Abstract
Introduction: Pain is highly prevalent among community-dwelling older adults and can undermine their ability to perform Instrumental Activities of Daily Living (IADL), which are essential for independent living. This systematic review aimed to summarize existing research to clarify the relationship between pain and IADL disability in community-dwelling older adults. Methods: We conducted a search of PubMed on 27 July 2025. Eligible studies met the following criteria: (1) assessed the association between pain and IADL disability; (2) included community-dwelling older adults aged 60 and older; and (3) were published in English. Results: Of the 400 records screened, 29 studies met the inclusion criteria. Of these, 23 studies (18 cross-sectional and 5 cohort studies) reported a significant association between pain and IADL disability, while 6 cross-sectional studies did not. Pain was assessed using diverse instruments across varying recall periods and thresholds, and IADL disability was measured using multiple scales. Such methodological heterogeneity precluded quantitative synthesis. Conclusions: In community-dwelling older adults, pain consistently predicts IADL disability across designs and settings. However, the lack of standardized, multidimensional measures and incomplete adjustment for treatment, multimorbidity, and polypharmacy limits precise effect estimation. Future research should adopt harmonized assessment tools, control comprehensively for relevant confounders, and perform meta-analyses where data permit to clarify pain’s true impact on functional independence.
1. Introduction
Pain is highly prevalent among community-dwelling older adults. A global systematic review found the prevalence to be between 25 and 76% across community-dwelling older population [1]. Estimates in the United States range from 27.6 to 33.6% among noninstitutionalized older adults [2], and up to half report pain in at least one body region within the past month [3]. In the United Kingdom, prevalence ranges from 35.0 to 51.3% (pooled estimate 43.5%; 95% CI: 38.4–48.6%) and reaches 62% among those aged 75 and older [4]. Across Europe, prevalence among adults aged 50 and older varies between 30% and 60%, with annual increases of 2.2 to 5.8% from 2004 to 2015 [5]. In China, the prevalence of pain among people aged 45 years or older was 60% [6]. In Japan, 39.0% of independent older individuals report pain (men 36.3%, women 41.8%), with prevalence increasing with age [7].
Instrumental Activities of Daily Living (IADL) are a frequently used measure to assess the ability of individuals, including older adults living independently, to perform the activities of daily living necessary for independent living [8,9]. In the United States, 37.1% of adults aged 60 years or older experienced difficulty with at least one IADL [10]. In the United Kingdom, limitations in at least one IADL were reported by 18.3% of men and 24.5% of women [11]. Across Europe, 23.8% of individuals aged 65 years or older had 1 or more IADL impairments, with a higher prevalence among women (27.1%) compared to men (17.6%), and rates rising to 51.5% among those aged 85 years and above [12]. The prevalence was 32% in China among adults aged 65 or older [13], and 15.9% in Japan among those aged 75 or older, with 10.7% developing functional disability over a 24-month period [14]. A decline in IADLs is associated with various adverse health outcomes, including dementia [15], mild cognitive impairment [16], frailty [17], depression [18], and mortality [19]. Experiencing pain may negatively affect IADL function through limited physical activity [20,21], impaired mental health [22,23,24,25], impaired cognitive function [26,27,28], and decreased social participation [29,30]. Pain management and maintenance of IADL function are crucial for promoting healthy aging and reducing the burden on healthcare systems.
Persistent or multisite pain sharply increases the risk of new IADL limitations in community-dwelling older adults, with cohort studies reporting two- to three-fold higher odds of decline within 2–5 years [31]. Several mechanisms plausibly link pain to IADL disability. Persistent pain is associated with attentional and short-term memory deficits [26,32], making complex instrumental tasks especially vulnerable to decline [27,28]. Pain also heightens depressive symptoms [23,33] and leads to reduced physical activity and social participation [20,21,29], pathways that further erode functional independence [30]. Clarifying the relationship between specific pain characteristics and functional independence remains essential; the present review addresses this gap.
Although multiple observational studies have examined the association between pain and IADL disability among older adults, their findings have been fragmented. To date, no comprehensive synthesis of the available evidence has been conducted. Therefore, this systematic review aimed to summarize existing research to clarify the relationship between pain and IADL disability in community-dwelling older adults.
2. Materials and Methods
This systematic review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta Analyses (PRISMA) guidelines and was registered on the PROSPERO platform under the code CRD420251072156.
2.1. Search Strategy
A systematic literature search was conducted in the PubMed electronic database on 27 July 2025. The search included studies published up to that date. The following Medical Subject Headings (MeSH) and free-text terms were used in combination: “pain” [MeSH Terms] AND (“Activities of Daily Living”[MeSH Terms] OR “instrumental activities of daily living”[All Fields] OR “IADL” [All Fields]).
2.2. Eligibility Criteria
Studies were included if they met the following criteria: (1) assessed the association between pain and disability in instrumental activities of daily living (IADL); (2) included community-dwelling older adults aged 60 and older [34] who were able to provide self-reported data, which effectively excluded individuals who were bedridden, in terminal stages, or severely ill, and (3) were published in English.
2.3. Study Selection
The studies identified through the systematic review were independently screened by two researchers (YM and SU) based on their titles, abstracts, and full texts. Any discrepancies were resolved through discussion.
2.4. Data Extraction
Two reviewers (YM and SU) independently extracted data from the included studies. The following information was collected: first author, publication year, country, study design, sample size, mean age of participants, pain assessment (e.g., presence, location, severity), IADL assessment method, covariates adjusted for, statistical methods used, and key findings regarding the association between pain and IADL disability. Any disagreements were resolved through discussion.
2.5. Data Synthesis
Because pain definitions, IADL instruments, and analytical approaches varied greatly, we conducted a narrative synthesis. Each study was classified as showing a significant association, a non-significant association, or inconsistent findings, using p < 0.05 as the threshold for statistical significance. The findings were then summarized by study design and by pain dimension to provide a narrative subgroup synthesis.
