Importance of Antioxidant Supplementation during In Vitro Maturation of Mammalian Oocytes
Abstract
Simple Summary
Abstract
1. Introduction
2. In Vitro Maturation of Mammalian Oocytes
3. Normal Production and Elimination of Reactive Oxygen Species (ROS) by Mammalian Oocytes
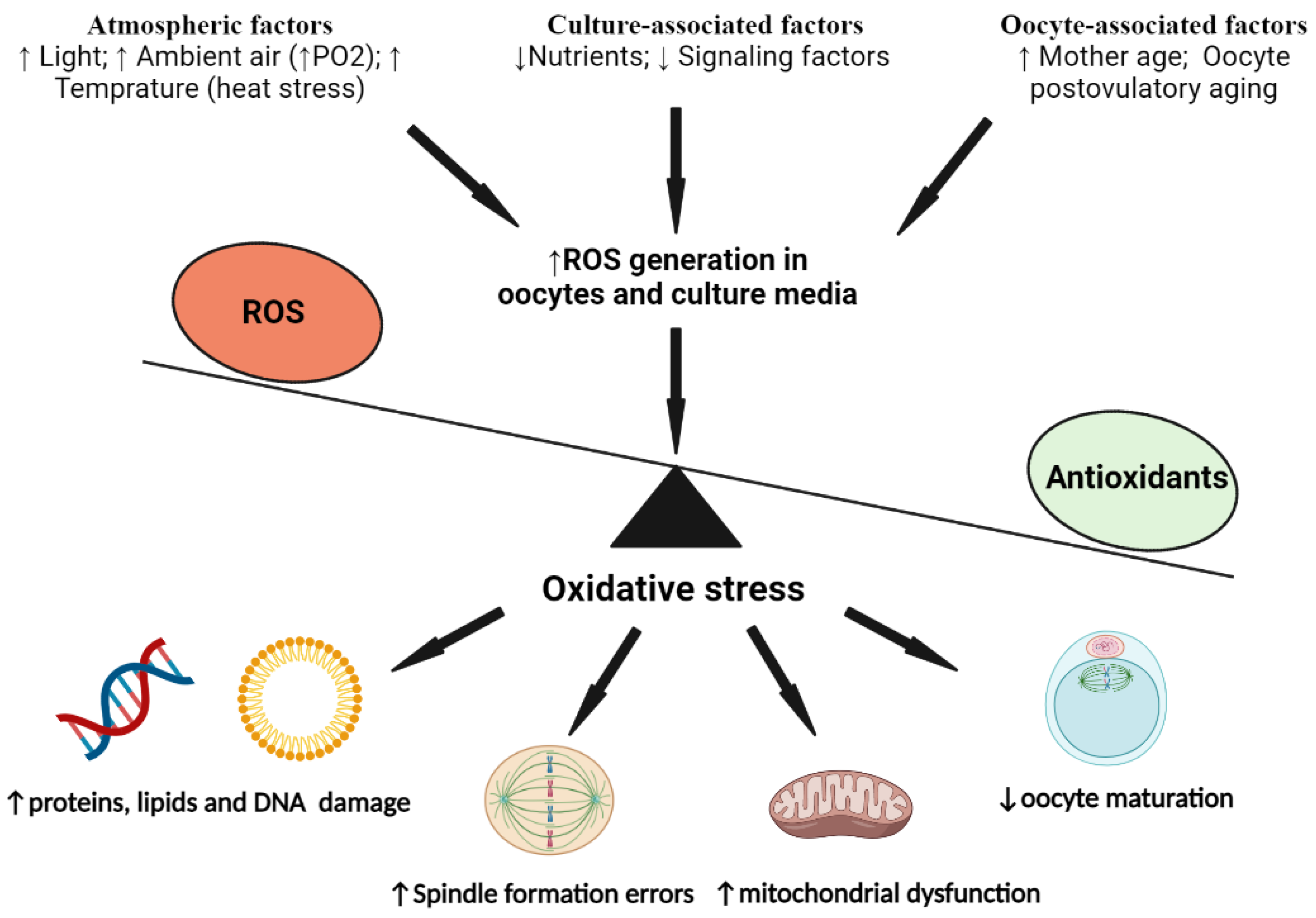
4. The Alteration in Oxidative Status of Mammalian Oocytes during Their IVM
5. Antioxidant Supplementation during IVM of Mammalian Oocytes to Counteract ROS-Induced Damage
6. Heat Stress and ROS Production by Mammalian Oocytes
6.1. Effect of Heat Stress on Oocyte ROS Production
6.2. Use of Antioxidants to Counteract ROS-Induced Damage of Heat-Stressed Oocytes
7. Maternal Aging and ROS Production by Mammalian Oocytes
7.1. Effect of Maternal Aging on Oocyte ROS Production
7.2. Use of Antioxidants to Counteract ROS-Induced Damage of Maternally Aged Oocytes
8. Postovulatory Aging and ROS Production by Mammalian Oocytes
8.1. Effect of Postovulatory Aging on Oocyte ROS Production
8.2. Use of Antioxidants to Counteract ROS-Induced Damage of Postovulatory Aged Oocytes
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baldassarre, H. Laparoscopic Ovum Pick-Up Followed by In Vitro Embryo Production and Transfer in Assisted Breeding Programs for Ruminants. Animals 2021, 11, 216. [Google Scholar] [CrossRef] [PubMed]
- Wani, N.A. In vitro embryo production (IVEP) in camelids, Present status and future perspectives. Reprod. Biol. 2021, 21, 100471. [Google Scholar] [CrossRef] [PubMed]
- Baldassarre, H. Practical aspects for implementing in vitro embryo production and cloning programs in sheep and goats. Anim. Reprod. 2018, 9, 188–194. [Google Scholar]
- Hatırnaz, Ş.; Ata, B.; Hatırnaz, E.S.; Dahan, M.H.; Tannus, S.; Tan, J.; Tan, S.L. Oocyte in vitro maturation, A sytematic review. Turk. J. Obstet. Gynecol. 2018, 15, 112–125. [Google Scholar] [CrossRef] [PubMed]
- Mikkelsen, A.L.; Smith, S.; Lindenberg, S. Possible factors affecting the development of oocytes in in-vitro maturation. Hum. Reprod. 2000, 15 (Suppl. 5), 11–17. [Google Scholar] [CrossRef][Green Version]
- Ye, R.; Xu, S.; Liu, Y.; Pang, L.; Lian, X.; Zhong, Y.; Su, Y.; Wang, S. Protective Effect of Icariin on the Development of Preimplantation Mouse Embryos against Hydrogen Peroxide-Induced Oxidative Injury. Oxid. Med. Cell. Longev. 2017, 2017, 2704532. [Google Scholar] [CrossRef]
- