Evaluation of the Acaricidal Effectiveness of Fipronil and Phoxim in Field Populations of Dermanyssus gallinae (De Geer, 1778) from Ornamental Poultry Farms in Italy
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ornamental Poultry Farms Tested
2.2. Populations of Dermanyssus gallinae Tested
2.3. Products Tested
2.4. Sensitivity Assay
2.5. Statistic Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Marangi, M.; Cafiero, M.A.; Capelli, G.; Camarda, A.; Sparagano, O.A.E.; Giangaspero, A. Evaluation of the Poultry Red Mite, Dermanyssus gallinae (Acari: Dermanyssidae) Susceptibility to Some Acaricides in Field Populations from Italy. Exp. Appl. Acarol. 2009, 48, 11–18. [Google Scholar] [CrossRef]
- Sigognault Flochlay, A.; Thomas, E.; Sparagano, O. Poultry Red Mite (Dermanyssus gallinae) Infestation: A Broad Impact Parasitological Disease That Still Remains a Significant Challenge for the Egg-Laying Industry in Europe. Parasites Vectors 2017, 10, 357. [Google Scholar] [CrossRef] [PubMed]
- Marangi, M.; Morelli, V.; Pati, S.; Camarda, A.; Cafiero, M.A.; Giangaspero, A. Acaricide Residues in Laying Hens Naturally Infested by Red Mite Dermanyssus gallinae. PLoS ONE 2012, 7, e31795. [Google Scholar] [CrossRef]
- Xu, X.; Wang, C.; Zhang, S.; Huang, Y.; Pan, T.; Wang, B.; Pan, B. Acaricidal Efficacy of Orally Administered Macrocyclic Lactones against Poultry Red Mites (Dermanyssus gallinae) on Chicks and Their Impacts on Mite Reproduction and Blood-Meal Digestion. Parasites Vectors 2019, 12, 345. [Google Scholar] [CrossRef] [PubMed]
- Kilpinen, O.; Roepstorff, A.; Permin, A.; Nørgaard-Nielsen, G.; Lawson, L.G.; Simonsen, H.B. Influence of Dermanyssus gallinae and Ascaridia galli infections on behaviour and health of laying hens (Gallus gallus domesticus). Brit. Poult. Sci. 2005, 46, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Valiente Moro, C.; Chauve, C.; Zenner, L. Experimental Infection of Salmonella enteritidis by the Poultry Red Mite, Dermanyssus gallinae. Vet. Parasitol. 2007, 146, 329–336. [Google Scholar] [CrossRef]
- Chirico, J.; Eriksson, H.; Fossum, O.; Jansson, D. The Poultry Red Mite, Dermanyssus gallinae, a Potential Vector of Erysipelothrix rhusiopathiae Causing Erysipelas in Hens. Med. Vet. Entomol. 2003, 17, 232–234. [Google Scholar] [CrossRef]
- George, D.R.; Finn, R.D.; Graham, K.M.; Mul, M.F.; Maurer, V.; Moro, C.V.; Sparagano, O.A. Should the Poultry Red Mite Dermanyssus Gallinae Be of Wider Concern for Veterinary and Medical Science? Parasites Vectors 2015, 8, 178. [Google Scholar] [CrossRef]
- Moroni, B.; Barlaam, A.; Misia, A.L.; Peano, A.; Rossi, L.; Giangaspero, A. Dermanyssus Gallinae in Non-Avian Hosts: A Case Report in a Dog and Review of the Literature. Parasitol. Int. 2021, 84, 102378. [Google Scholar] [CrossRef]
- Rosen, S.; Yeruham, I.; Braverman, Y. Dermatitis in Humans Associated with the Mites Pyemotes Tritici, Dermanyssus gallinae, Ornithonyssus bacoti and Androlaelaps casalis in Israel: Mite-Mediated Dermatitis in Humans. Med. Vet. Entomol. 2002, 16, 442–444. [Google Scholar] [CrossRef]