2.6. Quality Assessment
The methodological quality of the included studies was not formally assessed, as the purpose of this review was to provide a descriptive summary of the existing literature rather than to perform a meta-analysis.
3. Results
3.1. Identification of Studies
A total of 400 studies were identified through the PubMed database. After screening titles and abstracts, 334 articles were excluded. The full texts of 66 articles were reviewed, and 29 studies met the inclusion criteria [23,24,25,28,31,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58]. The main reasons for the exclusion of full-text articles were as follows: the study did not assess the association between pain and IADL disability, the participants were under 60 years of age, the participants were not community-dwelling older adults, or the study population consisted of patients. The numbers for each exclusion reason are shown in Figure 1.
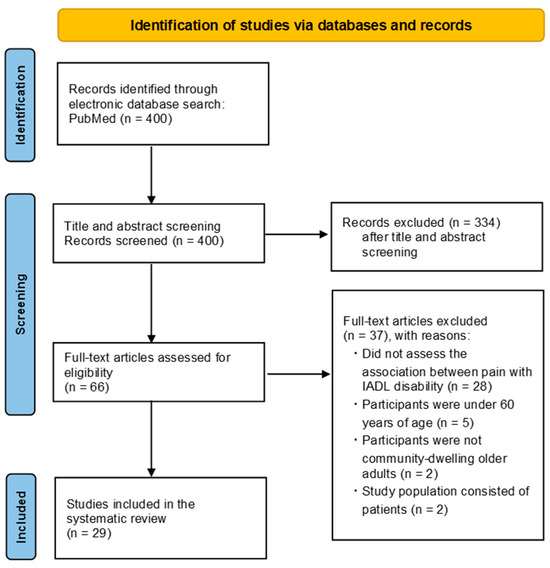
Figure 1.
Flow Diagram of the Study Selection Process.
3.2. Study Characteristics
Table 1, Table 2 and Table 3 summarize the characteristics of the included studies. 3 studies were published each in 2019 [31,46,47], 2014 [23,51,52], and 2010 [24,53,54]; 2 studies were published each in 2025 [35,36], 2024 [37,38], 2023 [39,40], 2021 [42,43], 2020 [44,45], and 2018 [48,49]; and 1 study was published each in 2022 [41], 2017 [28], 2016 [50], 2009 [55], 2006 [56], 2004 [25], 2000 [57], and 1992 [58]. 10 studies were conducted in the United States [23,31,37,38,48,51,52,53,55,57], 4 studies were conducted in China [25,35,41,45], 3 studies were conducted in Poland [36,46,49]; 2 studies each were conducted in Nigeria [40,56] and Canada [24,54]; and 1 study each was conducted in India [39], Australia [42], Sweden [43], Saudi Arabia [44], Spain [47], Ireland [28], Singapore [50], and France [58]. The sample sizes ranged from 171 [44] to 31,464 [39] (Table 1).

Table 1.
Characteristics of included studies.

Table 2.
Overview of pain assessment.

Table 3.
Association between pain and disability in instrumental activities of daily living (IADL).
3.3. Design
The included studies comprised both cohort [31,42,48,52,53] and cross-sectional designs [23,24,25,28,35,36,37,38,39,40,41,43,44,45,46,47,49,50,51,54,55,56,57,58] (Table 1).
3.4. Pain Assessment
3.4.1. Pain Presence Definition
The included studies used a variety of definitions and instruments to assess pain presence, such as pain scale [23,24,36,42,44,45,46,49,52,54,55,57], self-reported discomfort [35], bother [38] or trouble [25,28,39], pain restricting activity [48], pain present on most days [53], presence of pain without additional criteria [37,40,41,43,47,50,56,58], and pain quality [31] or pain location [51] (Table 2).
3.4.2. Pain Location
Pain location did not specify in some studies [28,31,36,37,39,40,45,46,47,49,57], while others reported specific location such as back, waist, buttocks, hips, thigh, legs, knees, feet, ankle, toes, shoulders, elbow, arms, hands, wrists, fingers, neck, head, generalized, face or tooth or jaw, stomach, abdominal, chest, body, spine, lower extremities, joints, muscles, and bones [23,24,25,35,38,41,42,43,44,48,50,51,52,53,54,55,56,58] (Table 2).
3.4.3. Pain Severity
Pain severity was assessed using several instruments, including the Visual Analog Scale [46,49,51], Verbal Descriptor Scale [23,24,54], Euroqol 5D quality of life assessment questionnaire pain scale [36], Short Form Health survey-12 [42], six-point Likert scale [44], Numeric Rating Scale [45], Brief Pain Inventory [52], Short Form Health survey-36 [55], and McGill Pain Questionnaire [57] (Table 2).
3.4.4. Pain Frequency/Quality
Pain frequency was categorized into four levels in one study [39], and pain quality was assessed using the MOBILIZE Boston Study pain quality instrument [31] in another study (Table 2).
3.5. IADL Assessment
IADL disability was assessed using a variety of scales, including the Lawton IADL scale [25,28,31,35,36,40,41,43,45,46,47,49,50,51,57,58], the Older Americans Resources and Services scale [24,53,54], the Rosow-Breslau IADL scale [42], the Nagi Physical Performance Scale and the Health Assessment Questionnaire [56], as well as a set of individual activities [23,37,38,39,44,48,52,55]. Response categories and scoring methods also varied, with some studies using dichotomized [24,28,35,38,39,40,41,42,45,46,47,48,49,50,52,53,56,58], or trichotomized [31,57] responses, and other evaluating total scores [23,25,36,37,43,44,51,54,55] (Table 3).
