Morpho-Histology and Morphometry of Chicken Testes and Seminiferous Tubules among Yellow-Feathered Broilers of Different Ages
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Animals
2.3. Sample Preparation for Anatomical Observations
2.4. Sample Preparation for Microscopic Observations
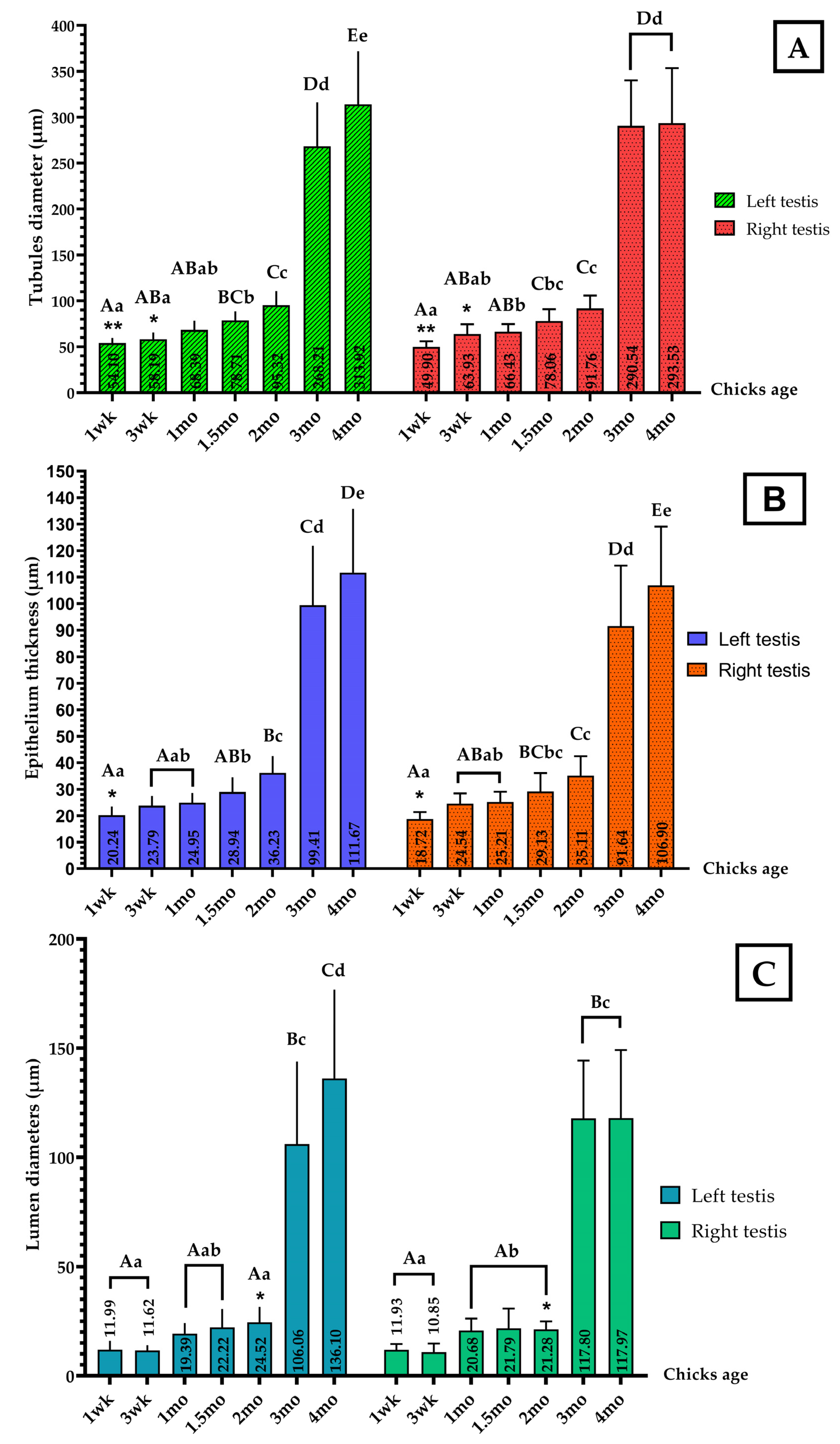
2.5. Data Collection
2.6. Statistical Analysis
3. Results
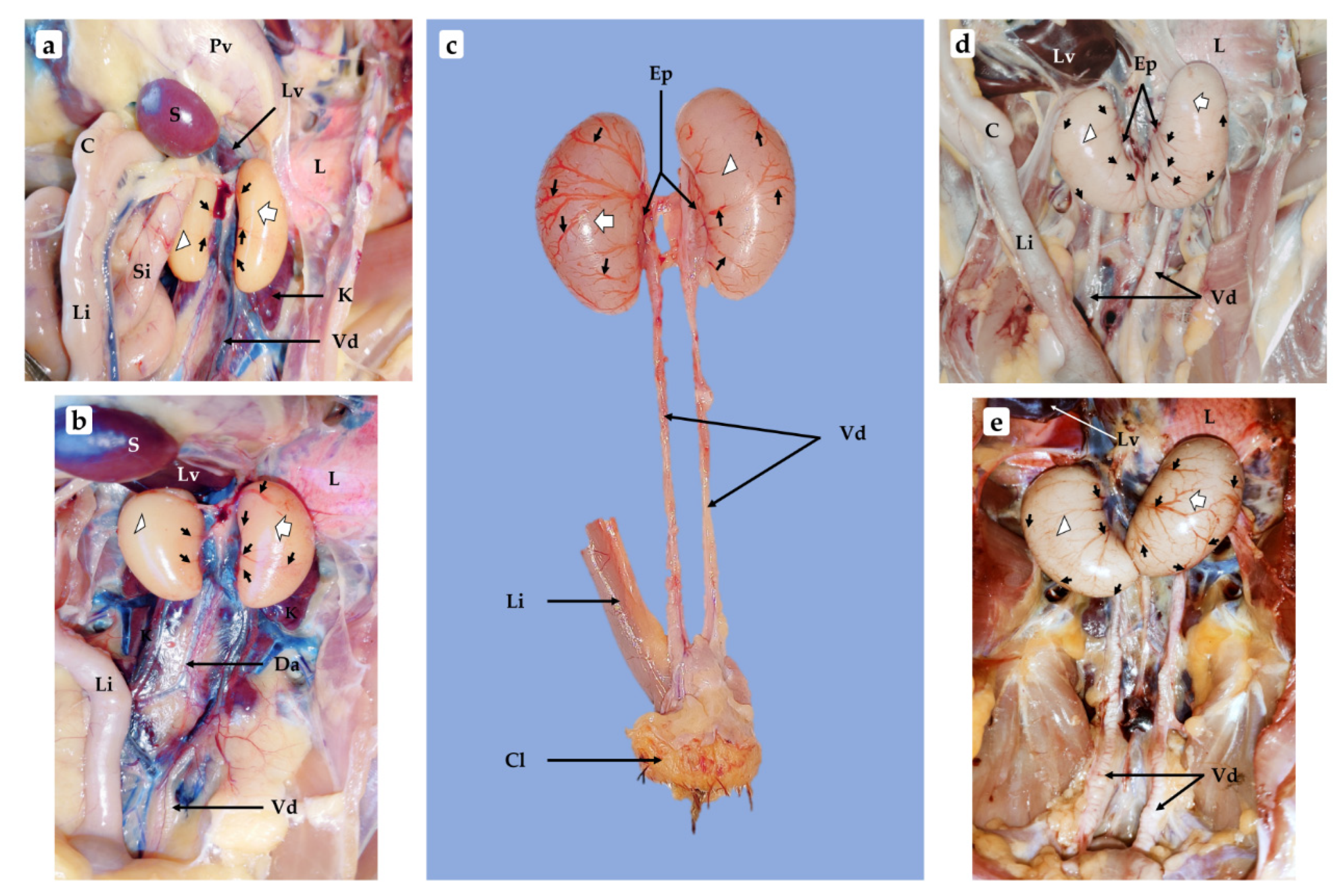
3.1. Anatomical Observations of Chicken Testes at Different Stages of Growth
3.1.1. One-Week-Old Chick
3.1.2. Three-Week-Old Chick
3.1.3. One-Month-Old Chick
3.1.4. One-Month and Half-Old (6 Weeks) Chick
3.1.5. Two-Month-Old Chick
3.1.6. Three-Month-Old Chick
3.1.7. Four-Month-Old Chick
3.1.8. Gonadosomatic Index (GSI) among Chickens at Different Stages of Growth
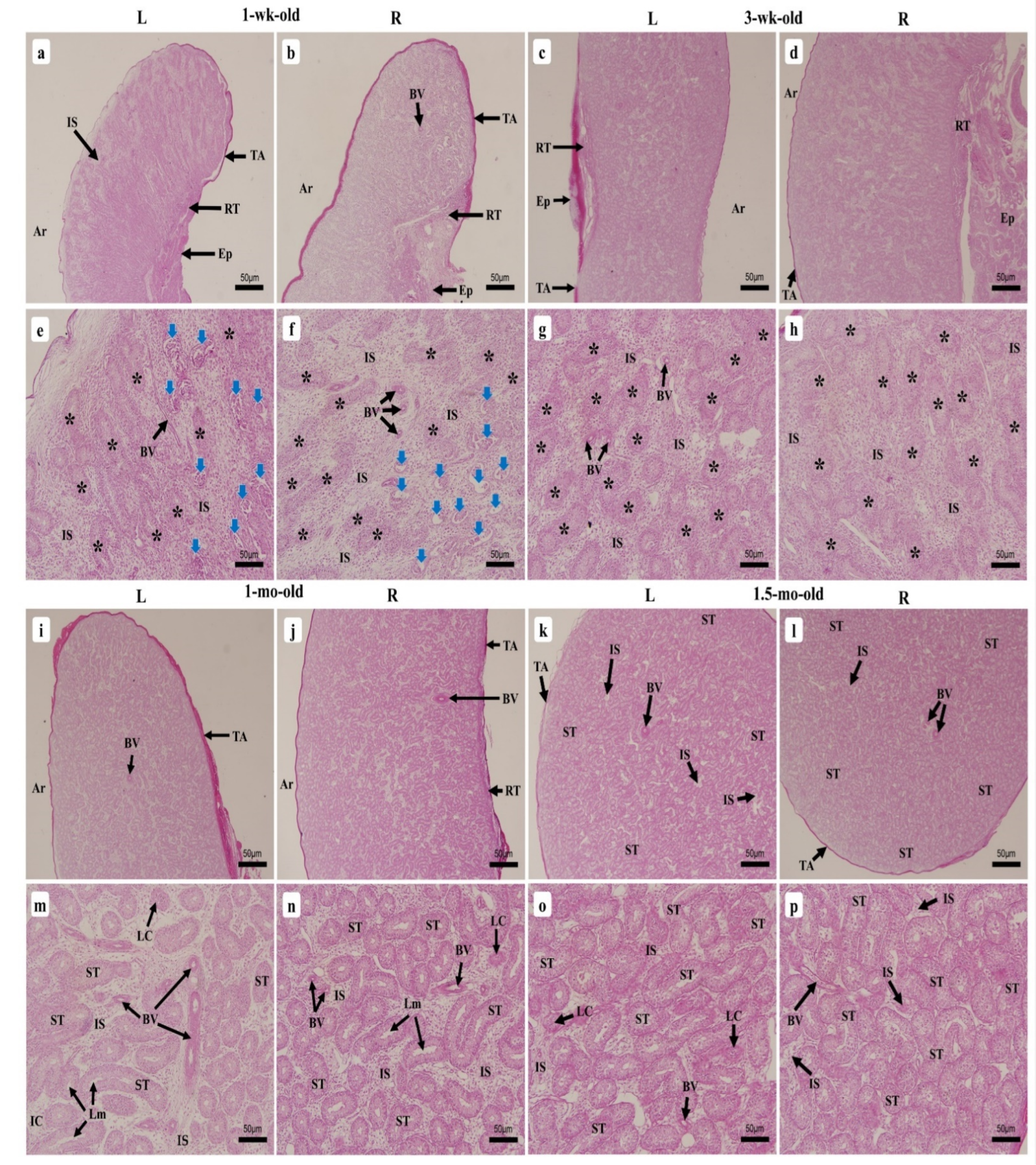
3.2. Histological Observation of Chicken Testes at Different Stages of Growth
3.2.1. Testicular Seminiferous Tubules’ Resorption by Apoptosis
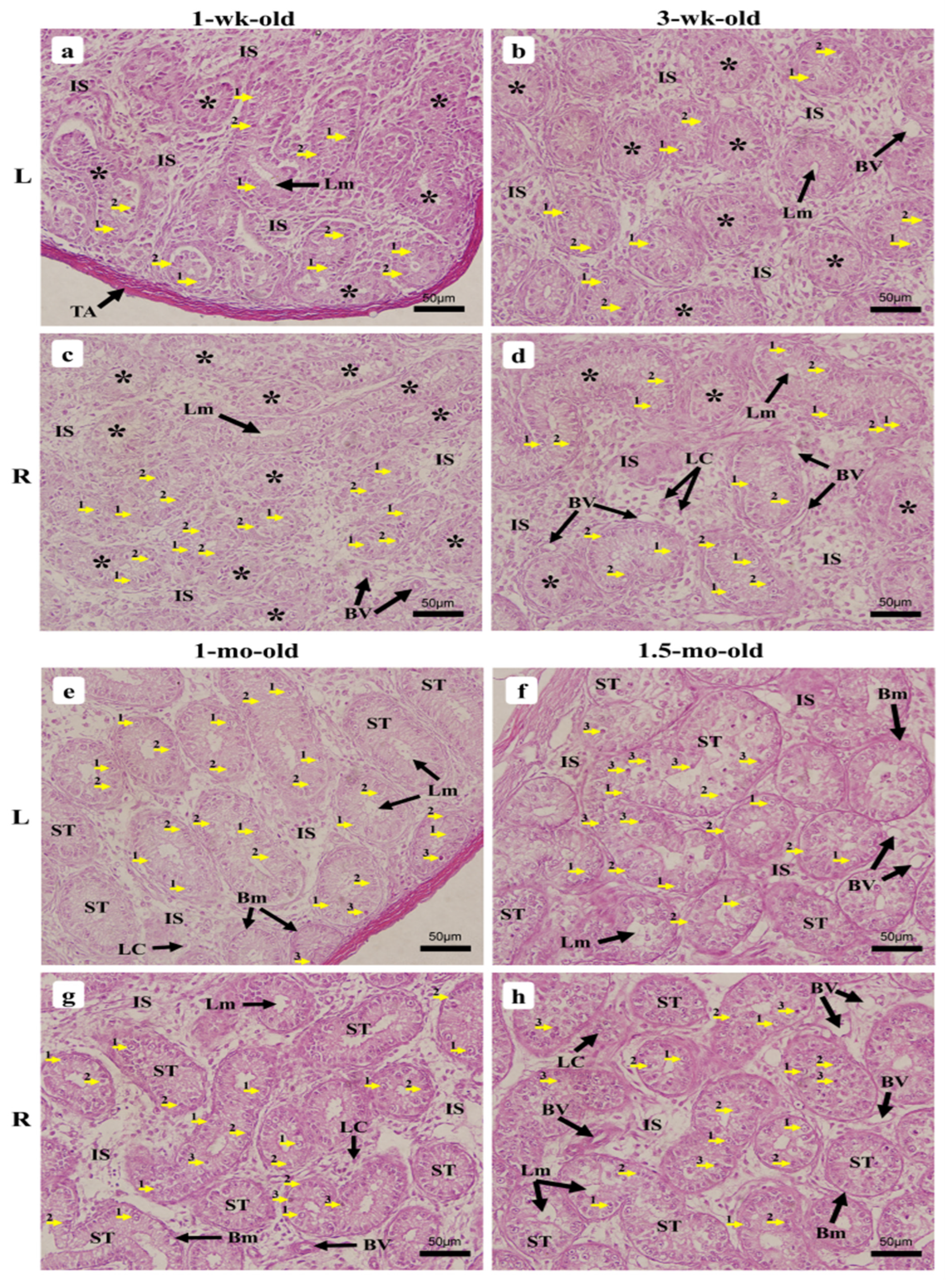
3.2.2. One-Week-Old Chick
3.2.3. Three-Week-Old Chick
3.2.4. One-Month-Old Chick
3.2.5. One-Month Half-Old Chicks (6 Weeks)
3.2.6. Two-Month-Old Chick
3.2.7. Three-Month-Old Chick
3.2.8. Four-Month-Old Chick
4. Discussion
4.1. Yellow-Feathered Broilers
4.2. Anatomo-Morphological Observation of the Testes and Seminiferous Tubule Morphometry at Different Stages of Growth
4.2.1. One-Week-Old Chick
4.2.2. Three-Week-Old Chick
4.2.3. One-Month-Old Chick
4.2.4. One-and-a-Half-Month- (6 Weeks-) Old Chicks
4.2.5. Two-Month-Old Chick
4.2.6. Three-Month-Old Chick
4.2.7. Four-Month-Old Chick
4.3. Seminiferous Tubules’ Degeneration by Apoptosis during Early Seminiferous Tubule Formation
4.4. Gonadosomatic Index (GSI) of the Growing Chickens at Different Stages of Growth