- Waap, H.; Nunes, T.; Mul, M.; Gomes, J.; Bartley, K. Survey on the Prevalence of Dermanyssus gallinae in Commercial Laying Farms in Portugal. Avian Pathol. 2019, 48, S2–S9. [Google Scholar] [CrossRef] [PubMed]
- Sparagano, O.A.E.; Tomley, F.M. The Impact of the COREMI Cost Action Network on the Progress towards the Control of the Poultry Red Mite, Dermanyssus gallinae. Avian Pathol. 2019, 48, S1. [Google Scholar] [CrossRef] [PubMed]
- Cafiero, M.A.; Barlaam, A.; Camarda, A.; Radeski, M.; Mul, M.; Sparagano, O.; Giangaspero, A. Dermanysuss Gallinae Attacks Humans. Mind the Gap! Avian Pathol. 2019, 48, S22–S34. [Google Scholar] [CrossRef] [PubMed]
- Locher, N.; Klimpel, S.; Abdel-Ghaffar, F.; Al Rasheid, K.A.S.; Mehlhorn, H. Light and Scanning Electron Microscopic Investigations on MiteStop®-Treated Poultry Red Mites. Parasitol. Res. 2010, 107, 433–437. [Google Scholar] [CrossRef]
- Kenyon, J. Red Mites in Backyard Chickens. Practice 2017, 39, 334–338. [Google Scholar] [CrossRef]
- Hamscher, G.; Prieß, B.; Nau, H. Determination of Phoxim Residues in Eggs by Using High-Performance Liquid Chromatography Diode Array Detection after Treatment of Stocked Housing Facilities for the Poultry Red Mite (Dermanyssus gallinae). Anal. Chim. Acta 2007, 586, 330–335. [Google Scholar] [CrossRef]
- Abdel-Ghaffar, F.; Semmler, M.; Al-Rasheid, K.; Mehlhorn, H. In Vitro Efficacy of ByeMite® and Mite-Stop® on Developmental Stages of the Red Chicken Mite Dermanyssus gallinae. Parasitol. Res. 2009, 105, 1469–1471. [Google Scholar] [CrossRef]
- Abdel-Ghaffar, F.; Sobhy, H.M.; Al-Quraishy, S.; Semmler, M. Field Study on the Efficacy of an Extract of Neem Seed (Mite-Stop®) against the Red Mite Dermanyssus gallinae Naturally Infecting Poultry in Egypt. Parasitol. Res. 2008, 103, 481–485. [Google Scholar] [CrossRef]
- Abbas, R.Z.; Colwell, D.D.; Iqbal, Z.; Khan, A. Acaricidal Drug Resistance in Poultry Red Mite (Dermanyssus gallinae) and Approaches to Its Management. World’s Poult. Sci. J. 2014, 70, 113–124. [Google Scholar] [CrossRef]
- Katsavou, E.; Vlogiannitis, S.; Karp-Tatham, E.; Blake, D.P.; Ilias, A.; Strube, C.; Kioulos, I.; Dermauw, W.; Van Leeuwen, T.; Vontas, J. Identification and Geographical Distribution of Pyrethroid Resistance Mutations in the Poultry Red Mite Dermanyssus gallinae. Pest. Manag. Sci. 2020, 76, 125–133. [Google Scholar] [CrossRef]
- Thind, B.B.; Ford, H.L. Assessment of Susceptibility of the Poultry Red Mite Dermanyssus gallinae (Acari: Dermanyssidae) to Some Acaricides Using an Adapted Filter Paper Based Bioassay. Vet. Parasitol. 2007, 144, 344–348. [Google Scholar] [CrossRef] [PubMed]
- Pavlicevic, A.; Pavlovic, I.; Stajkovic, N.; Pešic, B. Evidence for Resistance to Carbaryl in Poultry Red Mites from the Republic of Serbia and Montenegro. Sci. Pap. 2016, 49, 222–225. [Google Scholar]
- Sparagano, O.A.E.; George, D.R.; Harrington, D.W.J.; Giangaspero, A. Significance and Control of the Poultry Red Mite, Dermanyssus gallinae. Annu. Rev. Entomol. 2014, 59, 447–466. [Google Scholar] [CrossRef]