3.6. Confounders
A wide range of variables were adjusted for in the multivariable analysis: age [24,25,28,31,35,36,39,42,45,46,47,48,49,50,52,53,54,55,56,57,58], sex [31,35,36,39,45,47,48,50,52,53,54,55,56,57,58], education [24,28,31,35,36,39,45,47,48,49,50,52,53,54,55,58], marital status [28,36,39,45,50], living arrangements [28,39,48,50], work status [28,39,50], income [50], ethnicity or race [24,31,48,50,52], religion [39], place of residence [39,58], region [39], social group [39], and wealth quintiles [39]. Health-related factors included self-rated health [24,28,35,39,57], number of comorbidities [24,35,42,46,48,49,54,55,57], presence of chronic diseases [28,39,50,52], diabetes [31,50], hypertension [50], hyperlipidemia [50], lung disease [31], heart disease [31], vascular diseases [53], vascular risk factors [53], visual and hearing impairments [28,50,58], body mass index [28,31,42,45,50,52,53,55], depression [28,50,54,58], depressive symptoms [24,25,38,39,42,48,53,55], cognition [28,35,39,48,50,52,53,54,55], Mini-Mental State Examination score [31], self-rated memory [28], worry levels [28], social support [50], social contacts [46], social connectedness [28], loneliness [28], good relations with relatives [46], presence of barriers and obstacles [46], adaptation of the home environment [49], physical activity [28,31,35,39,42,46,49,52,53], exercise [40,45], restriction of habitual activity [47], bedridden status [47], frailty [48,55], history of falls [28,35,49,50], smoking [28,42], medication use (number of drugs or prescription medications) [28,36,42,52], assistive device use [49], time spent sitting [28], life satisfaction [35], and quality of life [28,49] (Table 3).
3.7. Statistical Analysis
15 studies were analyzed using the logistic regression [24,25,28,35,38,39,42,45,46,47,49,50,56,57,58]. 2 studies each were analyzed using the Poisson regression [31,52] and the Cox proportional hazards model [48,53]. 1 study each was analyzed using the Wald test [23], the Analysis of Variance [36], the Hierarchical regression [37], Fisher’s exact test [40], the chi-square test [41], the Mann–Whitney U test [43], the Kruskal–Wallis test [44], the t-test [51], the linear regression [54] and the negative binomial regression [55] (Table 3).
3.8. Synthesis of Results
A total of 29 studies were included in this review. Of these, 23 studies reported a significant association between pain and IADL disability; they consisted of 18 cross-sectional [23,24,35,36,37,38,39,41,44,45,46,47,49,50,51,54,55,56] and 5 cohort [31,42,48,52,53] studies, whereas the remaining 6 studies did not [25,28,40,43,57,58].
6 cross-sectional studies reported no significant relationship between pain presence and IADL disability [25,28,40,43,57,58]. In these studies, 3 defined pain presence simply as the occurrence of pain [40,43,58], 2 defined it as being troubled by pain [25,28], and 1 assessed pain presence using the McGill Pain Questionnaire pain scale [57]. In the studies reporting a significant association, pain presence was defined by a pain scale in 50% of the cross-sectional studies [23,24,36,44,45,46,49,54,55] and in 60% of the cohort studies [31,42,52]. Taken together, these observations provide a narrative summary, indicating that the association between pain and IADL disability is more consistently observed in cohort studies and in studies that defined pain with validated scales.
3.8.1. Association Between Pain Location and IADL Disability
5 studies that targeted 5 to 15 pain areas either classified participants into groups based on the number of pain location [35,38,41,52] or calculated the risk of IADL disability associated with each additional site [53]. 5 studies measured pain location by simply selecting 3 to 13 locations [25,42,50,56], or by using a pain map [23], and assessed IADL disability based on the presence of pain without considering the number of pain location. 5 studies measured body pain [24,44,54,55] or joint pain [58] without specifying exact locations; among these, 4 studies [24,44,54,55] evaluated pain severity using assessment scales. Overall, these 14 studies indicated that pain was significantly associated with IADL disability, except for 2 studies [25,58]. 2 studies measured pain at a specific location on the back [43,48]. Among these studies, 1 reported a significant association [48] while 1 reported no significant association [43]. 11 studies [28,31,36,37,39,40,45,46,47,49,57] that did not specify the pain location generally indicated a significant association between pain and IADL disability, except for 3 studies [28,40,57]. 1 study compared IADL scores between groups with pain at two locations (spinal pain versus those with lower extremity pain) [51] and found no significant difference between the groups.
3.8.2. Association Between Pain Severity and IADL Disability
9 studies did not use pain severity scales. Of these, 6 found that pain was significantly associated with IADL impairment [37,41,47,50,53,56], while 3 did not [40,43,58]. 5 studies evaluated pain severity by asking how discomfort [35], bothered [38] or troubled [25,28,39] participants were by pain; in 3 studies [35,38,39], a significant association was reported, while 2 studies [25,28] no significant association. 1 study defined pain severity as the restriction of activity due to pain [48], and found a significant association. 13 studies used pain severity scales [23,24,36,42,44,45,46,49,51,52,54,55,57]. Most of these studies reported a significant association between pain and IADL disability, except for 1 study [57]. 1 study found that a 1-point increase on the Visual Analog Scale was associated with 1.27 times higher odds of disability (95% CI: 1.22–1.33) [46], while another study reported that a 1-point increase was associated with a1.21 times higher odds of disability (95% CI: 1.06–1.36) [49].
3.8.3. Association Between Pain Frequency/Quality and IADL Disability
1 study categorized pain frequency into four levels and found that, compared with individuals without pain, the odds of IADL disability were 1.12 (95% CI: 1.02–1.23) for rare pain, 1.49 (95% CI: 1.38–1.61) for occasional pain, and 1.67 (95% CI: 1.53–1.82) for frequent pain [39]. 1 study measured pain quality by the MOBILIZE Boston Study and found that, at 18 months follow-up, individuals with 2 persistent pain qualities had a relative risk of 2.59 (95% CI: 1.10–6.09) and those with 3 had a relative risk of 2.69 (95% CI: 1.34–7.79) compared with 1 persistent pain quality [31].