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lara, N.L.; Costa, G.M.; Avelar, G.F.; Lacerda, S.M.; Hess, R.A.; França, L.R. Testis physiology-overview and histology. In Encyclopedia of Reproduction; Elseview: Amsterdam, The Netherlands, 2018; pp. 105–116. [Google Scholar]
- Tagami, T.; Miyahara, D.; Nakamura, Y. Avian Primordial Germ Cells. Adv. Exp. Med. Biol. 2017, 1001, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Ginsburg, M.; Eyal-Giladi, H. Primordial germ cells of the young chick blastoderm originate from the central zone of the area pellucida irrespective of the embryo-forming process. Development 1987, 101, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Romanoff, A.L. The avian embryo. In The Urogenital System; Romanoff, A.L., Ed.; Macmillan: New York, NY, USA, 1960; pp. 783–862. [Google Scholar] [CrossRef]
- King, A.S.; McLelland, J. Outlines of Avian Anatomy; Bailliere Tindall: London, UK, 1975; p. 65. [Google Scholar]
- Luo, D.; Zhang, M.; Su, X.; Liu, L.; Zhou, X.; Zhang, X.; Guan, Q. High fat diet impairs spermatogenesis by regulating glucose and lipid metabolism in Sertoli cells. Life Sci. 2020, 257, 118028. [Google Scholar] [CrossRef]
- Hu, C.; Zuo, Q.; Jin, K.; Zhao, Z.; Wu, Y.; Gao, J.; Zhang, Y. Retinoic acid promotes the formation of chicken (Gallus gallus) Spermatogonial stem cells by regulating the ECM-receptor interaction signaling pathway. Gene 2022, 820, 146227. [Google Scholar] [CrossRef] [PubMed]
- Lan, W.E.I.; Peng, K.M.; Liu, H.; Song, H.; Wang, Y.; Tang, L. Histological examination of testicular cell development and apoptosis in the ostrich chick. Turk. J. Vet. Anim. Sci. 2011, 35, 7–14. [Google Scholar] [CrossRef]
