Abstract
Objectives: This study aimed to investigate the relationship between osteoporosis and cerebral small vessel disease (CSVD) burden in stroke-free individuals, as well as its specific imaging markers, including lacunes, enlarged perivascular spaces (EPVSs), white matter hyperintensities (WMHs), and brain atrophy (BA). Methods: A total of 684 stroke-free patients who underwent both bone mineral density (BMD) assessments and brain MRI were included. Clinical data, CSVD burden scores, imaging markers of CSVD, and bone density parameters were collected. Logistic regression models were used to evaluate the relationship between BMD and CSVD burden and imaging markers. Results: Osteoporosis, including hip and vertebral osteoporosis, was independently associated with CSVD burden (OR = 2.332, 95%CI: [1.345, 4.039], p = 0.003; OR = 2.598, 95%CI: [1.540, 4.384], p < 0.001; OR = 1.515, 95%CI: [1.010, 2.272], p = 0.044). Increased BMD in the hip and spine correlated with reduced CSVD burden (OR = 0.929, 95%CI: [0.887, 0.972], p = 0.001; OR = 0.952, 95%CI: [0.917, 0.989], p = 0.012). Hip osteoporosis was a risk factor for lacunes (OR = 2.215, 95%CI: [1.197, 4.1], p = 0.011), multiple lacunes (OR = 2.274, 95%CI: [1.039, 4.980], p = 0.04), severe WMH (OR = 2.611, 95%CI: [1.171, 5.823], p = 0.019), and EPVS ≥ 2 (OR = 1.99, 95%CI: [1.133, 3.495], p = 0.017). No significant association was found between osteoporosis and BA (p = 0.928). In sex-stratified analyses, both hip and vertebral osteoporosis were independently associated with a higher CSVD burden in female patients (hip: OR = 2.529, 95%CI: [1.122, 5.703], p = 0.025; vertebral: OR = 3.129, 95%CI: [1.517, 6.455], p = 0.002; general osteoporosis: OR = 1.755, 95%CI: [1.057, 2.912], p = 0.03), whereas no significant association was observed in male patients (all p > 0.05). Conclusions: Osteoporosis was independently associated with an increased burden of CSVD, particularly evident in female patients. These findings suggest that bone health may be important in CSVD management, particularly for women.
1. Introduction
Cerebral small vessel disease (CSVD) is a group of disorders marked by abnormalities in the cerebral small vessels. Characteristic magnetic resonance imaging (MRI) features are used to define CSVD, including lacunes, enlarged perivascular spaces (EPVSs), cerebral microbleeds (CMBs), white matter hyperintensities (WMHs), and brain atrophy (BA). CSVD causes about 25% of ischemic strokes, most vascular dementia, and most intracerebral hemorrhages in the elderly over 65, and is associated with motor impairment, gait apraxia, neurobehavioral abnormalities, and mood disorders [1]. Previous research has identified a range of conventional vascular risk factors for CSVD [2]. However, despite extensive studies in this area, the biological mechanisms underlying CSVD remain insufficiently understood [3,4,5]. Thus, identifying new biomarkers could significantly enhance our understanding of CSVD, laying the groundwork for advances in clinical management.
Osteoporosis, a systemic bone disease characterized by low bone mass and deterioration of bone tissue microarchitecture, leading to increased bone fragility and a higher risk of fractures [6], is linked to cardiovascular diseases [7,8,9,10], including coronary artery disease and carotid atherosclerosis. Moreover, evidence suggests that osteoporosis is related to neurological disorders [11,12,13,14,15], such as Alzheimer’s and Parkinson’s disease. Therefore, we hypothesized that osteoporosis might be associated with CSVD. Only a few studies have explored the relationship between osteoporosis and CSVD. Existing studies have primarily focused on stroke populations [16] or examined only specific CSVD features in healthy individuals. Research in non-stroke populations has linked low bone mineral density to various brain structural changes, including gray matter atrophy [17], brain parenchymal atrophy [18], ventricular enlargement [19], and white matter hyperintensities [20]. However, there is currently a lack of comprehensive studies investigating the overall association between osteoporosis and CSVD burden in stroke-free individuals. Given the importance of early prevention, our study aims to fill this gap by systematically assessing the relationship between osteoporosis and both total CSVD burden and its specific imaging markers. We aim to provide new evidence on the connection between osteoporosis and CSVD. Understanding this link will help us gain a more comprehensive understanding of the pathophysiology of these diseases, ultimately leading to more effective prevention and treatment approaches.
2. Materials and Methods
2.1. Study Participants and Data Collection
We retrospectively included patients aged 50 years and older who underwent both brain MRI and BMD assessments at Zhongshan Hospital Affiliated to Xiamen University between 2015 and 2024. Exclusion criteria were as follows: (1) neurological disorders or structural brain abnormalities (e.g., acute or prior cerebral infarction, cerebral hemorrhage, traumatic brain injury, intracranial mass lesions, history of brain surgery, encephalitis, cerebral vasculitis, cerebral vascular malformations, or multiple sclerosis); (2) connective tissue diseases or conditions associated with secondary osteoporosis (e.g., systemic lupus erythematosus, rheumatoid arthritis, multiple myeloma, primary hyperparathyroidism, or long-term corticosteroid use); (3) malignancies (e.g., breast, lung, or colorectal cancer, or other active cancers); (4) severe comorbidities or systemic conditions (e.g., severe hepatic or renal insufficiency, recent infections, or other serious illnesses); (5) poor imaging quality; and (6) incomplete data. In total, 684 patients were ultimately included, as illustrated in the patient selection flowchart (Figure 1). The study population primarily consisted of (1) individuals undergoing routine health check-ups, who opted for both tests as part of a comprehensive evaluation of bone and cerebrovascular health; (2) patients with symptoms such as lower back pain, dizziness, or balance issues, prompting DXA or brain MRI for further assessment; and (3) hospitalized or outpatient individuals for whom physicians recommended both examinations due to risk factors like advanced age, hypertension, or diabetes.