4. Discussion
This systematic review synthesized findings from 29 studies investigating the association between pain and IADL disability in community-dwelling older adults. The majority of these studies (23 out of 29) reported a significant relationship, highlighting the considerable impact of pain on functional independence.
Several potential mechanisms may explain the observed association between pain and IADL dysfunction. First, persistent pain has been associated with memory decline [26], which in turn is linked to IADL disability [28]. Key features of cognitive dysfunction related to chronic pain include reduced attentional capacity and impaired short-term memory [32]. Pain may compete for limited cognitive resources and divert attention from cognitive tasks, especially in cases of severe pain or frequent rumination, leading to memory impairment due to incomplete encoding [26,32,59]. Given that IADL tasks require more complex neuropsychological processing than basic ADLs, these cognitive deficits make IADLs particularly vulnerable to decline [27]. Second, older adults with pain are more likely to experience depressive symptoms [23], which in turn are associated with an increased risk of IADL limitations [33]. Because IADL tasks place greater cognitive demands than basic ADLs or mobility tasks, this may partly explain the association between depressive symptoms and IADL limitations. Structural equation modeling suggests that depressive symptoms may impair IADL performance indirectly by reducing cognitive function [33]. Third, chronic pain is associated with reduced physical activity [20], which may lead to IADL impairment through mechanisms such as increased fatigue, decreased motivation, and cognitive decline [21,28]. It is also linked to social frailty, as older adults with pain are less likely to engage in social activities like going out or visiting friends [29]. In contrast, greater social participation is associated with lower IADL disability by encouraging daily instrumental activities, enhancing access to resources and health-related information, and reducing psychological stress through emotional support [30,60].
Pain measurement in the included studies had several systematic and methodological limitations. All 29 studies relied solely on self-reported scales without incorporating objective measures of pain or disability, which may have introduced recall and reporting bias and led to underestimation of the true pain burden. Because pain is inherently subjective and no standardized instrument exists, heterogeneity may have arisen from variation in question wording, the use of unvalidated tools with inconsistent recall periods (e.g., past 3 months vs. 4 weeks) [42]. Furthermore, simplifying explanatory variables into binary categories and using ordinal verbal descriptor scales [23] limit interpretability by obscuring differences between levels of severity. Limitations in existing studies include the inability to determine the temporal relationship between persistent pain and functional decline [31], missing information on pain location [24], and the absence of physiological indicators in tools like the Short Form Health survey-36 [55]. Self-reported pain is particularly prone to underreporting among older adults with cognitive impairment, who may struggle to perceive or articulate pain [61]. Non-standardized definitions further obscure prevalence: for example, merely altering the knee pain question wording changed prevalence from 19% to 28% in the same population [62], and extending the recall period from 3 to 6 months increased prevalence estimates by 30% [63]. If pain is under-recognized or inconsistently defined, its true impact on IADL function may be underestimated. Therefore, addressing this issue requires the use of standardized, validated, and objective tools capable of capturing the multidimensional nature of pain in older adults.
Although most studies adjusted for demographic and health covariates, only one [52] accounted for daily analgesic use, and none considered non-pharmacological treatments such as physical or occupational therapy. Similarly, only two studies adjusted for the number of prescription medications [42] or polypharmacy (defined as ≥5 medications) [28]. Regarding multimorbidity, 9 studies used a comorbidity count [24,35,42,46,48,49,54,55,57]. This limited adjustment likely results in residual confounding by overall health burden, as older adults with complex disease profiles are predisposed to both pain and IADL disability. Inadequate adjustment for factors such as pain treatment, polypharmacy, and disease clustering may bias current estimates of pain’s impact on IADL disability. The association could be overstated if untreated pain is unaccounted for, or understated if comorbidities are over-adjusted. Future research should gather detailed data on pain management, use comprehensive multimorbidity indices, and consider medication burden to better isolate pain’s independent contribution to IADL decline.
This review has several strengths. First, the review examined multiple dimensions of pain, including location, frequency, severity, quality, and presence, in relation to IADL disability. This approach provided a comprehensive understanding of how various aspects of pain may influence functional independence. Second, by incorporating both cross-sectional and cohort studies, the review captured concurrent associations as well as temporal relationships between pain and IADL decline. However, this review also has certain limitations. First, the search for relevant studies was limited to those published in English, which may have resulted in the exclusion of relevant studies published in other languages. Second, we did not conduct a formal risk-of-bias assessment or perform a quantitative meta-analysis. A meta-analysis was not feasible due to considerable heterogeneity among the 29 included studies. Pain was assessed using various instruments, with differing recall periods and thresholds. IADL disability was measured using a range of tools and categorized as dichotomous, trichotomous, or continuous outcomes. Effect estimates were reported in multiple formats, including odds ratios, relative risks, hazard ratios, and regression coefficients, and were often presented without sufficient variance measures. Additionally, the timing of pain and IADL assessments varied widely, and covariate adjustment differed considerably between studies. These methodological inconsistencies made it inappropriate to pool the data. Future research should apply standardized measurement tools, report effect estimates with accompanying variances and conduct structured quality appraisals. Third, this review was based on a literature search conducted using only PubMed. Although efforts were made to identify relevant studies through broad search terms and reference list screening, the possibility remains that some relevant studies indexed in other databases were not captured. Future reviews may benefit from incorporating additional databases such as Embase, CINAHL, PEDro, or Web of Science to enhance comprehensiveness and reduce the risk of publication bias. Fourth, the search for relevant studies was limited to those published in English, based on the recommendation of the Cochrane Handbook for Systematic Reviews of Interventions [64], which notes that excluding in other languages usually does not affect review conclusions. However, we acknowledge that this criterion may still have introduced language bias and could have resulted in the exclusion of pertinent non-English studies. Fifth, because IADLs are more complex than basic ADLs and require a certain level of independence, our findings may not be generalizable to older adults who are bedridden or have severe illnesses. Sixth, we did not conduct a formal methodological quality assessment of the included studies due to the diversity in study designs and outcomes. This may have introduced additional bias into our findings. Seventh, we were unable to conduct a quantitative subgroup meta-analysis owing to substantial heterogeneity in outcome scales and reported effect estimates. Eighth, our findings may not be generalizable to older adults who are bedridden, at the end of life, or severely ill, because the requirement for independent self-report effectively excluded these groups.