- Pritchard, J.; Kuster, T.; Sparagano, O.; Tomley, F. Understanding the Biology and Control of the Poultry Red Mite Dermanyssus gallinae: A Review. Avian Pathol. 2015, 44, 143–153. [Google Scholar] [CrossRef]
- Angeli Alves, L.F.; Oliveira, D.G.P.; Kasburg, C.R.; Nardelli, M.S. Acaricidal activity of inert powders against the poultry red mite Dermanyssus gallinae (De Geer, 1778) (Mesostigmata: Dermanyssidae). AVS 2019, 24, 81–92. [Google Scholar] [CrossRef]
- Thomas, E.; Zoller, H.; Liebisch, G.; Alves, L.F.A.; Vettorato, L.; Chiummo, R.M.; Sigognault-Flochlay, A. In Vitro Activity of Fluralaner and Commonly Used Acaricides against Dermanyssus gallinae Isolates from Europe and Brazil. Parasites Vectors 2018, 11, 361. [Google Scholar] [CrossRef] [PubMed]
- Stafford, E.G.; Tell, L.A.; Lin, Z.; Davis, J.L.; Vickroy, T.W.; Riviere, J.E.; Baynes, R.E. Consequences of Fipronil Exposure in Egg-Laying Hens. J. Am. Vet. Med. Assoc. 2018, 253, 57–60. [Google Scholar] [CrossRef] [PubMed]
- Arioli, F.; Negro, V.; Roncada, P.; Guerrini, A.; Villa, R.; Nobile, M.; Chiesa, L.; Panseri, S. Presence of Fipronil and Metabolites in Eggs and Feathers of Ornamental Hens from Italian Family Farms. Food Control 2022, 138, 109034. [Google Scholar] [CrossRef]
- Di Palma, A.; Giangaspero, A.; Cafiero, M.A.; Germinara, S.G. A gallery of the key characters to ease identification of Dermanyssus gallinae (Acari: Gamasida: Dermanyssidae) and allow differentiation from Ornithonyssus sylviarum (Acari: Gamasida: Macronyssidae). Parasites Vectors 2012, 5, 104. [Google Scholar] [CrossRef]
- Nunn, F.; Baganz, J.; Bartley, K.; Hall, S.; Burgess, S.; Nisbet, A.J. An Improved Method for in Vitro Feeding of Adult Female Dermanyssus gallinae (Poultry Red Mite) Using Baudruche Membrane (Goldbeater’s Skin). Parasites Vectors 2020, 13, 585. [Google Scholar] [CrossRef]
- Wang, C.; Xu, X.; Huang, Y.; Yu, H.; Li, H.; Wan, Q.; Li, H.; Wang, L.; Sun, Y.; Pan, B. Susceptibility of Dermanyssus gallinae from China to Acaricides and Functional Analysis of Glutathione S-Transferases Associated with Beta-Cypermethrin Resistance. Pestic. Biochem. Physiol. 2021, 171, 104724. [Google Scholar] [CrossRef] [PubMed]
- Zdybel, J.; Karamon, J.; Cencek, T. In vitro effectiveness of selected acaricides against red poultry mites (Dermanyssus gallinae, De Geer, 1778) isolated from laying hen battery cage farms localised in different regions of poland. Bull. Vet. Inst. Pulawy. 2011, 55, 411–416. [Google Scholar]
- Boffey, D.; Connolly, K. Egg Contamination Scandal Widens as 15 EU States, Switzerland and Hong Kong Affected. 2017. Available online: https://www.theguardian.com/world/2017/aug/11/tainted-eggs-found-in-hong-kong-switzerland-and-15-eu-countries (accessed on 25 July 2022).
- BBC News. Eggs Containin Fipronil Found in 15 EU Countries and Hong Kong. 2017. Available online: http://www.bbc.com/news/world-europe-40896899 (accessed on 25 July 2022).