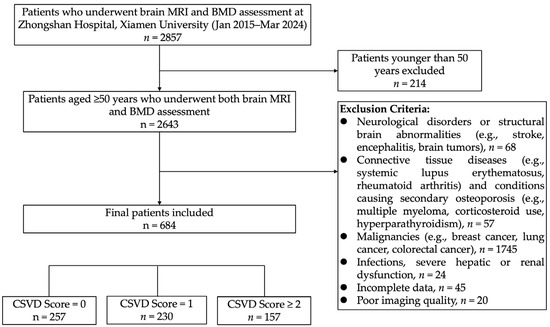
Figure 1.
Flowchart of participant selection for this study.
We collected baseline data from the Hospital Information System, including demographic characteristics, medical history (diabetes, hypertension, dyslipidemia, coronary heart disease (CHD), atrial fibrillation (AF), peripheral artery disease (PAD), hyperuricemia), blood laboratory parameters (white blood cell count (WBC), red blood cell count (RBC), hemoglobin (Hb), low-density lipoprotein cholesterol (LDL-C), triglycerides (TGs), total cholesterol (TC), fasting plasma glucose (FPG), alanine aminotransferase (ALT), estimated glomerular filtration rate (eGFR), C-reactive protein (CRP), D dimer (DD), total protein (TP), albumin (Alb), fibrinogen (Fbg), etc.), current smoking and alcohol consumption status, body mass index (BMI), and systolic and diastolic blood pressures (SBP and DBP).
2.2. Measurement of CSVD
Brain MRI sequences included T1-weighted images (T1WI), T2-weighted images (T2WI), fluid-attenuated inversion recovery (FLAIR), diffusion-weighted imaging (DWI), and susceptibility-weighted imaging (SWI). The field of view (FOV) was 240 mm × 240 mm, with a matrix size of 320 × 256. Coronal and transaxial views of T1WI and T2WI and transaxial views of T2-FLAIR, DWI, and SWI were collected. All MRI data were obtained from the Picture Archiving and Communication Systems and independently reviewed by an experienced chief radiologist and a senior neurologist with extensive experience in cerebrovascular diseases. Both evaluators were blinded to the clinical information of the subjects. Any discrepancies in interpretation were resolved through discussion and consensus.
All neuroimaging markers of CSVD, including lacunes, WMH, EPVS, CMBs, and BA, were strictly diagnosed according to the Standards for Reporting Vascular Changes on Neuroimaging (STRIVE) guidelines [21,22]. Lacunes were defined as round or oval lesions of cerebrospinal fluid signal on T1WI and T2WI measuring 3 to 20 mm in diameter, always with a hyperintense ring on FLAIR. WMH was defined as abnormal signals of varying sizes in periventricular and deep white matter regions, which were hyperintense on T2WI and FLAIR and isointense or hypointense on T1WI. The severity of periventricular (PVWMH) and deep WMH (DWMH) were assessed according to the Fazekas scale from 0 to 3 [23]. EPVS appeared as small (<3 mm) punctate or linear structures with CSF signal intensity on T1WI, T2WI, and FLAIR in the centrum semiovale (CS-EPVS) and basal ganglia (BG-EPVS). The severity of EPVS in both regions was assessed separately by a previously validated grading system (0–4) [24], and the final grade was determined by the worse of the two. CMBs were defined as round lesions less than 10 mm in diameter with hypointensity on SWI. A visual cortical atrophy rating scale (0–3) was used to assess whole-brain cortical atrophy. Due to the low number of participants rated as Grade 3 (only 4 cases) in this study, these were combined with Grade 2 for analysis.
Finally, the total burden score of CSVD was assessed according to the STRIVE-1 criteria: 1 point was assigned for a DWMH score of 2 or a PVWMH score of 3; 1 point for a CS-EPVS or BG-EPVS score of 2 or higher; 1 point for the presence of CMB; and 1 point for the presence of a lacune. The sum of these four items yielded the total CSVD burden score, ranging from 0 to 4 points, with higher scores indicating a more severe burden of CSVD. For research purposes, we further categorized the CSVD burden into three grades: Grade 0 (0 points), Grade 1 (1 point), and Grade 2 (2–4 points).
2.3. BMD Measurement
Dual-energy X-ray Absorptiometry (DXA) is the gold standard for diagnosing osteoporosis. We used a DXA bone densitometer (Lunar Prodigy, GE Healthcare, Madison, WI, USA) to measure BMD at the lumbar spine (L1–L4) and the hip. The diagnosis was based on the corresponding T-scores: normal bone mass was defined as a T-score of −1.0 or higher; osteopenia was defined as a T-score between −1.0 and −2.5; and osteoporosis was defined as a T-score of −2.5 or lower. A patient was diagnosed with osteoporosis if the BMD at either the lumbar spine or the hip met the criteria for osteoporosis. If both sites showed normal BMD, the patient was classified in the normal group. Patients who did not meet the criteria for normal BMD or osteoporosis were classified in the osteopenia group.