- Kumaran, J.D.D.; Turner, C.W. The normal development of the testes in the White Plymouth Rock. Poult. Sci. 1949, 28, 511–520. [Google Scholar] [CrossRef]
- Razi, M.; Hassanzadeh, S.H.; Najafi, G.R.; Feyzi, S.; Amin, M.; Moshtagion, M.; Janbaz, H. Histological and anatomical study of the White Rooster of testis, epididymis and ductus deferens. Int. J. Vet. Res. 2010, 4, 229–236. [Google Scholar]
- Ibrahim, M.I.; Zakariah, M.; Molele, R.A.; Mahdy, M.A.; Williams, J.H.; Botha, C.J. Ontogeny of the testicular excurrent duct system of male Japanese quail (Coturnix japonica): A histological, ultrastructural, and histometric study. Microsc. Res. Tech. 2022, 85, 1160–1170. [Google Scholar] [CrossRef]
- Chang, G.B.; Chen, R.; Qin, Y.R.; Zhang, Y.; Dai, A.Q.; Chen, G.H. The Development of Primordial Germ Cells (PGCs) and Testis in the Quail Embryo. Pak. Vet. J. 2012, 32, 88–92. [Google Scholar]
- Obeid, A.K.; Al-Bazii, S.; Alsafy, A.H.M. Histological and Morphomterical Features of Domesticus Duck Testes (Anas Platyrhynchos). Ann. Rom. Soc. Cell Biol. 2021, 25, 336–341. [Google Scholar]
- Gerzilov, V.; Bochukov, A.; Penchev, G.; Petrov, P. Testicular development in the muscovy duck (Cairina moschata). Bulg. J. Vet. Med. 2016, 19, 8–18. [Google Scholar] [CrossRef]
- Aire, T.A.; Ozegbe, P.C. The testicular capsule and peritubular tissue of birds: Morphometry, histology, ultrastructure and immunohistochemistry. J. Anat. 2007, 210, 731–740. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, F.E.Z.A.; Elhanbaly, R. Histological, histochemical, immunohistochemical and ultrastructural characterization of the testes of the dove. Zygote 2021, 29, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, S. From cyst to tubule: Innovations in vertebrate spermatogenesis. Wiley Interdiscip. Rev. Dev. Biol. 2016, 5, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Aire, T.A. Spermiogenesis in birds. Spermatogenesis 2014, 4, e959392. [Google Scholar] [CrossRef]