5. Conclusions
Pain is a consistent predictor of IADL disability in community-dwelling older adults. Despite variability in populations, pain definitions, and functional assessments, both cross-sectional and longitudinal data support this association. However, heterogeneity in measurement tools and limited adjustment for treatment, multimorbidity, and polypharmacy precluded meta-analysis and precise effect estimation. Future research should use standardized, multidimensional measures, account for relevant confounders, and conduct meta-analyses where possible to better determine pain’s impact on independent functioning in later life.
Author Contributions
Conceptualization, Y.M. and S.U.; methodology, Y.M. and S.U.; writing—original draft preparation, Y.M.; writing—review and editing, S.U.; supervision, S.U. All authors have read and agreed to the published version of the manuscript.
Funding
S.U. was funded by a Grant-in-Aid for Scientific Research (grant number 24K05554[CoBiA]) from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest, financial or otherwise.
References
- Abdulla, A.; Adams, N.; Bone, M.; Elliott, A.M.; Gaffin, J.; Jones, D.; Knaggs, R.; Martin, D.; Sampson, L.; Schofield, P. Guidance on the Management of Pain in Older People. Age Ageing 2013, 42, i1–i57. [Google Scholar] [CrossRef]
- Dahlhamer, J.; Lucas, J.; Zelaya, C.; Nahin, R.; Mackey, S.; DeBar, L.; Kerns, R.; Von Korff, M.; Porter, L.; Helmick, C. Prevalence of Chronic Pain and High-Impact Chronic Pain Among Adults—United States, 2016. Morb. Mortal. Wkly. Rep. 2018, 67, 1001–1006. [Google Scholar] [CrossRef]
- Patel, K.V.; Guralnik, J.M.; Dansie, E.J.; Turk, D.C. Prevalence and Impact of Pain among Older Adults in the United States: Findings from the 2011 National Health and Aging Trends Study. Pain 2013, 154, 2649–2657. [Google Scholar] [CrossRef]
- Fayaz, A.; Croft, P.; Langford, R.M.; Donaldson, L.J.; Jones, G.T. Prevalence of Chronic Pain in the UK: A Systematic Review and Meta-Analysis of Population Studies. BMJ Open 2016, 6, e010364. [Google Scholar] [CrossRef]
- Zimmer, Z.; Zajacova, A.; Grol-Prokopczyk, H. Trends in Pain Prevalence among Adults Aged 50 and Older across Europe, 2004 to 2015. J. Aging Health 2020, 32, 1419–1432. [Google Scholar] [CrossRef] [PubMed]
- Ai, Z.; Tang, C.; Peng, P.; Wen, X.; Tang, S. Prevalence and Influencing Factors of Chronic Pain in Middle-Aged and Older Adults in China: Results of a Nationally Representative Survey. Front. Public Health 2023, 11, 1110216. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.; Fujii, T.; Kubota, Y.; Ikeda, T.; Hanazato, M.; Kondo, N.; Matsudaira, K.; Kondo, K. Prevalence and Municipal Variation in Chronic Musculoskeletal Pain among Independent Older People: Data from the Japan Gerontological Evaluation Study (JAGES). BMC Musculoskelet. Disord. 2022, 23, 755. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.J.; Sapra, A. Instrumental Activity of Daily Living. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Hopkins, R.O.; Suchyta, M.R.; Kamdar, B.B.; Darowski, E.; Jackson, J.C.; Needham, D.M. Instrumental Activities of Daily Living after Critical Illness: A Systematic Review. Ann. Am. Thorac. Soc. 2017, 14, 1332–1343. [Google Scholar] [CrossRef]
- Germain, C.M.; Vasquez, E.; Batsis, J.A.; McQuoid, D.R. Sex, Race and Age Differences in Muscle Strength and Limitations in Community Dwelling Older Adults: Data from the Health and Retirement Survey (HRS). Arch. Gerontol. Geriatr. 2016, 65, 98–103. [Google Scholar] [CrossRef]
- Banks, J.; Batty, G.D.; Nazroo, J.; Oskala, A.; Steptoe, A. Evidence from the English Longitudinal Study of Ageing 2002–16 (Wave 8). 2018. Available online: https://www.elsa-project.ac.uk/wave-reports-and-tables (accessed on 25 April 2025).