2.4. Statistical Analysis
SPSS 21.0 statistical software was used for data analysis. Continuous variables following a normal distribution were expressed as mean ± standard deviation (SD) and compared among groups using independent-sample t-tests or analysis of variance (ANOVA). Non-normally distributed continuous variables were expressed as the median and interquartile range (IQR), with group comparisons made using non-parametric tests. Categorical variables were expressed as frequencies and percentages, and comparisons among groups were conducted using the chi-square test.
The inter-rater reliability of CSVD imaging marker assessments was evaluated using Cohen’s kappa statistic, with values greater than 0.8 indicating a high level of agreement. Ordered and binary logistic regression models were utilized to assess the relationship between BMD, osteoporosis, and both the overall CSVD burden and individual CSVD imaging features. In the ordered logistic regression model, common odds ratios (ORs) and 95% confidence intervals (CIs) were estimated using two models. Model 1 was unadjusted for covariates. In Model 2, adjustments were made for variables showing intergroup differences in univariate analyses, including age, SBP, TP, LDL-C, TG, FPG, ALT, eGFR, WBC count, RBC count, CRP, Fbg, gender, hypertension, PAD, AF, and CHD. Despite the absence of significant intergroup differences in diabetes, it was included due to its well-known impact on vascular health. Binary variables (PAD, AF, CHD, and diabetes) were coded as 1 = yes, and 0 = no. Osteoporosis status was reverse-coded (0 = osteoporosis, 1 = osteopenia, and 2 = normal) to allow for a more intuitive interpretation of the associations. To ensure the validity of the multivariable models, multicollinearity was assessed using variance inflation factors (VIFs). All included variables had VIF values less than 5, indicating no significant collinearity. For other CSVD imaging features, binary or multinomial logistic regression analyses were performed, with the OR and 95%CI reported. Similarly, Model 1 remained unadjusted for covariates, while Model 2 was adjusted for variables that exhibited intergroup differences in univariate analyses.
3. Results
This study included a total of 684 participants. The inter-rater reliability for CSVD imaging markers was high, with Cohen’s kappa values for brain atrophy, estimated lacunes, periventricular WMH, deep WMH, EPVS, and CMBs being 0.931, 0.914, 0.949, 0.940, 0.959, and 0.832, respectively, indicating strong agreement. The CSVD burden scores were distributed as follows: 257 participants with a score of 0 (CSVD = 0 group), 230 with a score of 1 (CSVD = 1 group), and 197 with scores of 2 or 3 (CSVD = 2 group). Among these participants, 90 had normal bone density, 288 had osteopenia, and 306 were diagnosed with osteoporosis. The number of hip osteoporosis cases was 239, and vertebral osteoporosis cases totaled 216. The participants had an average age of 68.4 years (SD = 10.3), with 222 males (32.5%) and 462 females (67.5%).
Table 1 compared the clinical characteristics and laboratory indicators across groups based on total CSVD burden scores. An increase in the total CSVD burden score was significantly associated with higher levels of age, SBP, FPG, WBC count, CRP, Fbg, and DD (p < 0.05). Conversely, TP, Alb, ALT, TC, HDL-C, LDL-C, eGFR, and RBC count decreased significantly (p < 0.05). As the total CSVD burden score increased, the proportions of osteoporosis, hip osteoporosis, and vertebral osteoporosis rose, while the proportions of normal bone mass and osteopenia in the hip and vertebrae decreased. Additionally, a heavier CSVD burden was associated with lower BMD values in both the hip and vertebrae (p < 0.05). TG levels also varied significantly across the three groups. Moreover, the proportions of patients with hypertension, PAD, AF, and CHD were higher in the groups with a greater CSVD burden score (p < 0.05).

Table 1.
Demographic and clinical characteristics according to the CSVD burden.
On multivariable ordinal logistic regression analysis (Table 2), osteoporosis (β = 0.847, OR = 2.332, 95%CI: [1.345, 4.039], p = 0.003), hip osteopenia (β = 0.690, OR = 1.787, 95%CI: [1.125, 2.806], p = 0.014), hip osteoporosis (β = 0.955, OR = 2.598, 95%CI: [1.540, 4.384], p < 0.001), and vertebral osteoporosis (β = 0.416, OR = 1.515, 95%CI: [1.010, 2.272], p = 0.044) were significantly and independently associated with a greater CSVD burden (reference: CSVD = 0). Higher BMD at the hip (β = −0.073, OR = 0.929, 95%CI: [0.887, 0.972], p = 0.001) and at the vertebrae (β = −0.049, OR = 0.952, 95%CI: [0.917, 0.989], p = 0.012) was associated with lower odds of increased CSVD burden.

Table 2.
Association between osteoporosis and CSVD burden.
In sex-stratified analyses (Table 3), both hip and vertebral osteoporosis were independently associated with a higher CSVD burden in female patients (hip: OR = 2.529, 95%CI: [1.122, 5.703], p = 0.025; spine: OR = 3.129, 95%CI: [1.517, 6.455], p = 0.002; general osteoporosis: OR = 1.755, 95%CI: [1.057, 2.912], p = 0.03), and increased hip and vertebral BMD were correlated with lower odds of increased CSVD burden (OR = 0.907, 95%CI: [0.854, 0.963], p = 0.001; OR = 0.944, 95%CI: [0.899, 0.993], p = 0.025, respectively). In contrast, no significant association was observed in male patients (hip: OR = 1.964, 95%CI: [0.801, 4.816], p = 0.140; spine: OR = 1.625, 95%CI: [0.732,3.603], p = 0.232; general osteoporosis: OR = 2.332, 95%CI: [0.981, 5.540], p = 0.055), and changes in hip and vertebral BMD were not significantly associated with CSVD burden (OR = 0.973, 95%CI: [0.903, 1.050], p = 0.491; OR = 0.959, 95%CI: [0.901, 1.021], p = 0.197, respectively).

