, R.; Gonzalez, A.; Jamieson, E.; MacMillan, H.L. Preliminary evaluation of the childhood experiences of violence questionnaire short form. J. Interpers. Violence 2012, 27, 396–407. [Google Scholar] [CrossRef] [PubMed]
- Walsh, C.A.; MacMillan, H.L.; Trocmé, N.; Jamieson, E.; Boyle, M.H. Measurement of victimization in adolescence: Development and validation of the Childhood Experiences of Violence Questionnaire. Child Abus. Negl. 2008, 32, 1037–1057. [Google Scholar] [CrossRef] [PubMed]
- McQueen, M.B.; Boardman, J.D.; Domingue, B.W.; Smolen, A.; Tabor, J.; Killeya-Jones, L.; Halpern, C.T.; Whitsel, E.A.; Harris, K.M. The National Longitudinal Study of Adolescent to Adult Health (Add Health) sibling pairs genome-wide data. Behav. Genet. 2015, 45, 12–23. [Google Scholar] [CrossRef]
- Joshi, D.; Raina, P.; Tonmyr, L.; MacMillan, H.L.; Gonzalez, A. Prevalence of adverse childhood experiences among individuals aged 45 to 85 years: A cross-sectional analysis of the Canadian Longitudinal Study on Aging. CMAJ Open 2021, 9, E158–E166. [Google Scholar] [CrossRef]
- Papadopoulou, S.K.; Tsintavis, P.; Potsaki, G.; Papandreou, D. Differences in the Prevalence of Sarcopenia in Community-Dwelling, Nursing Home and Hospitalized Individuals. A Systematic Review and Meta-Analysis. J. Nutr. Health Aging 2020, 24, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.E.; Chen, E.; Parker, K.J. Psychological stress in childhood and susceptibility to the chronic diseases of aging: Moving toward a model of behavioral and biological mechanisms. Psychol. Bull. 2011, 137, 959–997. [Google Scholar] [CrossRef] [PubMed]
- Baumeister, D.; Akhtar, R.; Ciufolini, S.; Pariante, C.M.; Mondelli, V. Childhood trauma and adulthood inflammation: A meta-analysis of peripheral C-reactive protein, interleukin-6 and tumour necrosis factor-α. Mol. Psychiatry 2016, 21, 642–649. [Google Scholar] [CrossRef]
- Raina, P.S.; Wolfson, C.; Kirkland, S.A.; Keshavarz, H.; Griffith, L.E.; Patterson, C.; Tuokko, H.; Penning, M.; Balion, C.M.; Hogan, D.; et al. Ascertainment of Chronic Diseases in the Canadian Longitudinal Study on Aging (CLSA), Systematic Review*. Can. J. Aging 2009, 28, 275–285. [Google Scholar] [CrossRef]
- Washburn, R.A.; Smith, K.W.; Jette, A.M.; Janney, C.A. The Physical Activity Scale for the Elderly (PASE): Development and evaluation. J. Clin. Epidemiol. 1993, 46, 153–162. [Google Scholar] [CrossRef]