- Arisov, M.; Indyuhova, E.; Arisova, G. Pharmacokinetics of Combination Antiparasitic Drug Preparation for Dogs and Cats in the Form of Spot-on Solution. J. Adv. Vet. Anim. Res. 2019, 6, 25. [Google Scholar] [CrossRef] [PubMed]
- Gols, R.; WallisDeVries, M.F.; van Loon, J.J.A. Reprotoxic Effects of the Systemic Insecticide Fipronil on the Butterfly Pieris Brassicae. Proc. R. Soc. B 2020, 287, 20192665. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-I.; Na, Y.-E.; Yi, J.-H.; Kim, B.-S.; Ahn, Y.-J. Contact and Fumigant Toxicity of Oriental Medicinal Plant Extracts against Dermanyssus gallinae (Acari: Dermanyssidae). Vet. Parasitol. 2007, 145, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Mullens, B.A.; Murillo, A.C.; Zoller, H.; Heckeroth, A.R.; Jirjis, F.; Flochlay-Sigognault, A. Comparative in Vitro Evaluation of Contact Activity of Fluralaner, Spinosad, Phoxim, Propoxur, Permethrin and Deltamethrin against the Northern Fowl Mite, Ornithonyssus sylviarum. Parasites Vectors 2017, 10, 358. [Google Scholar] [CrossRef]
- Li, M.; Lu, W.C.; Feng, H.Z.; He, L. Molecular BlackwellPublishingLtd Characterization and Expression of Three Heat Shock Protein70 Genes from the Carmine Spider Mite, Tetranychus cinnabarinus (Boisduval). Insect Mol. Biol. 2009, 18, 183–194. [Google Scholar] [CrossRef]
- Kim, J.-R.; Perumalsamy, H.; Lee, J.-H.; Ahn, Y.-J.; Lee, Y.S.; Lee, S.-G. Acaricidal Activity of Asarum Heterotropoides Root-Derived Compounds and Hydrodistillate Constitutes toward Dermanyssus gallinae (Mesostigmata: Dermanyssidae). Exp. Appl. Acarol. 2016, 68, 485–495. [Google Scholar] [CrossRef]