Table 3.
Sex-stratified analysis of the association between osteoporosis and CSVD burden.
We performed logistic regression analyses on various imaging markers of CSVD, including lacunes, EPVS, WMH, and BA. As shown in Table 4, the results indicated that hip osteoporosis was an independent risk factor for lacunes (β = 0.795, OR = 2.215, 95%CI: [1.197, 4.1], p = 0.011), multiple lacunes (β = 0.822, OR = 2.274, 95%CI: [1.039, 4.98], p = 0.04), and severe WMH (β = 0.96, OR = 2.611, 95%CI: [1.171, 5.823], p = 0.019). Osteoporosis was independently associated with grade 2–4 EPVS (β = 0.799, OR = 2.222, 95%CI: [1.234, 4.004], p = 0.008). Additionally, hip osteopenia, hip osteoporosis, vertebral osteopenia, and vertebral osteoporosis were each independently associated with grade 2–4 EPVS (β = 0.551, OR = 1.735, 95%CI: [1.058, 2.844], p = 0.029; β = 0.688, OR = 1.99, 95%CI: [1.133, 3.495], p = 0.017; β = 0.415, OR = 1.514, 95%CI: [1.004, 2.284], p = 0.048; β = 0.502, OR = 1.652, 95%CI: [1.075, 2.538], p = 0.022). However, no significant association was found between BMD and BA (p > 0.05).

Table 4.
Association between osteoporosis and CSVD imaging features.
4. Discussion
Our study examined the relationship between osteoporosis and the burden of CSVD, including various imaging features of CSVD. While BA was analyzed, it was not found to be associated with osteoporosis. The findings underscore a significant association between osteoporosis and the overall CSVD burden, suggesting that bone health may influence cerebrovascular integrity. Subgroup analysis further revealed that this association was significant in female patients but not in males, indicating a potential sex-specific relationship. Given the observational nature of this study, causal inference is limited, and further longitudinal and interventional studies are needed to determine whether managing osteoporosis can causally impact CSVD risk.
4.1. Association Between Osteoporosis and CSVD Burden
The positive correlation between osteoporosis and increased CSVD burden observed in this study aligns with previous research suggesting that BMD and cerebrovascular health are interconnected [16,20]. Osteoporosis, characterized by reduced bone mass and microarchitectural deterioration, may reflect broader systemic processes that also affect cerebral vasculature. The pathophysiological mechanisms underlying this association may involve shared risk factors [25,26,27] such as aging, diabetes [27], and chronic inflammation, which can simultaneously contribute to bone loss and cerebrovascular damage [2]. Additionally, reduced BMD may indicate impaired calcium homeostasis and vitamin D deficiency, both of which have been implicated in endothelial dysfunction and increased vascular stiffness [28,29], leading to CSVD.
Our study found that even after adjusting for factors such as age, hypertension, and diabetes, the impact of osteoporosis on CSVD remained significant. This suggests that osteoporosis may influence cerebral small vessels through additional mechanisms. The underlying mechanisms of the association between osteoporosis and CSVD are not well elucidated; we propose that the influence of osteoporosis on CSVD primarily involves “bone–vascular crosstalk” and “bone–brain interaction”.
Firstly, the skeleton acts as an atypical endocrine organ. Osteocytes secrete various regulatory factors, such as sclerostin and osteoprotegerin (OPG), which play crucial roles in the interaction between bone, vasculature, and the brain. Sclerostin may disrupt the Wnt/β-catenin pathway in the brain, potentially contributing to aging and the progression of Alzheimer’s disease [11]. OPG, released by osteoblasts to inhibit bone resorption, also plays a significant role in the development of vascular wall inflammation and endothelial dysfunction [30]. Elevated OPG levels are associated with an increased risk of cardiovascular events [31]. Additionally, studies suggest that other proteins secreted by osteocytes, such as lipocalin-2 and fibroblast growth factor 23, may influence neuroinflammation, synaptic plasticity, and neuronal degeneration [32]. Aging-related bone factors may promote the development of atherosclerosis by affecting vascular smooth muscle cells [33]. These findings further reveal the link between osteoporosis, the central nervous system, and vascular diseases.
Furthermore, as a key site for stem cell maintenance and hematopoiesis, bone marrow provides endothelial progenitor cells (EPCs) and inflammatory cells. Research has found that aging-related extracellular vesicles (AB-EVs) from the bone matrix can infiltrate vascular tissues, promoting a bone–fat imbalance and vascular calcification [34]. We hypothesize that osteoporosis may disrupt the bone marrow microenvironment, thereby affecting stem cells critical for vascular repair and contributing to the progression of CSVD.
Finally, a recent study revealed that a loss-of-function mutation in the ARHGEF15 gene may inhibit the Wnt/β-catenin signaling pathway in osteoblasts, leading to dysfunction in vascular smooth muscle cells and osteoblasts [35]. This dysfunction manifests as CSVD accompanied by osteoporotic fractures, suggesting a potential shared genetic basis between osteoporosis and CSVD.