- Jones, R.C.; Lin, M. Spermatogenesis in birds. Oxf. Rev. Reprod. Biol. Biol. 1993, 15, 233–264. [Google Scholar]
- Pudney, J. Spermatogenesis in nonmammalian vertebrates. Microsc. Res. Tech. 1995, 32, 459–497. [Google Scholar] [CrossRef]
- Bachmid, N.A.; Purba, F.Y.; Apada, A.M.S.; Sari, D.K. Morphology and histomorphometric study of 1-to 4-month-old Gaga’chicken’s testes. In Proceedings of the IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2019; Volume 343, p. 012031. [Google Scholar]
- Okpe, G.C.; Nwatu, U.; Anya, K. Morphometric study of the testes of the Nigerian local breed of chicken. Anim. Res. Inter. 2010, 7, 1163–1168. [Google Scholar]
- Tamilselvan, S.; Dhote, B.S.; Singh, I.; Mrigesh, M.; Sathapathy, S.; Mahanta, D. Gross morphology of testes and gonadosomatic index (GSI) of guinea fowl (Numida meleagris). J. Entomol. Zool. Stud. 2018, 6, 156–159. [Google Scholar]
- Masyitha, D.; Akmal, M.; Gholib, G.; Wahyuni, S. Morphoanatomy and Gonadosomatic Index (GSI) of Testis of Turkey (Meleagris gallopavo) at Different Ages. In Proceedings of the 2nd International Conference on Veterinary, Animal, and Environmental Sciences, ICVAES, Banda Aceh, Indonesia, 22–23 October 2020; Atlantis Press: Amsterdam, The Netherlands, 2021; pp. 140–142. [Google Scholar]
- Flores, A.; Wiff, R.; Díaz, E. Using the gonadosomatic index to estimate the maturity ogive: Application to Chilean hake (Merluccius gayi gayi). ICES J. Mar. Sci. 2015, 72, 508–514. [Google Scholar] [CrossRef]
- Hassanin, A.; Kuwahara, S.; Tsukamoto, Y.; Ogawa, K.; Hiramatsu, K.; Sasaki, F. Gonadosomatic index and testis morphology of common carp (Cyprinus carpio) in rivers contaminated with estrogenic chemicals. J. Vet. Med. Sci. 2002, 64, 921–926. [Google Scholar] [CrossRef] [PubMed]