- Hayes, A.F.; Preacher, K.J. Statistical mediation analysis with a multicategorical independent variable. Br. J. Math. Stat. Psychol. 2014, 67, 451–470. [Google Scholar] [CrossRef] [PubMed]
- Arts, M.H.L.; Collard, R.M.; Comijs, H.C.; Naudé, P.J.W.; Risselada, R.; Naarding, P.; Oude Voshaar, R.C. Relationship Between Physical Frailty and Low-Grade Inflammation in Late-Life Depression. J. Am. Geriatr. Soc. 2015, 63, 1652–1657. [Google Scholar] [CrossRef]
- Yang, G.; Cao, X.; Yu, J.; Li, X.; Zhang, L.; Zhang, J.; Ma, C.; Zhang, N.; Lu, Q.; Wu, C.; et al. Association of Childhood Adversity With Frailty and the Mediating Role of Unhealthy Lifestyle: A Lifespan Analysis. Am. J. Geriatr. Psychiatry Off. J. Am. Assoc. Geriatr. Psychiatry 2023, 32, 71–82. [Google Scholar] [CrossRef]
- Kuzminskaite, E.; Penninx, B.W.J.H.; van Harmelen, A.L.; Elzinga, B.M.; Hovens, J.G.F.M.; Vinkers, C.H. Childhood Trauma in Adult Depressive and Anxiety Disorders: An Integrated Review on Psychological and Biological Mechanisms in the NESDA Cohort. J. Affect. Disord. 2021, 283, 179–191. [Google Scholar] [CrossRef]
- Souama, C.; Lamers, F.; Milaneschi, Y.; Vinkers, C.H.; Defina, S.; Garvert, L.; Stein, F.; Woofenden, T.; Brosch, K.; Dannlowski, U.; et al. Depression, cardiometabolic disease, and their co-occurrence after childhood maltreatment: An individual participant data meta-analysis including over 200,000 participants. BMC Med. 2023, 21, 93. [Google Scholar] [CrossRef] [PubMed]
- Wielaard, I.; Stek, M.L.; Comijs, H.C.; Rhebergen, D. Reliability of retrospectiv reports on childhood abuse and its determinants in older adults during a 6-year follow-up. J. Psychiatr. Res. 2018, 105, 9–16. [Google Scholar] [CrossRef] [PubMed]
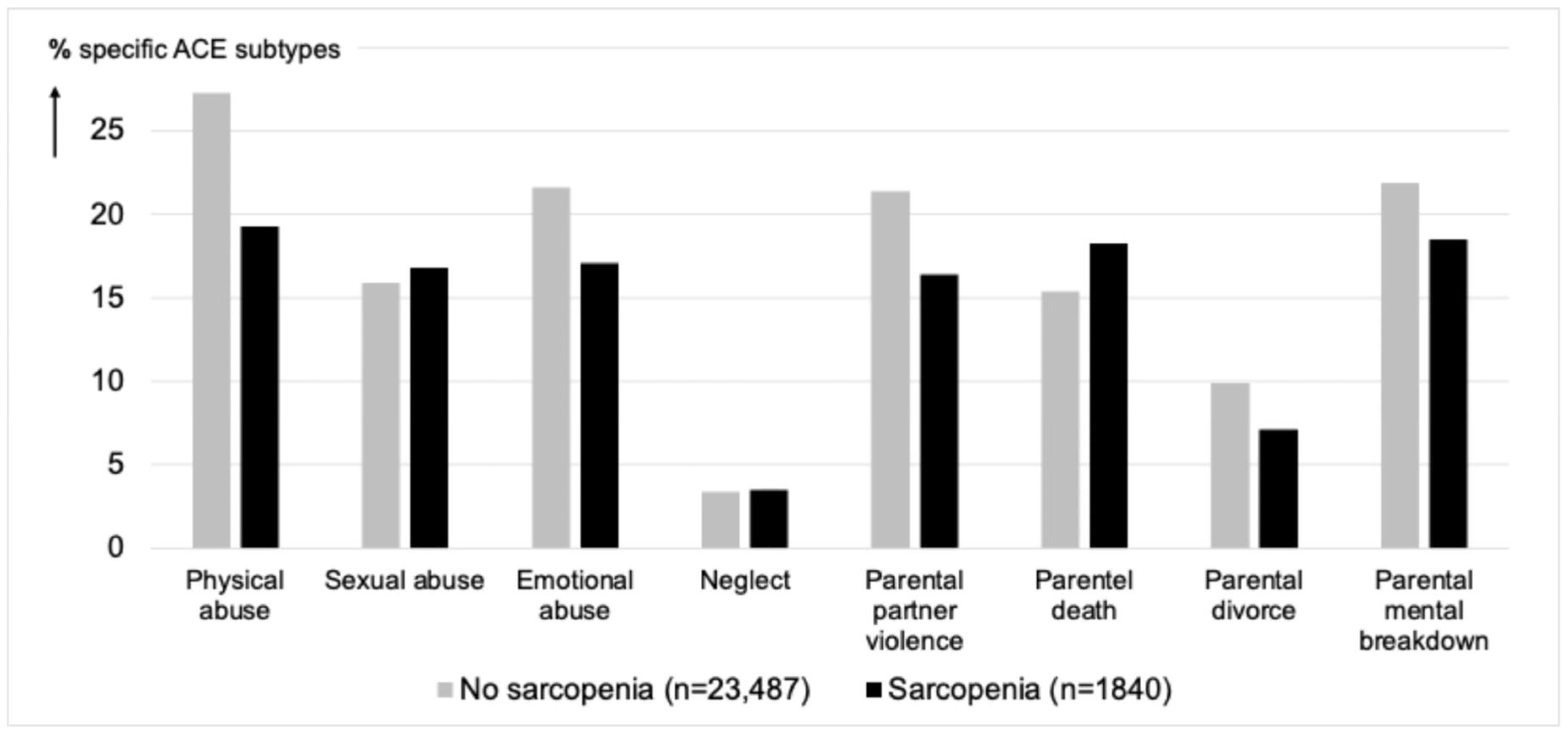
| Presence of Sarcopenia | ||||
|---|---|---|---|---|
| Characteristics | No (n = 23,487) | Yes (n = 1840) | Statistics | |
| Socio-demographics: | ||||
| mean (SD) | 61.8 (9.8) | 69.5 (10.2) | t = −32.5, df = 25,325, p < 0.001 |
| n (%) | 11,462 (48.8) | 1284 (69.8) | Chi2 = 300.5, df = 1, p < 0.001 |
| Chi2 = 146.4, df = 4, p < 0.001 | |||
| ◦ Low | n (%) | 4125 (17.6) | 366 (19.9) | |
| ◦ Middle | n (%) | 5001 (21.3) | 417 (22.7) | |
| ◦ Bachelor level | n (%) | 5812 (24.7) | 350 (19.0) | |
| ◦ Master level | n (%) | 5425 (23.1) | 308 (16.7) | |
| ◦ Missing | n (%) | 3124 (13.3) | 308 (21.7) | |
| Chi2 = 518.6, df = 3, p < 0.001 | |||
| ◦ Low | n (%) | 5302 (22.6) | 759 (41.3) | |
| ◦ Middle | n (%) | 7889 (33.6) | 599 (32.6) | |
| ◦ High | n (%) | 9002 (38.3) | 305 (16.6) | |
| ◦ Missing | n (%) | 1294 (5.5) | 177 (9.6) | |