Importantly, our sex-stratified analysis revealed that the association between osteoporosis and CSVD burden was significant in female patients but not in males. This sex-specific disparity may be explained by the pivotal role of estrogen in maintaining both bone and vascular health [36]. In women, especially postmenopausal individuals, the abrupt decline in estrogen—a hormone known for its vasoprotective and neuroprotective properties [37]—can accelerate bone resorption while simultaneously promoting endothelial dysfunction [38], microvascular inflammation [39], and increased arterial stiffness. These processes may contribute to heightened susceptibility to CSVD. In contrast, men experience a more gradual decline in sex hormones, which may result in a lesser impact on the bone–vascular axis. Additionally, differences in body composition [40] and physical activity patterns, as well as the potential underdiagnosis of osteoporosis in men [41], may contribute to the observed disparities and warrant further investigation.
These findings indicate that it may be important to consider sex as a biological variable when studying the bone–brain axis, and suggest that postmenopausal women with osteoporosis could represent a particularly vulnerable group for CSVD-related brain changes. Future research should aim to elucidate the role of estrogen signaling in this interaction and assess whether sex-specific therapeutic strategies can mitigate cerebrovascular risk in osteoporotic populations.
4.2. Osteoporosis and Specific CSVD Imaging Markers
Our logistic regression analysis further revealed that hip osteoporosis is an independent risk factor for the presence of a lacune, multiple lacunes, and severe WMH. This is particularly noteworthy as lacunes and WMH are well-established markers of CSVD, associated with cognitive decline and an increased stroke risk [42,43,44]. The protective role of increased hip BMD against lacunes and severe WMH emphasizes the importance of maintaining bone health to potentially mitigate CSVD progression.
Additionally, the dose–response relationship observed between osteoporosis (including hip and vertebral osteoporosis) and higher grades of EPVS suggests that reduced bone density may contribute to the pathological expansion of EPVS. This relationship highlights the multifaceted nature of CSVD, where different imaging markers may be influenced by bone health through distinct mechanisms.
Interestingly, no significant association was found between BMD and brain atrophy in our study, diverging from previous reports [17,18,19] suggesting a link between osteoporosis and cortical thinning or brain volume reduction. This discrepancy may be due to differences in study populations or diagnostic criteria. Additionally, brain atrophy may lack specificity as a marker of CSVD, given that it can result from a range of other conditions. Beyond neurodegenerative disorders such as Alzheimer’s disease and chronic alcohol use, contributing factors include vitamin B12 deficiency, which has been linked to neurodegeneration and reduced brain volume. Moreover, age-related physiological brain volume loss further complicates the interpretation of its relationship with CSVD. However, the association between osteoporosis and the overall CSVD burden suggests that bone health may exert a cumulative effect on multiple CSVD subtypes. Further research is needed to clarify the relationship between osteoporosis and BA, especially in diverse cohorts and using more sensitive imaging modalities.
The notably low incidence of CMBs observed in our study differs from previous research. This could be due to our study population, which included non-stroke individuals, some from general health check-up groups. Consequently, the risk of hemorrhagic transformation was likely lower, and antiplatelet medication use was less common, contributing to the reduced CMB occurrence.
Despite this study’s merits, such as thorough imaging evaluations and meticulous clinical and laboratory data collection, several limitations should be acknowledged. The retrospective, hospital-based design may limit the generalizability of our findings to the broader population, as selection bias cannot be excluded. Additionally, the modest sample size constrains the statistical power and external validity, underscoring the need for multicenter validations. The cross-sectional nature of this study precludes causal inferences, making it unclear whether osteoporosis contributes to increased CSVD burden or vice versa. Residual confounding from unmeasured variables also cannot be ruled out. Moreover, we did not exclude individuals with a history of fractures, which may have influenced bone mineral density independently of osteoporosis and introduced additional variability. Furthermore, the exclusion of bone-related biomarkers, such as vitamin D and osteocalcin, due to incomplete data, hampers a deeper understanding of the mechanisms linking osteoporosis with cerebrovascular disease. Additionally, some odds ratios had relatively wide confidence intervals, suggesting potential estimate variability due to the modest sample size or population heterogeneity. Future studies with larger, more diverse cohorts and refined analyses are needed to validate these findings and enhance estimate precision.
These findings carry important clinical implications, suggesting that osteoporosis management may play a role in preventing or slowing the progression of CSVD. With the increasing recognition of CSVD as a major contributor to cognitive decline and stroke, interventions aimed at improving bone health—such as calcium and vitamin D supplementation, weight-bearing exercises, and pharmacological treatments for osteoporosis—could have the added benefit of preserving both bone and cerebrovascular health.
5. Conclusions
Our study demonstrates a significant association between osteoporosis and an increased CSVD burden, including specific CSVD imaging markers, with the exception of BA. This association with total CSVD burden was particularly evident in female patients, indicating a potential sex-related difference. These results suggest a potential link between osteoporosis and increased CSVD burden, particularly in women, warranting further investigation into the underlying mechanisms.
Author Contributions
Conceptualization, X.X. and C.S.; data curation, J.L. and J.C.; formal analysis, L.C. and M.D.; investigation, J.L. and J.C.; methodology, X.X. and C.S.; project administration, X.X. and L.C.; supervision, L.C. and M.D.; visualization, X.X. and C.S.; writing—original draft, X.X.; writing—review and editing, X.X. and C.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki, and approved by the Scientific Subcommittee of the Medical Ethics Committee of Zhongshan Hospital Xiamen University (protocol code 2024-081, date 24 May 2024).