- Dupont, J.; Métayer-Coustard, S.; Ji, B.; Ramé, C.; Gespach, C.; Voy, B.; Simon, J. Characterization of major elements of insulin signaling cascade in chicken adipose tissue: Apparent insulin refractoriness. Gen. Comp. Endocrinol. 2012, 176, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Orlu, E.E.; Egbunike, G.N. Breed and seasonal variations in the testicular morphometry, gonadal and extragonadal sperm reserves of the barred Plymouth rock and Nigerian indigenous breeds of the domestic fowl. Pak. J. Biol. Sci. 2010, 13, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods. 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- França, L.R.; Godinho, C.L. Testis Morphometry, Seminiferous Epithelium Cycle Length, and Daily Sperm Production in Domestic Cats (Felis catus). Biol. Reprod. 2003, 68, 1554–1561. [Google Scholar] [CrossRef] [PubMed]
- Neves, E.S.; Chiarini-Garcia, H.; França, L.R. Comparative testis morphometry and seminiferous epithelium cycle length in donkeys and mules. Biol. Reprod. 2002, 67, 247–255. [Google Scholar] [CrossRef][Green Version]
- Gou, Z.Y.; Jiang, S.Q.; Jiang, Z.Y.; Zheng, C.T.; Li, L.; Ruan, D. Effects of High Peanut Meal with Different Crude Protein Level Supplemented with Amino Acids on Performance, Carcass Traits and Nitrogen Retention of Chinese Yellow Broilers. J. Anim. Physiol. Anim. Nutr. 2016, 100, 657–664. [Google Scholar] [CrossRef]
- Current Situation of China’s Yellow-Feathered Broiler Industry: It Is Extending to Hubei, Hunan, Sichuan, Shandong, Henan, and Other Markets. Available online: https://www.jbzyw.com/view/324204 (accessed on 18 August 2022). (In Chinese).