- Portela, D.; Almada, M.; Midão, L.; Costa, E. Instrumental Activities of Daily Living (iADL) Limitations in Europe: An Assessment of SHARE Data. Int. J. Environ. Res. Public Health 2020, 17, 7387. [Google Scholar] [CrossRef]
- Zhang, X.; Dupre, M.E.; Qiu, L.; Zhou, W.; Zhao, Y.; Gu, D. Urban-Rural Differences in the Association between Access to Healthcare and Health Outcomes among Older Adults in China. BMC Geriatr. 2017, 17, 151. [Google Scholar] [CrossRef] [PubMed]
- Makino, K.; Lee, S.; Bae, S.; Shinkai, Y.; Chiba, I.; Shimada, H. Predictive Validity of a New Instrumental Activities of Daily Living Scale for Detecting the Incidence of Functional Disability among Community-Dwelling Older Japanese Adults: A Prospective Cohort Study. Int. J. Environ. Res. Public Health 2020, 17, 2291. [Google Scholar] [CrossRef] [PubMed]
- Pérès, K.; Helmer, C.; Amieva, H.; Orgogozo, J.-M.; Rouch, I.; Dartigues, J.-F.; Barberger-Gateau, P. Natural History of Decline in Instrumental Activities of Daily Living Performance over the 10 Years Preceding the Clinical Diagnosis of Dementia: A Prospective Population-Based Study. J. Am. Geriatr. Soc. 2008, 56, 37–44. [Google Scholar] [CrossRef]
- Jekel, K.; Damian, M.; Wattmo, C.; Hausner, L.; Bullock, R.; Connelly, P.J.; Dubois, B.; Eriksdotter, M.; Ewers, M.; Graessel, E.; et al. Mild Cognitive Impairment and Deficits in Instrumental Activities of Daily Living: A Systematic Review. Alzheimers Res. Ther. 2015, 7, 17. [Google Scholar] [CrossRef]
- Kojima, G. Frailty as a Predictor of Disabilities among Community-Dwelling Older People: A Systematic Review and Meta-Analysis. Disabil. Rehabil. 2017, 39, 1897–1908. [Google Scholar] [CrossRef]
- Kiyoshige, E.; Kabayama, M.; Gondo, Y.; Masui, Y.; Inagaki, H.; Ogawa, M.; Nakagawa, T.; Yasumoto, S.; Akasaka, H.; Sugimoto, K.; et al. Age Group Differences in Association between IADL Decline and Depressive Symptoms in Community-Dwelling Elderly. BMC Geriatr. 2019, 19, 309. [Google Scholar] [CrossRef]
- Millán-Calenti, J.C.; Tubío, J.; Pita-Fernández, S.; González-Abraldes, I.; Lorenzo, T.; Fernández-Arruty, T.; Maseda, A. Prevalence of Functional Disability in Activities of Daily Living (ADL), Instrumental Activities of Daily Living (IADL) and Associated Factors, as Predictors of Morbidity and Mortality. Arch. Gerontol. Geriatr. 2010, 50, 306–310. [Google Scholar] [CrossRef]
- Stubbs, B.; Binnekade, T.T.; Soundy, A.; Schofield, P.; Huijnen, I.P.J.; Eggermont, L.H.P. Are Older Adults with Chronic Musculoskeletal Pain Less Active than Older Adults Without Pain? A Systematic Review and Meta-Analysis. Pain Med. 2013, 14, 1316–1331. [Google Scholar] [CrossRef]
- Boyle, P.A.; Buchman, A.S.; Wilson, R.S.; Bienias, J.L.; Bennett, D.A. Physical Activity Is Associated with Incident Disability in Community-Based Older Persons. J. Am. Geriatr. Soc. 2007, 55, 195–201. [Google Scholar] [CrossRef]
- Muhammad, T.; Rashid, M. Prevalence and Correlates of Pain and Associated Depression among Community-Dwelling Older Adults: Cross-Sectional Findings from LASI, 2017–2018. Depress. Anxiety 2022, 39, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Shega, J.W.; Tiedt, A.D.; Grant, K.; Dale, W. Pain Measurement in the National Social Life, Health, and Aging Project: Presence, Intensity, and Location. J. Gerontol. B Psychol. Sci. Soc. Sci. 2014, 69, S191–S197. [Google Scholar] [CrossRef]
- Shega, J.W.; Ersek, M.; Herr, K.; Paice, J.A.; Rockwood, K.; Weiner, D.K.; Dale, W. The Multidimensional Experience of Noncancer Pain: Does Cognitive Status Matter? Pain Med. 2010, 11, 1680–1687. [Google Scholar] [CrossRef]
- Miu, D.; Chan, T.; Chan, M. Pain and Disability in a Group of Chinese Elderly Out-Patients in Hong Kong. Hong Kong Med. J. 2004, 10, 160–165. [Google Scholar] [PubMed]
- Whitlock, E.L.; Diaz-Ramirez, L.G.; Glymour, M.M.; Boscardin, W.J.; Covinsky, K.E.; Smith, A.K. Association Between Persistent Pain and Memory Decline and Dementia in a Longitudinal Cohort of Elders. JAMA Intern. Med. 2017, 177, 1146. [Google Scholar] [CrossRef] [PubMed]
- Njegovan, V.; Man-Son-Hing, M.; Mitchell, S.L.; Molnar, F.J. The Hierarchy of Functional Loss Associated With Cognitive Decline in Older Persons. J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, M638–M643. [Google Scholar] [CrossRef]
- Connolly, D.; Garvey, J.; McKee, G. Factors Associated with ADL/IADL Disability in Community Dwelling Older Adults in the Irish Longitudinal Study on Ageing (TILDA). Disabil. Rehabil. 2017, 39, 809–816. [Google Scholar] [CrossRef]
- Hirase, T.; Makizako, H.; Okubo, Y.; Lord, S.R.; Inokuchi, S.; Okita, M. Chronic Pain Is Independently Associated with Social Frailty in Community-dwelling Older Adults. Geriatr. Gerontol. Int. 2019, 19, 1153–1156. [Google Scholar] [CrossRef]