Informed Consent Statement
Patient consent was waived due to de-identification of data.
Data Availability Statement
The datasets analyzed in this study are available from the corresponding author upon reasonable request.
Acknowledgments
The authors thank all colleagues and reviewers for their support and valuable feedback during the preparation of this manuscript. Special thanks to Xiaoqiang Hong from Xiamen University for his invaluable advice during the writing process.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| CSVD | Cerebral Small Vessel Disease |
| EPVSs | Enlarged Perivascular Spaces |
| WMHs | White Matter Hyperintensities |
| BA | Brain Atrophy |
| BMD | Bone Mineral Density |
| CHD | Coronary Heart Disease |
| AF | Atrial Fibrillation |
| PAD | Peripheral Artery Disease |
| BMI | Body Mass Index |
| SBP | Systolic Blood Pressure |
| DBP | Diastolic Blood Pressure |
| WBC | White Blood Cell Count |
| RBC | Red Blood Cell Count |
| Hb | Hemoglobin |
| LDL-C | Low-Density Lipoprotein Cholesterol |
| TGs | Triglycerides |
| TC | Total Cholesterol |
| FPG | Fasting Plasma Glucose |
| ALT | Alanine Aminotransferase |
| eGFR | Estimated Glomerular Filtration Rate |
| CRP | C-Reactive Protein |
| DD | D-Dimer |
| TP | Total Protein |
| Alb | Albumin |
| Fbg | Fibrinogen |
| AST | Aspartate Aminotransferase |
| ALP | Alkaline Phosphatase |
| HDL-C | High-Density Lipoprotein Cholesterol |
| UA | Uric Acid |
References
- Wardlaw, J.M.; Smith, C.; Dichgans, M. Small vessel disease: Mechanisms and clinical implications. Lancet Neurol. 2019, 18, 684–696. [Google Scholar] [CrossRef] [PubMed]
- Staals, J.; Makin, S.D.; Doubal, F.N.; Dennis, M.S.; Wardlaw, J.M. Stroke subtype, vascular risk factors, and total MRI brain small-vessel disease burden. Neurology 2014, 83, 1228–1234. [Google Scholar] [CrossRef] [PubMed]
- Cuadrado-Godia, E.; Dwivedi, P.; Sharma, S.; Ois Santiago, A.; Roquer Gonzalez, J.; Balcells, M.; Laird, J.; Turk, M.; Suri, H.S.; Nicolaides, A.; et al. Cerebral Small Vessel Disease: A Review Focusing on Pathophysiology, Biomarkers, and Machine Learning Strategies. J. Stroke 2018, 20, 302–320. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Su, Y.; Fu, J.; Shen, Y.; Dong, Q.; Cheng, X. Glymphatic pathway in sporadic cerebral small vessel diseases: From bench to bedside. Ageing Res. Rev. 2023, 86, 101885. [Google Scholar] [CrossRef]
- Kumar, A.A.; Yeo, N.; Whittaker, M.; Attra, P.; Barrick, T.R.; Bridges, L.R.; Dickson, D.W.; Esiri, M.M.; Farris, C.W.; Graham, D.; et al. Vascular Collagen Type-IV in Hypertension and Cerebral Small Vessel Disease. Stroke 2022, 53, 3696–3705. [Google Scholar] [CrossRef]
- Consensus development conference: Diagnosis, prophylaxis, and treatment of osteoporosis. Am. J. Med. 1993, 94, 646–650. [CrossRef]
- Hu, X.; Ma, S.; Chen, L.; Tian, C.; Wang, W. Association between osteoporosis and cardiovascular disease in elderly people: Evidence from a retrospective study. PeerJ 2023, 11, e16546. [Google Scholar] [CrossRef]
- Geng, G.; Li, Z.; Wang, S.; Yuan, T.; Quan, G. Association between bone mineral density and coronary plaque burden in patients with coronary artery disease: A cross-sectional study using quantitative computed tomography. Coron. Artery Dis. 2024, 35, 105–113. [Google Scholar] [CrossRef]
- Han, S.; Kim, N.R.; Kang, J.W.; Eun, J.S.; Kang, Y.M. Radial BMD and serum CTX-I can predict the progression of carotid plaque in rheumatoid arthritis: A 3-year prospective cohort study. Arthritis Res. Ther. 2021, 23, 258. [Google Scholar] [CrossRef]
- Liu, D.; Chen, L.; Dong, S.; Peng, Z.; Yang, H.; Chen, Y.; Li, L.; Zhou, H.; Zhou, R. Bone mass density and bone metabolism marker are associated with progression of carotid and cardiac calcified plaque in Chinese elderly population. Osteoporos. Int. 2019, 30, 1807–1815. [Google Scholar] [CrossRef]
- Shi, T.; Shen, S.; Shi, Y.; Wang, Q.; Zhang, G.; Lin, J.; Chen, J.; Bai, F.; Zhang, L.; Wang, Y.; et al. Osteocyte-derived sclerostin impairs cognitive function during ageing and Alzheimer’s disease progression. Nat. Metab. 2024, 6, 531–549. [Google Scholar] [CrossRef] [PubMed]