- Kim, H.K.; Lee, S.J.; Hwang, B.-Y.; Yoon, J.U.; Kim, G.-H. Acaricidal and Repellent Effects of Cnidium Officinale-Derived Material against Dermanyssus gallinae (Acari: Dermanyssidae). Exp. Appl. Acarol. 2018, 74, 403–414. [Google Scholar] [CrossRef]
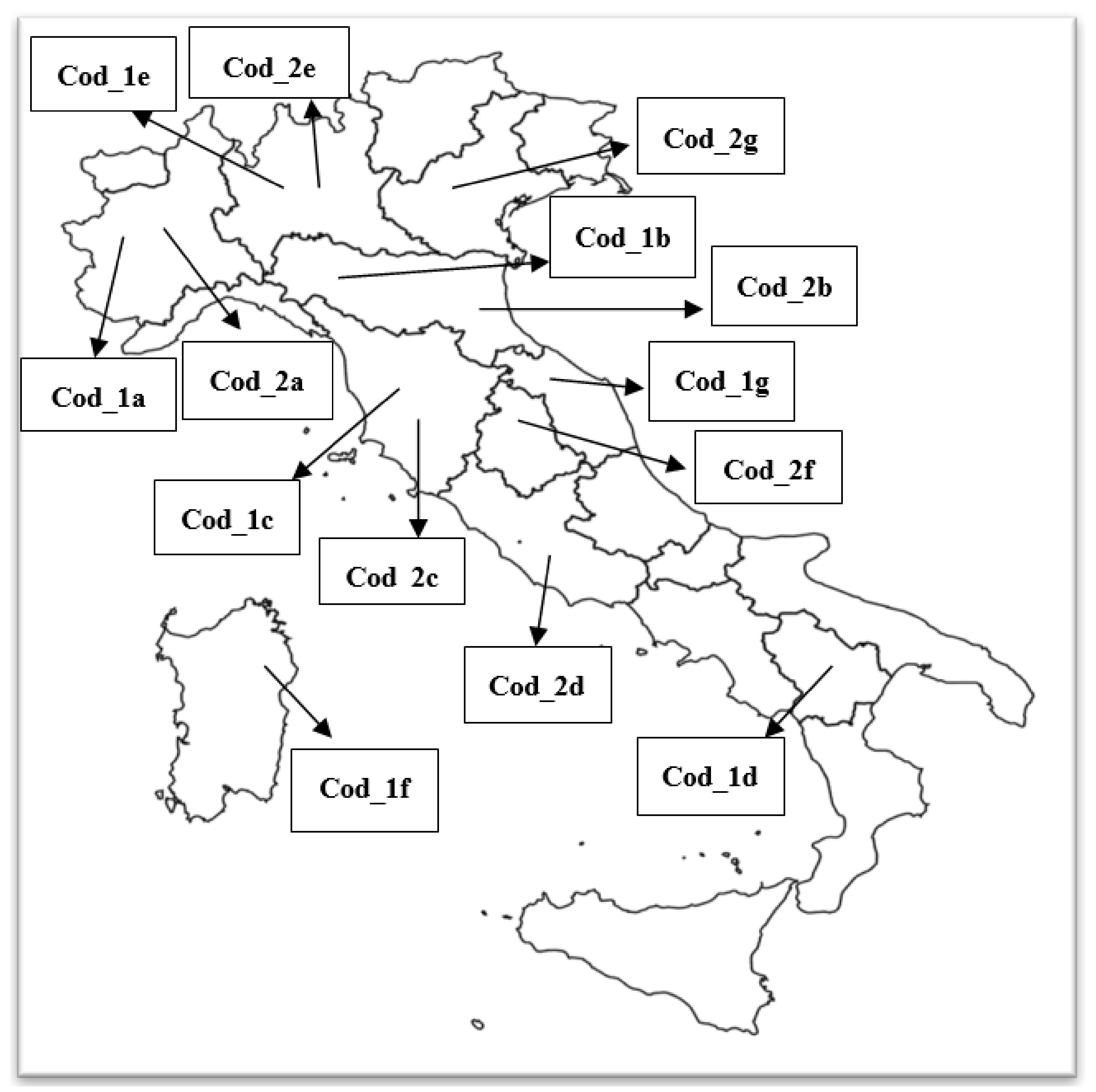
| Farm Code | Region | Breeds | N° Chickens * | Pesticides Used ** |
|---|---|---|---|---|
| Cod_1a | Piedmont | Silkie, Cocin, Polish | 120 | ByeMite® (Phoxim) Neem Oil |
| Cod_2a | Piedmont | Orpington, Brahma | 60–70 | ByeMite® (Phoxim) |
| Cod_1b | Emilia-Romagna | Orpington, Brahma | 130 | nrǂ |
| Cod_2b | Emilia-Romagna | Polish | 180 | nr |
| Cod_1c | Tuscany | Leghorn | 100–110 | nr |
| Cod_2c | Tuscany | Leghorn, Ancona | 100 | ByeMite® (Phoxim) Neem Oil |
| Cod_1d | Basilicata | Ko-shamo, Cornish | 40–60 | ByeMite® (Phoxim) |
| Cod_2d | Lazio | Silkie, Cocin, Cemani | 250 | ByeMite® (Phoxim) |
| Cod_1e | Lombardy | Barnevelder | 80–100 | nr |
| Cod_2e | Lombardy | Faverolles, Cocin, Brahma | 120–225 | ByeMite® (Phoxim) Neem Oil |
| Cod_1f | Sardinia | Silkie, Sebright | 60 | nr |
| Cod_2f | Umbria | Romagnola, Ancona, Polish | 170 | nr |
| Cod_1g | Marche | Barbuta d’Anversa | 200–230 | ByeMite® (Phoxim) |
| Cod_2g | Veneto | Ermellinata di Rovigo | 50–60 | ByeMite® (Phoxim) |
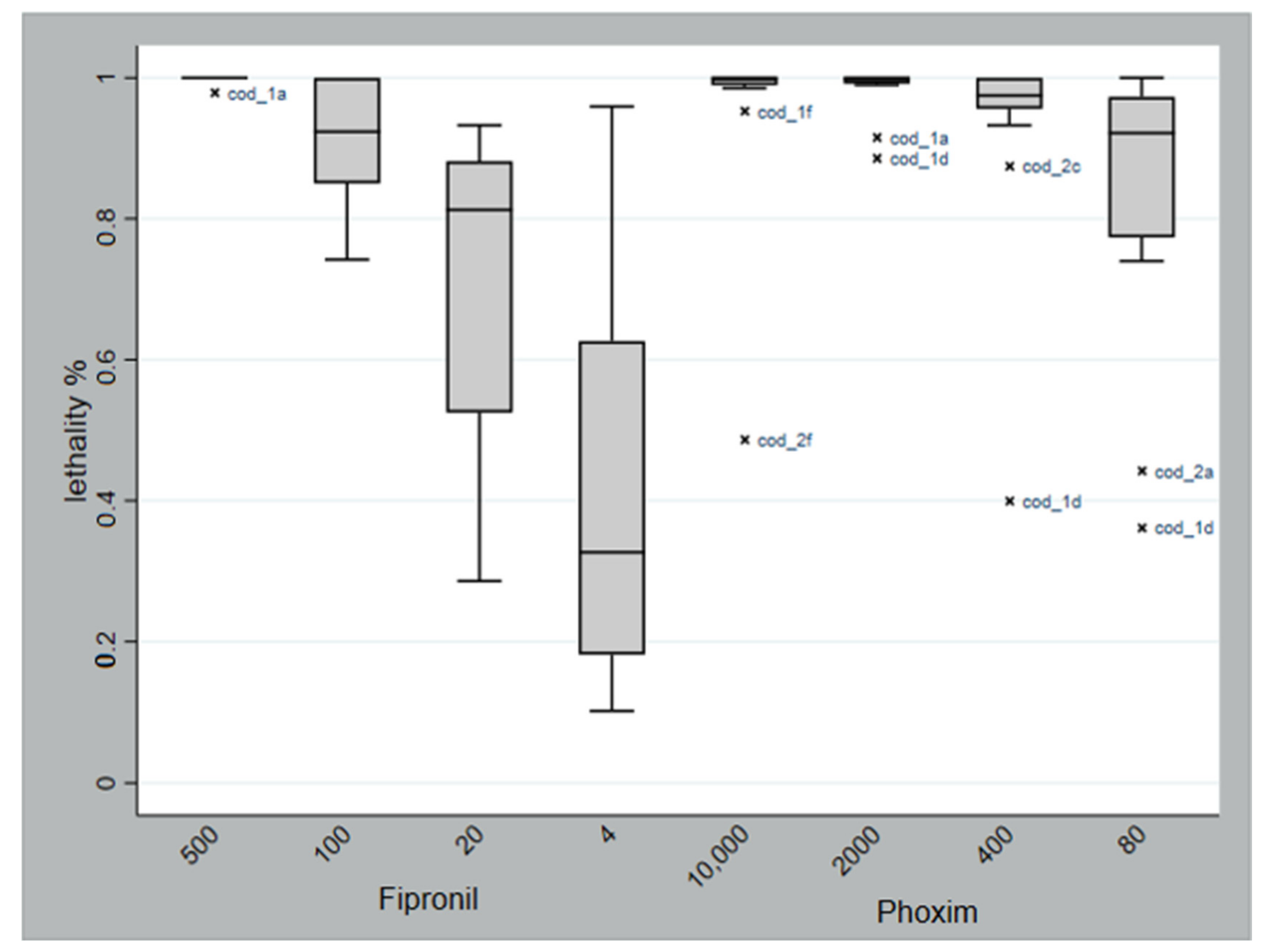
| Treatment | Concentration (ppm) | Lethality Rate (%) |
|---|---|---|
| Fipronil | 500 | 99.8 |
| 100 | 91.2 | |
| 20 | 72.4 | |
| 4 | 43.4 | |
| Phoxim | 10,000 | 95.4 |
| 2000 | 98.4 | |
| 400 | 93.1 | |
| 80 | 83.4 | |
| Control | - | 22.2 |
| Treatment | |||
|---|---|---|---|