- Tomioka, K.; Kurumatani, N.; Hosoi, H. Association Between Social Participation and Instrumental Activities of Daily Living Among Community-Dwelling Older Adults. J. Epidemiol. 2016, 26, 553–561. [Google Scholar] [CrossRef]
- Thakral, M.; Shi, L.; Foust, J.B.; Patel, K.V.; Shmerling, R.H.; Bean, J.F.; Leveille, S.G. Persistent Pain Quality as a Novel Approach to Assessing Risk for Disability in Community-Dwelling Elders With Chronic Pain. J. Gerontol. Ser. A 2019, 74, 733–741. [Google Scholar] [CrossRef]
- Dick, B.D.; Rashiq, S. Disruption of Attention and Working Memory Traces in Individuals with Chronic Pain. Anesth. Analg. 2007, 104, 1223. [Google Scholar] [CrossRef]
- Hybels, C.F.; Pieper, C.F.; Blazer, D.G. The Complex Relationship between Depressive Symptoms and Functional Limitations in Community-Dwelling Older Adults: The Impact of Subthreshold Depression. Psychol. Med. 2009, 39, 1677–1688. [Google Scholar] [CrossRef]
- World Health Organization. Decade of Healthy Ageing: Baseline Report; World Health Organization: Geneva, Switzerland, 2020; ISBN 978-92-4-001790-0. [Google Scholar]
- Chu, J.; Weng, L.; Jin, W.; Yin, X.; Xu, Q.; Xu, Z. Pain Status and Disability in Activities of Daily Living Among Older Adults in China: Evidence from CHARLS 2020. Pain Res. Manag. 2025, 2025, 4974163. [Google Scholar] [CrossRef]
- Balicki, P.; Sołtysik, B.K.; Borowiak, E.; Kostka, T.; Kostka, J. Activities of Daily Living Limitations in Relation to the Presence of Pain in Community-Dwelling Older Adults. Sci. Rep. 2025, 15, 15027. [Google Scholar] [CrossRef] [PubMed]
- Ord, A.S.; Coddington, K.; Maksad, G.P.; Swiatek, S.R.; Saunders, J.; Netz, D.; Washburn, D.; Braud, S.; Holland, J.; Eldridge, A.H.; et al. Neuropsychological Symptoms and Functional Capacity in Older Adults with Chronic Pain. Gerontol. Geriatr. Med. 2024, 10, 23337214241307537. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Chen, H.; Tong, B.; Zhu, D.; Cong, X.; Shang, S. Association between Multisite Musculoskeletal Pain and Disability Trajectories among Community-Dwelling Older Adults. Aging Clin. Exp. Res. 2024, 36, 115. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, T.; Rashid, M.; Zanwar, P.P. Examining the Association of Pain and Pain Frequency With Self-Reported Difficulty in Activities of Daily Living and Instrumental Activities of Daily Living Among Community-Dwelling Older Adults: Findings from the Longitudinal Aging Study in India. J. Gerontol. Ser. B 2023, 78, 1545–1554. [Google Scholar] [CrossRef]
- Olawumi, A.L.; Grema, B.A.; Suleiman, A.K.; Michael, G.C.; Umar, Z.A.; Damagum, F.M.; Haruna, A.I.; Abdulkadir, Z.; Kwaku, A. Socio-Economic and Lifestyle Determinants of Functional Capacity of the Senior Attendees of an Outpatient Clinic in Northern Nigeria: A Cross-Sectional Study. WEST Afr. J. Med. 2023, 40, 581–589. [Google Scholar]
- Lu, Z.; Ye, P.; Er, Y.; Zhan, Y.; Deng, X.; Duan, L. Body Pain and Functional Disability Predict Falls in Chinese Older Adults: A Population-Based Cohort Study. Aging Clin. Exp. Res. 2022, 34, 2515–2523. [Google Scholar] [CrossRef]
- Scott, D.; Blyth, F.; Naganathan, V.; Le Couteur, D.G.; Handelsman, D.J.; Seibel, M.J.; Waite, L.M.; Hirani, V. Prospective Associations of Chronic and Intrusive Pain with Sarcopenia and Physical Disability amongst Older Australian Men: The Concord Health and Ageing in Men Project. Exp. Gerontol. 2021, 153, 111501. [Google Scholar] [CrossRef]
- Svensson, H.K.; Karlsson, J.; Sterner, T.R.; Ahlner, F.; Skoog, I.; Erhag, H.F. Self-Perceived Functional Ability and Performance-Based Testing of Physical Function in Older Women with or without Long-Term Back Pain—Results of the H70 Study. BMC Geriatr. 2021, 21, 229. [Google Scholar] [CrossRef]
- Al-Qahtani, A.M. Health Status and Functional Abilities of Elderly Males Visiting Primary Health-Care Centers in Khamis Mushait, Saudi Arabia. Clin. Interv. Aging 2020, 15, 2129–2143. [Google Scholar] [CrossRef]
- Peng, X.; Bao, X.; Xie, Y.; Zhang, X.; Huang, J.; Liu, Y.; Cheng, M.; Liu, N.; Wang, P. The Mediating Effect of Pain on the Association between Multimorbidity and Disability and Impaired Physical Performance among Community-Dwelling Older Adults in Southern China. Aging Clin. Exp. Res. 2020, 32, 1327–1334. [Google Scholar] [CrossRef]
- Ćwirlej-Sozańska, A.; Wiśniowska-Szurlej, A.; Wilmowska-Pietruszyńska, A.; Sozański, B. Determinants of ADL and IADL Disability in Older Adults in Southeastern Poland. BMC Geriatr. 2019, 19, 297. [Google Scholar] [CrossRef]
- Carmona-Torres, J.M.; Rodríguez-Borrego, M.A.; Laredo-Aguilera, J.A.; López-Soto, P.J.; Santacruz-Salas, E.; Cobo-Cuenca, A.I. Disability for Basic and Instrumental Activities of Daily Living in Older Individuals. PLoS ONE 2019, 14, e0220157. [Google Scholar] [CrossRef]