- Dauphinot, V.; Laurent, M.; Prodel, M.; Civet, A.; Vainchtock, A.; Moutet, C.; Krolak-Salmon, P.; Garnier-Crussard, A. Identification of profiles associated with conversions between the Alzheimer’s disease stages, using a machine learning approach. Alzheimers Res. Ther. 2024, 16, 166. [Google Scholar] [CrossRef]
- Tang, A.S.; Rankin, K.P.; Cerono, G.; Miramontes, S.; Mills, H.; Roger, J.; Zeng, B.; Nelson, C.; Soman, K.; Woldemariam, S.; et al. Leveraging electronic health records and knowledge networks for Alzheimer’s disease prediction and sex-specific biological insights. Nat. Aging 2024, 4, 379–395. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.H.; Huang, Y.P.; Yeh, K.C.; Pan, S.L. Osteoporosis and the Risk of Parkinson’s Disease: A Nationwide, Propensity Score-Matched, Longitudinal Follow-up Study. J. Clin. Endocrinol. Metab. 2021, 106, e763–e771. [Google Scholar] [CrossRef] [PubMed]
- Park, K.Y.; Jung, J.H.; Hwang, H.S.; Park, H.K.; Han, K.; Nam, G.E. Bone Mineral Density and the Risk of Parkinson’s Disease in Postmenopausal Women. Mov. Disord. 2023, 38, 1606–1614. [Google Scholar] [CrossRef]
- Kim, J.M.; Park, K.Y.; Kim, H.R.; Ahn, H.Y.; Pantoni, L.; Park, M.S.; Han, S.H.; Jung, H.B.; Bae, J. Association of Bone Mineral Density to Cerebral Small Vessel Disease Burden. Neurology 2021, 96, e1290–e1300. [Google Scholar] [CrossRef]
- Kalc, P.; Dahnke, R.; Hoffstaedter, F.; Gaser, C. Low bone mineral density is associated with gray matter volume decrease in UK Biobank. Front. Aging Neurosci. 2023, 15, 1287304. [Google Scholar] [CrossRef]
- Pan, H.; Cao, J.; Wu, C.; Huang, F.; Wu, P.; Lang, J.; Liu, Y. Osteoporosis is associated with elevated baseline cerebrospinal fluid biomarkers and accelerated brain structural atrophy among older people. Front. Aging Neurosci. 2022, 14, 958050. [Google Scholar] [CrossRef]
- Bae, I.S.; Kim, J.M.; Cheong, J.H.; Ryu, J.I.; Han, M.H. Association between bone mineral density and brain parenchymal atrophy and ventricular enlargement in healthy individuals. Aging 2019, 11, 8217–8238. [Google Scholar] [CrossRef]
- Li, X.; Wu, X.; Zhou, G.; Mo, D.; Lin, X.; Wang, P.; Zeng, Y.; Luo, M. Estimated bone mineral density and white matter hyperintensities: A bidirectional Mendelian randomization study. Bone 2024, 187, 117138. [Google Scholar] [CrossRef]
- Duering, M.; Biessels, G.J.; Brodtmann, A.; Chen, C.; Cordonnier, C.; de Leeuw, F.E.; Debette, S.; Frayne, R.; Jouvent, E.; Rost, N.S.; et al. Neuroimaging standards for research into small vessel disease-advances since 2013. Lancet Neurol. 2023, 22, 602–618. [Google Scholar] [CrossRef]
- Wardlaw, J.M.; Smith, E.E.; Biessels, G.J.; Cordonnier, C.; Fazekas, F.; Frayne, R.; Lindley, R.I.; O’Brien, J.T.; Barkhof, F.; Benavente, O.R.; et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013, 12, 822–838. [Google Scholar] [CrossRef]
- Fazekas, F.; Chawluk, J.B.; Alavi, A.; Hurtig, H.I.; Zimmerman, R.A. MR signal abnormalities at 1.5 T in Alzheimer’s dementia and normal aging. AJR Am. J. Roentgenol. 1987, 149, 351–356. [Google Scholar] [CrossRef]
- Doubal, F.N.; MacLullich, A.M.; Ferguson, K.J.; Dennis, M.S.; Wardlaw, J.M. Enlarged perivascular spaces on MRI are a feature of cerebral small vessel disease. Stroke 2010, 41, 450–454. [Google Scholar] [CrossRef] [PubMed]
- Gregson, C.L.; Armstrong, D.J.; Bowden, J.; Cooper, C.; Edwards, J.; Gittoes, N.J.L.; Harvey, N.; Kanis, J.; Leyland, S.; Low, R.; et al. UK clinical guideline for the prevention and treatment of osteoporosis. Arch. Osteoporos. 2022, 17, 58. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Wang, Y.; Wang, H.; Wang, L.; Huan, B.; Liu, C. Correlation study: Bone density and circulating inflammatory markers in postmenopausal patients. Immun. Inflamm. Dis. 2024, 12, e1365. [Google Scholar] [CrossRef]
- Leungsuwan, D.S.; Chandran, M. Bone Fragility in Diabetes and its Management: A Narrative Review. Drugs 2024, 84, 1111–1134. [Google Scholar] [CrossRef] [PubMed]
- Croll, P.H.; Boelens, M.; Vernooij, M.W.; van de Rest, O.; Zillikens, M.C.; Ikram, M.A.; Voortman, T. Associations of vitamin D deficiency with MRI markers of brain health in a community sample. Clin. Nutr. 2021, 40, 72–78. [Google Scholar] [CrossRef]