| n (%) | 22,569 (96.1) | 1738 (94.5) | Chi2 = 11.8, df = 1, p < 0.001 |
| Independent variables: | ||||
| mean (SD) | 1.4 (1.5) | 1.2 (1.4) | t = 5.4, df = 25,325, p < 0.001 |
| Chi2 = 35.7, df = 3, p < 0.001 | |||
| ◦ No ACE | n (%) | 8742 (39.2) | 769 (43.6) | |
| ◦ One ACE | n (%) | 6425 (28.8) | 551 (31.2) | |
| ◦ Two ACEs | n (%) | 3592 (16.1) | 230 (13.0) | |
| ◦ Three or more ACEs | n (%) | 3540 (15.9) | 214 (12.1) | |
| Potential mediators: | ||||
| n (%) | 3683 (15.7) | 324 (17.7) | Chi2 = 4.7, df = 1, p = 0.031 |
| mean (SD) | 2.1 (1.8) | 2.8 (2.1) | T = −15.7, df = 23574, p < 0.001 |
| mean (SD) | 379.9 (98.4) | 341.0 (91.8) | t = 16.4, df = 25,325, p < 0.001 |
| mean (SD) | 2.4 (4.7) | 2.7 (6.3) | t = −2.1, df = 23,037, p = 0.102 |
| 45–54 Years Old (n = 6714) | 55–64 Years Old (n = 8560) | 65–74 Years Old (n = 6123) | 75+ Years Old (n = 3930) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR | [95% CI] | p | OR | [95% CI] | p | OR | [95% CI] | p | OR | [95% CI] | p | |
| Main model | ||||||||||||
| 0.99 | [0.90–1.07] | 0.737 | 0.99 | [0.93–1.06] | 0.791 | 0.97 | [0.91–1.04] | 0.419 | 0.93 | [0.86–1.00] | 0.043 |
| Sensitivity analysis: | ||||||||||||
| REF | 0.030 | REF | 0.481 | REF | 0.355 | REF | 0.032 | ||||
| 1.05 | [0.72–1.53] | 0.819 | 0.95 | [0.73–1.25] | 0.721 | 1.22 | [0.98–1.51] | 0.073 | 1.00 | [0.83–1.21] | 0.991 |
| 0.45 | [0.26–0.80] | 0.006 | 1.14 | [0.85–1.53] | 0.391 | 1.07 | [0.80–1.42] | 0.653 | 0.72 | [0.54–0.95] | 0.021 |
| 0.54 | [0.54–1.28] | 0.407 | 0.87 | [0.63–1.19] | 0.381 | 1.08 | [0.81–1.45] | 0.597 | 0.72 | [0.52–1.00] | 0.052 |
| 45–54 Years | 55–64 Years | 65–74 Years | 75+ Years | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ACE Subtypes | OR | [95% CI] | p | OR | [95% CI] | p | OR | [95% CI] | p | OR | [95% CI] | p |
| 1.00 | [0.77–1.30] | 0.986 | 1.00 | [0.77–1.30] | 0.986 | 0.82 | [0.64–1.06] | 0.130 | 0.63 | [0.49–0.82] | <0.001 |
| 1.06 | [0.82–1.38] | 0.641 | 1.06 | [0.82–1.38] | 0.641 | 0.91 | [0.70–1.17] | 0.451 | 1.24 | [0.96–1.61] | 0.106 |
| 1.06 | [0.80–1.42] | 0.679 | 1.06 | [0.80–1.42] | 0.679 | 0.99 | [0.75–1.32] | 0.961 | 1.04 | [0.76–1.41] | 0.809 |
| 0.91 | [0.54–1.53] | 0.708 | 0.91 | [0.54–1.53] | 0.708 | 1.12 | [0.67–1.88] | 0.664 | 1.11 | [0.65–1.91] | 0.702 |
| 0.92 | [0.69–1.23] | 0.586 | 0.92 | [0.69–1.23] | 0.586 | 0.93 | [0.70–1.23] | 0.606 | 0.83 | [0.60–1.14] | 0.240 |
| 0.87 | [0.65–1.17] | 0.353 | 0.87 | [0.65–1.17] | 0.353 | 1.12 | [0.88–1.41] | 0.366 | 1.13 | [0.92–1.39] | 0.231 |