| Concentration | Fipronil | Phoxim | p-Value |
| 1st dilution | 0.998 | 0.954 | p = 0.148 |
| 2nd dilution | 0.912 | 0.984 | p = 0.01 |
| 3rd dilution | 0.724 | 0.931 | p < 0.001 |
| 4th dilution | 0.434 | 0.834 | p = 0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guerrini, A.; Morandi, B.; Roncada, P.; Brambilla, G.; Dini, F.M.; Galuppi, R. Evaluation of the Acaricidal Effectiveness of Fipronil and Phoxim in Field Populations of Dermanyssus gallinae (De Geer, 1778) from Ornamental Poultry Farms in Italy. Vet. Sci. 2022, 9, 486. https://doi.org/10.3390/vetsci9090486
Guerrini A, Morandi B, Roncada P, Brambilla G, Dini FM, Galuppi R. Evaluation of the Acaricidal Effectiveness of Fipronil and Phoxim in Field Populations of Dermanyssus gallinae (De Geer, 1778) from Ornamental Poultry Farms in Italy. Veterinary Sciences. 2022; 9(9):486. https://doi.org/10.3390/vetsci9090486
Chicago/Turabian StyleGuerrini, Alessandro, Benedetto Morandi, Paola Roncada, Gianfranco Brambilla, Filippo Maria Dini, and Roberta Galuppi. 2022. "Evaluation of the Acaricidal Effectiveness of Fipronil and Phoxim in Field Populations of Dermanyssus gallinae (De Geer, 1778) from Ornamental Poultry Farms in Italy" Veterinary Sciences 9, no. 9: 486. https://doi.org/10.3390/vetsci9090486
APA StyleGuerrini, A., Morandi, B., Roncada, P., Brambilla, G., Dini, F. M., & Galuppi, R. (2022). Evaluation of the Acaricidal Effectiveness of Fipronil and Phoxim in Field Populations of Dermanyssus gallinae (De Geer, 1778) from Ornamental Poultry Farms in Italy. Veterinary Sciences, 9(9), 486. https://doi.org/10.3390/vetsci9090486