- Wang, H.; Zhang, X.; Wang, G.; Jia, K.; Xu, X.; Zhou, G. Bacterial community and spoilage profiles shift in response to packaging in yellow-feather broiler, a highly popular meat in Asia. Front. Microbiol. 2017, 8, 2588. [Google Scholar] [CrossRef]
- Wang, Y.; Gou, Z.; Lin, X.; Fan, Q.; Ye, J.; Jiang, S. Optimal Level of Supplemental Manganese for Yellow-Feathered Broilers during the Growth Phase. Animals 2021, 11, 1389. [Google Scholar] [CrossRef]
- Liu, W.; Yuan, Y.; Sun, C.; Balasubramanian, B.; Zhao, Z.; An, L. Effects of Dietary Betaine on Growth Performance, Digestive Function, Carcass Traits, and Meat Quality in Indigenous Yellow-Feathered Broilers under Long-Term Heat Stress. Animals 2019, 9, 506. [Google Scholar] [CrossRef]
- Liu, W.C.; Huang, M.Y.; Balasubramanian, B.; Jha, R. Heat Stress Affects Jejunal Immunity of Yellow-Feathered Broilers and Is Potentially Mediated by the Microbiome. Front. Physiol. 2022, 13, 1022. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, S.S.; Rashamol, V.P.; Bagath, M.; Sejian, V.; Dunshea, F.R. Impacts of Heat Stress on Immune Responses and Oxidative Stress in Farm Animals and Nutritional Strategies for Amelioration. Int. J. Biometeorol. 2021, 65, 1231–1244. [Google Scholar] [CrossRef] [PubMed]
- Mfoundou, J.D.L.; Guo, Y.J.; Liu, M.M.; Ran, X.R.; Fu, D.H.; Yan, Z.Q.; Li, M.N.; Wang, X.R. The morphological and histological study of chicken left ovary during growth and development among Hy-line brown layers of different ages. Poult. Sci. 2021, 100, 101191. [Google Scholar] [CrossRef] [PubMed]
- Elbajory, S.I.A. Morphometric study of the testis of adult Sudanese chicken (Gallus domesticus) and duck (Anas platyrhynchos). Curr. Res. J. Biol. Sci. 2011, 3, 393–397. [Google Scholar]
- Rizzi, C.; Verdiglione, R. Testicular growth and comb and wattles development in three Italian chicken genotypes reared under free-range conditions. Ital. J. Anim. Sci. 2015, 14, 3653. [Google Scholar] [CrossRef]
- Steger, K. Transcriptional and translational regulation of gene expression in haploid spermatids. Anat. Embryol. 1999, 199, 471–487. [Google Scholar] [CrossRef]
- Nishimura, H.; L’Hernault, S.W. Spermatogenesis. Curr. Biol. 2017, 27, R988–R994. [Google Scholar] [CrossRef]
- Parker, J.E.; McKenzie, F.F.; Kempster, H.L. Development of the Testes and Combs of White Leghorn and New Hampshire Cockerels. Poult. Sci. 1942, 21, 35–44. [Google Scholar] [CrossRef]
- Bakst, M.R.; Akuffo, V.; Trefil, P.; Brillard, J.P. Morphological and histochemical characterization of the seminiferous epithelial and Leydig cells of the turkey. Anim. Reprod. Sci. 2007, 97, 303–313. [Google Scholar] [CrossRef]