- Chung, P.W.; Park, K.Y.; Kim, J.M.; Shin, D.W.; Park, M.S.; Chung, Y.J.; Ha, S.Y.; Ahn, S.W.; Shin, H.W.; Kim, Y.B.; et al. 25-hydroxyvitamin D status is associated with chronic cerebral small vessel disease. Stroke 2015, 46, 248–251. [Google Scholar] [CrossRef]
- Dutka, M.; Garczorz, W.; Kosowska, A.; Buczek, E.; Godek, P.; Wojakowski, W.; Francuz, T. Osteoprotegerin Is Essential for the Development of Endothelial Dysfunction Induced by Angiotensin II in Mice. Int. J. Mol. Sci. 2024, 25, 6434. [Google Scholar] [CrossRef]
- Tschiderer, L.; Klingenschmid, G.; Nagrani, R.; Willeit, J.; Laukkanen, J.A.; Schett, G.; Kiechl, S.; Willeit, P. Osteoprotegerin and Cardiovascular Events in High-Risk Populations: Meta-Analysis of 19 Prospective Studies Involving 27,450 Participants. J. Am. Heart Assoc. 2018, 7, e009012. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.T.; Liu, M.H.; Xiong, Y.; Wang, Y.J.; Bu, X.L. Crosstalk between bone and brain in Alzheimer’s disease: Mechanisms, applications, and perspectives. Alzheimers Dement. 2024, 20, 5720–5739. [Google Scholar] [CrossRef]
- Liang, W.; Wei, T.; Hu, L.; Chen, M.; Tong, L.; Zhou, W.; Duan, X.; Zhao, X.; Zhou, W.; Jiang, Q.; et al. An integrated multi-omics analysis reveals osteokines involved in global regulation. Cell Metab. 2024, 36, 1144–1163.e1147. [Google Scholar] [CrossRef]
- Wang, Z.X.; Luo, Z.W.; Li, F.X.; Cao, J.; Rao, S.S.; Liu, Y.W.; Wang, Y.Y.; Zhu, G.Q.; Gong, J.S.; Zou, J.T.; et al. Aged bone matrix-derived extracellular vesicles as a messenger for calcification paradox. Nat. Commun. 2022, 13, 1453. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Chen, Y.; Guo, C.; Fu, Y.; Qin, C.; Zhu, Q.; Wang, J.; Zhang, R.; Tian, H.; Feng, R.; et al. Mutations in ARHGEF15 cause autosomal dominant hereditary cerebral small vessel disease and osteoporotic fracture. Acta Neuropathol. 2023, 145, 681–705. [Google Scholar] [CrossRef]
- Camon, C.; Garratt, M.; Correa, S.M. Exploring the effects of estrogen deficiency and aging on organismal homeostasis during menopause. Nat. Aging 2024, 4, 1731–1744. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.T.; Wang, Z.J.; Cai, H.Y.; Yuan, L.; Hu, M.M.; Wu, M.N.; Qi, J.S. Sex Differences in Neuropathology and Cognitive Behavior in APP/PS1/tau Triple-Transgenic Mouse Model of Alzheimer’s Disease. Neurosci. Bull. 2018, 34, 736–746. [Google Scholar] [CrossRef]
- Chakrabarti, S.; Morton, J.S.; Davidge, S.T. Mechanisms of estrogen effects on the endothelium: An overview. Can. J. Cardiol. 2014, 30, 705–712. [Google Scholar] [CrossRef]
- McCarthy, M.; Raval, A.P. The peri-menopause in a woman’s life: A systemic inflammatory phase that enables later neurodegenerative disease. J. Neuroinflamm. 2020, 17, 317. [Google Scholar] [CrossRef]
- Andrew, T.W.; Koepke, L.S.; Wang, Y.; Lopez, M.; Steininger, H.; Struck, D.; Boyko, T.; Ambrosi, T.H.; Tong, X.; Sun, Y.; et al. Sexually dimorphic estrogen sensing in skeletal stem cells controls skeletal regeneration. Nat. Commun. 2022, 13, 6491. [Google Scholar] [CrossRef]
- Rinonapoli, G.; Ruggiero, C.; Meccariello, L.; Bisaccia, M.; Ceccarini, P.; Caraffa, A. Osteoporosis in Men: A Review of an Underestimated Bone Condition. Int. J. Mol. Sci. 2021, 4, 2105. [Google Scholar] [CrossRef] [PubMed]
- Fleischman, D.A.; Arfanakis, K.; Leurgans, S.E.; Arvanitakis, Z.; Lamar, M.; Han, S.D.; Poole, V.N.; Bennett, D.A.; Barnes, L.L. Cerebral arteriolosclerosis, lacunar infarcts, and cognition in older Black adults. Alzheimers Dement. 2024, 20, 5375–5384. [Google Scholar] [CrossRef] [PubMed]
- Alber, J.; Alladi, S.; Bae, H.J.; Barton, D.A.; Beckett, L.A.; Bell, J.M.; Berman, S.E.; Biessels, G.J.; Black, S.E.; Bos, I.; et al. White matter hyperintensities in vascular contributions to cognitive impairment and dementia (VCID): Knowledge gaps and opportunities. Alzheimers Dement. 2019, 5, 107–117. [Google Scholar] [CrossRef]
- Georgakis, M.K.; Duering, M.; Wardlaw, J.M.; Dichgans, M. WMH and long-term outcomes in ischemic stroke: A systematic review and meta-analysis. Neurology 2019, 92, e1298–e1308. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).