| 1.11 | [0.78–1.59] | 0.553 | 1.11 | [0.78–1.59] | 0.553 | 0.82 | [0.55–1.23] | 0.343 | 0.64 | [0.42–0.96] | 0.032 |
| 0.95 | [0.74–1.22] | 0.947 | 0.95 | [0.74–1.22] | 0.673 | 1.25 | [0.99–1.59] | 0.061 | 1.08 | [0.83–1.40] | 0.587 |
| 45–54 Years Old (n = 6714) | 55–64 Years Old (n = 8560) | 65–74 Years Old (n = 6123) | 75+ Years Old (n = 3930) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR | [95% CI] | p | OR | [95% CI] | p | OR | [95% CI] | p | OR | [95% CI] | p | |
| Main model | ||||||||||||
| 1.02 | [0.98–1.06] | 0.364 | 1.02 | [0.98–1.05] | 0.344 | 0.98 | [0.95–1.02] | 0.388 | 0.99 | [0.94–1.05] | 0.770 |
| Sensitivity analysis: | ||||||||||||
| REF | 0.785 | REF | 0.873 | REF | 0.783 | REF | 0.353 | ||||
| 1.02 | [0.87–1.21] | 0.780 | 0.96 | [0.84–1.09] | 0.513 | 0.98 | [0.86–1.11] | 0.706 | 1.08 | [0.93–1.25] | 0.321 |
| 0.93 | [0.77–1.12] | 0.430 | 1.02 | [0.88–1.18] | 0.811 | 1.07 | [0.91–1.26] | 0.434 | 1.06 | [0.87–1.29] | 0.565 |
| 0.97 | [0.81–1.17] | 0.764 | 0.99 | [0.85–1.15] | 0.869 | 0.99 | [0.84–1.18] | 0.936 | 0.87 | [0.69–1.10] | 0.251 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dimitriadis, M.M.; Kokkeler, K.J.E.; Hoogendijk, E.O.; Marijnissen, R.M.; Aprahamian, I.; Jeuring, H.W.; Oude Voshaar, R.C. Adverse Childhood Experiences and Sarcopenia in Later Life: Baseline Data from the Canadian Longitudinal Study on Aging. Geriatrics 2025, 10, 111. https://doi.org/10.3390/geriatrics10040111
Dimitriadis MM, Kokkeler KJE, Hoogendijk EO, Marijnissen RM, Aprahamian I, Jeuring HW, Oude Voshaar RC. Adverse Childhood Experiences and Sarcopenia in Later Life: Baseline Data from the Canadian Longitudinal Study on Aging. Geriatrics. 2025; 10(4):111. https://doi.org/10.3390/geriatrics10040111
Chicago/Turabian StyleDimitriadis, Menelaos M., Kitty J. E. Kokkeler, Emiel O. Hoogendijk, Radboud M. Marijnissen, Ivan Aprahamian, Hans W. Jeuring, and Richard C. Oude Voshaar. 2025. "Adverse Childhood Experiences and Sarcopenia in Later Life: Baseline Data from the Canadian Longitudinal Study on Aging" Geriatrics 10, no. 4: 111. https://doi.org/10.3390/geriatrics10040111
APA StyleDimitriadis, M. M., Kokkeler, K. J. E., Hoogendijk, E. O., Marijnissen, R. M., Aprahamian, I., Jeuring, H. W., & Oude Voshaar, R. C. (2025). Adverse Childhood Experiences and Sarcopenia in Later Life: Baseline Data from the Canadian Longitudinal Study on Aging. Geriatrics, 10(4), 111. https://doi.org/10.3390/geriatrics10040111