- González-Morán, M.G. Histological and stereological changes in growing and regressing chicken ovaries during development. Anat. Rec. 2011, 294, 893–904. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.L.; Woods, D.C. Ovarian dynamics and follicle development. In Reproductive Biology and Physiology; Jamieson, B.G.M., Ed.; Science Publisher: Queensland, Australia, 2007; Volume 6A, pp. 243–277. [Google Scholar]
- Hogue, R.L.; Schnetzler, E.E. Development of fertility in young Barred Plymouth Rock males. Poult. Sci. 1937, 16, 62–67. [Google Scholar] [CrossRef]
- McCartney, M.G. Sexual maturity in broiler breeder males. Poult. Sci. 1978, 57, 1720–1722. [Google Scholar] [CrossRef] [PubMed]
- Kareem, D.A.; Jassem, E.S.; Daaj, S.A.; Al-Khalad, W.J. Morphological and histological study of the testes in adult duck. Plant Arch. 2020, 20, 751–755. [Google Scholar]
- Yahaya, M.S.; Nwannenna, A.I.; Fadason, S.T.; Rekwot, P.I. Testicular morphometry and sperm reserves of local turkey toms fed varying levels of protein in the diet. Sokoto J. Vet. Sci. 2017, 15, 10–14. [Google Scholar] [CrossRef][Green Version]
- Kerr, J.F.; Wyllie, A.H.; Currie, A.R. Apoptosis: A basic biological phenomenon with wideranging implications in tissue kinetics. Br. J. Cancer. 1972, 26, 239–257. [Google Scholar] [CrossRef] [PubMed]
- Qi, A.; Zhang, Z.P.; Cao, G.F.; Zhang, Y. Histological study of germ cells development and apoptosis in Mongolian sheep fetal ovaries. Anim. Reprod. Sci. 2008, 103, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Arends, M.J.; Morris, R.G.; Wyllie, A. Apoptosis. The role of the endonuclease. Am. J. Pathol. 1990, 136, 593. [Google Scholar] [PubMed]
- Shaha, C.; Tripathi, R.; Mishra, D.P. Male germ cell apoptosis: Regulation and biology. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 1501–1515. [Google Scholar] [CrossRef]
- MacLean, G.; Li, H.; Metzger, D.; Chambon, P.; Petkovich, M. Apoptotic extinction of germ cells in testes of Cyp26b1 knockout mice. Endocrinology 2007, 148, 4560–4567. [Google Scholar] [CrossRef]
- Hikim, A.P.S.; Lue, Y.; Diaz-Romero, M.; Yen, P.H.; Wang, C.; Swerdloff, R.S. Deciphering the pathways of germ cell apoptosis in the testis. J. Steroid Biochem. Mol. Biol. 2003, 85, 175–182. [Google Scholar] [CrossRef]
- Billig, H. Apoptosis in testis germ cells: Developmental changes in gonadotropin dependence and localization to selective tubule stages. Endocrinology 1995, 136, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Hawkins, K.L.; Dewolf, W.C.; Morgentaler, A. Heat stress causes testicular germ cell apoptosis in adult mice. J. Androl. 1997, 18, 159–165. [Google Scholar] [PubMed]