- Makris, U.E.; Weinreich, M.A.; Fraenkel, L.; Han, L.; Leo-Summers, L.; Gill, T.M. Restricting Back Pain and Subsequent Disability in Activities of Daily Living Among Community-Living Older Adults. J. Aging Health 2018, 30, 1482–1494. [Google Scholar] [CrossRef] [PubMed]
- Ćwirlej-Sozańska, A.; Sozański, B.; Wiśniowska-Szurlej, A.; Wilmowska-Pietruszyńska, A. An Assessment of Factors Related to Disability in ADL and IADL in Elderly Inhabitants of Rural Areas of South-Eastern Poland. Ann. Agric. Environ. Med. 2018, 25, 504–511. [Google Scholar] [CrossRef]
- Liang En, W.; Sin, D.; Wen Qi, C.; Zong Chen, L.; Shibli, S.; Choon-Huat Koh, G. Chronic Pain in a Low Socioeconomic Status Population in Singapore: A Cross-Sectional Study. Pain Med. 2016, 17, 864–876. [Google Scholar] [CrossRef] [PubMed]
- Yağci, N.; Duymaz, T.; Cavlak, U. How Does Pain Localization Affect Physical Functioning, Emotional Status and Independency in Older Adults with Chronic Musculoskeletal Pain? J. Phys. Ther. Sci. 2014, 26, 1189–1192. [Google Scholar] [CrossRef]
- Eggermont, L.H.P.; Leveille, S.G.; Shi, L.; Kiely, D.K.; Shmerling, R.H.; Jones, R.N.; Guralnik, J.M.; Bean, J.F. Pain Characteristics Associated with the Onset of Disability in Older Adults: The Maintenance of Balance, Independent Living, Intellect, and Zest in the Elderly Boston Study. J. Am. Geriatr. Soc. 2014, 62, 1007–1016. [Google Scholar] [CrossRef]
- Buchman, A.S.; Shah, R.C.; Leurgans, S.E.; Boyle, P.A.; Wilson, R.S.; Bennett, D.A. Musculoskeletal Pain and Incident Disability in Community-dwelling Older Adults. Arthritis Care Res. 2010, 62, 1287–1293. [Google Scholar] [CrossRef] [PubMed]
- Shega, J.W.; Weiner, D.K.; Paice, J.A.; Bilir, S.P.; Rockwood, K.; Herr, K.; Ersek, M.; Emanuel, L.; Dale, W. The Association Between Noncancer Pain, Cognitive Impairment, and Functional Disability: An Analysis of the Canadian Study of Health and Aging. J. Gerontol. A Biol. Sci. Med. Sci. 2010, 65A, 880–886. [Google Scholar] [CrossRef]
- Weaver, G.D.; Kuo, Y.; Raji, M.A.; Al Snih, S.; Ray, L.; Torres, E.; Ottenbacher, K.J. Pain and Disability in Mexican-American Older Adults. J. Am. Geriatr. Soc. 2009, 57, 992–999. [Google Scholar] [CrossRef] [PubMed]
- Gureje, O.; Ogunniyi, A.; Kola, L.; Afolabi, E. Functional Disability in Elderly Nigerians: Results from the Ibadan Study of Aging. J. Am. Geriatr. Soc. 2006, 54, 1784–1789. [Google Scholar] [CrossRef]
- Mossey, J.M.; Gallagher, R.M.; Tirumalasetti, F. The Effects of Pain and Depression on Physical Functioning in Elderly Residents of a Continuing Care Retirement Community. Pain Med. 2000, 1, 340–350. [Google Scholar] [CrossRef] [PubMed]
- Barberger-Gateau, P.; Chaslerie, A.; Dartigues, J.F.; Commenges, D.; Gagnon, M.; Salamon, R. Health Measures Correlates in a French Elderly Community Population: The PAQUID Study. J. Gerontol. 1992, 47, S88–S95. [Google Scholar] [CrossRef]
- van der Leeuw, G.; Eggermont, L.H.P.; Shi, L.; Milberg, W.P.; Gross, A.L.; Hausdorff, J.M.; Bean, J.F.; Leveille, S.G. Pain and Cognitive Function Among Older Adults Living in the Community. J. Gerontol. A Biol. Sci. Med. Sci. 2016, 71, 398–405. [Google Scholar] [CrossRef]
- Cohen, S. Social Relationships and Health. Am. Psychol. 2004, 59, 676–684. [Google Scholar] [CrossRef]
- Stubbs, B.; Thompson, T.; Solmi, M.; Vancampfort, D.; Sergi, G.; Luchini, C.; Veronese, N. Is Pain Sensitivity Altered in People with Alzheimer’s Disease? A Systematic Review and Meta-Analysis of Experimental Pain Research. Exp. Gerontol. 2016, 82, 30–38. [Google Scholar] [CrossRef]
- O’Reilly, S.C.; Muir, K.R.; Doherty, M. Screening for Pain in Knee Osteoarthritis: Which Question? Ann. Rheum. Dis. 1996, 55, 931–933. [Google Scholar] [CrossRef] [PubMed]
- Sá, K.N.; Moreira, L.; Baptista, A.F.; Yeng, L.T.; Teixeira, M.J.; Galhardoni, R.; de Andrade, D.C. Prevalence of Chronic Pain in Developing Countries: Systematic Review and Meta-Analysis. Pain Rep. 2019, 4, e779. [Google Scholar] [CrossRef]
- Lefebvre, C.; Glanville, J.; Briscoe, S.; Featherstone, R.; Littlewood, A.; Metzendorf, M.-I.; Noel-Storr, A.; Paynter, R.; Rader, T.; Thomas, J.; et al. Section 4.4.5 Language, date and document format restrictions [last updated March 2025]. In Cochrane Handbook for Systematic Reviews of Interventions Version 6.5.1; Higgins, J.P.T., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M.J., Welch, V.A., Eds.; Cochrane: Calgary, AB, Canada, 2025; Available online: https://www.cochrane.org/authors/handbooks-and-manuals/handbook/current/chapter-04#section-4-4-5 (accessed on 3 June 2025).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).