- Yan, W.; Suominen, J.; Toppari, J. Stem cell factor protects germ cells from apoptosis in vitro. J. Cell Sci. 2000, 113, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Dharani, P.; Ushakumary, S.; Sundaram, V.; Joseph, C.; Ramesh, G. Morphological Analysis of Testis of the Guinea Fowl (Numida meleagris) Under Tropical Savannah Climate of India. Int. J. Morphol. 2018, 36, 909–914. [Google Scholar] [CrossRef]
- França, L.R.; Russell, L.D. The testis of domestic mammals. In Male Reproduction: A Multidisciplinary Overview; Martinez-Garcia, F.J., Regadera, E., Eds.; Churchill Living-Stone Communication: Madrid, Spain, 1998; pp. 197–219. [Google Scholar]
- Kenagy, G.J.; Trombulack, S.C. Size and function of mammalian testes in relation to body size. J. Mammal. 1986, 67, 1–22. [Google Scholar] [CrossRef]
- Segatell, T.M.; Franca, L.R.; Inheiro, F.P.; Alemida, C.C.D.; Martinez, M.; Martinez, F.E. Spermatogenic Cycle length and spermatogenic efficiency in the Gerbil (Meriones unguiculatus). J. Androl. 2004, 2, 13. [Google Scholar] [CrossRef]
- Lunstra, D.D.; Wise, T.H.; Ford, J.J. Sertoli cells in the boar testis: Changes during development and compensatory hypertrophy after hemicastration at different ages. Biol. Reprod. 2003, 68, 140–150. [Google Scholar] [CrossRef]

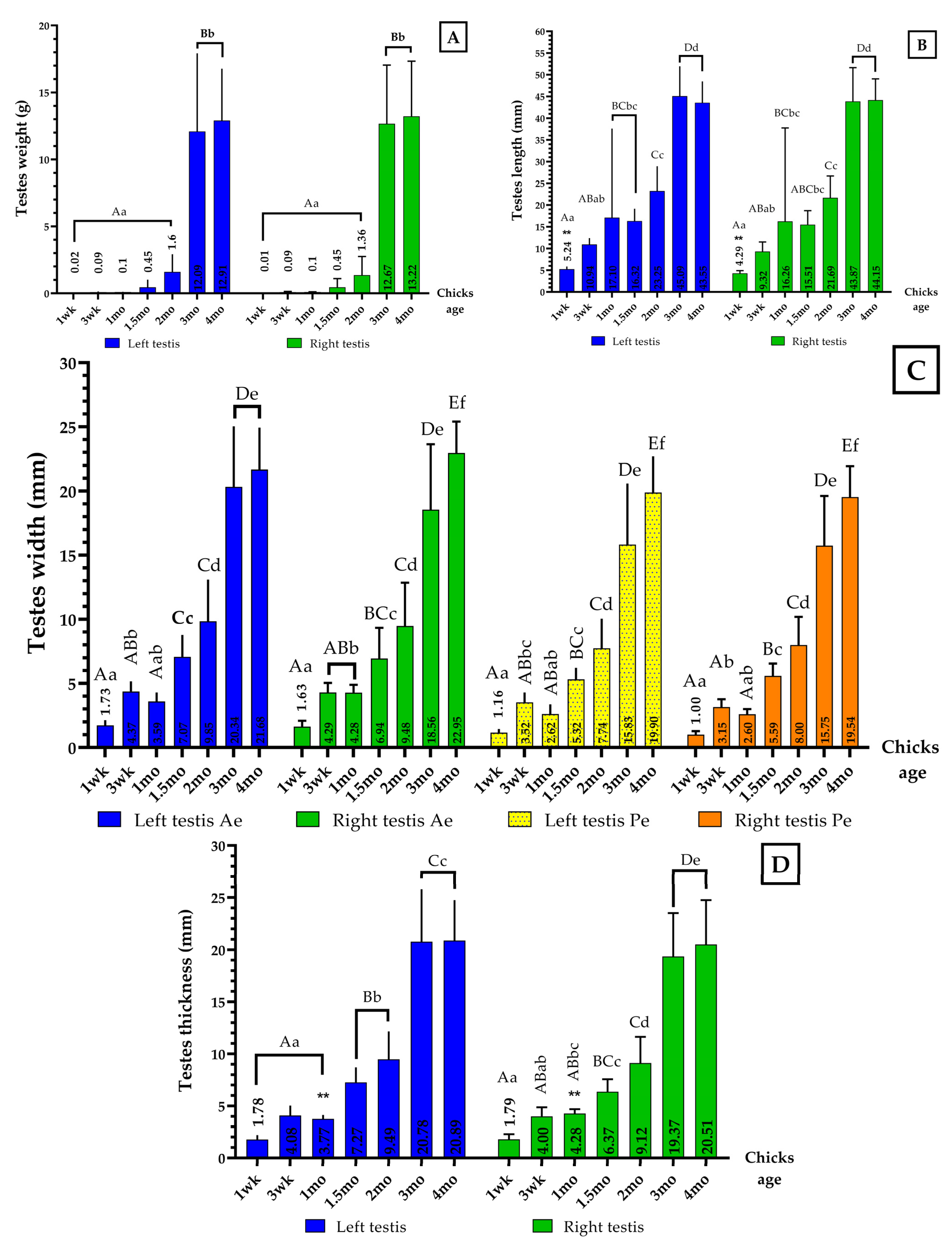



| Parameters | Both Testes Weight (g) | Bodyweight (g) | GSI (%) |
|---|---|---|---|
| wk 1 | 0.02 ± 0.01 Aa | 89.67 ± 7.42 Aa | 0.02 |
| wk 3 | 0.09 ± 0.05 Aa | 541.50 ± 66.91 Bb | 0.02 |
| mo 1 | 0.10 ± 0.02 Aa | 686.26 ± 70.30 Bb | 0.01 |
| mo 1.5 | 0.45 ± 0.60 Aa | 1557.64 ± 134.78 Cc | 0.03 |
| mo 2 | 1.48 ± 1.33 Aa | 2361.00 ± 295.88 Dd | 0.06 |
| mo 3 | 12.38 ± 5.02 Bb | 3332.00 ± 436.85 Ee | 0.37 |
| mo 4 | 13.07 ± 3.89 Bb | 4282.00 ± 419.01 Ff | 0.31 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mfoundou, J.D.L.; Guo, Y.; Yan, Z.; Wang, X. Morpho-Histology and Morphometry of Chicken Testes and Seminiferous Tubules among Yellow-Feathered Broilers of Different Ages. Vet. Sci. 2022, 9, 485. https://doi.org/10.3390/vetsci9090485
Mfoundou JDL, Guo Y, Yan Z, Wang X. Morpho-Histology and Morphometry of Chicken Testes and Seminiferous Tubules among Yellow-Feathered Broilers of Different Ages. Veterinary Sciences. 2022; 9(9):485. https://doi.org/10.3390/vetsci9090485
Chicago/Turabian StyleMfoundou, Jos Dorian Lawson, Yajun Guo, Zunqiang Yan, and Xinrong Wang. 2022. "Morpho-Histology and Morphometry of Chicken Testes and Seminiferous Tubules among Yellow-Feathered Broilers of Different Ages" Veterinary Sciences 9, no. 9: 485. https://doi.org/10.3390/vetsci9090485
APA StyleMfoundou, J. D. L., Guo, Y., Yan, Z., & Wang, X. (2022). Morpho-Histology and Morphometry of Chicken Testes and Seminiferous Tubules among Yellow-Feathered Broilers of Different Ages. Veterinary Sciences, 9(9), 485. https://doi.org/10.3390/vetsci9090485






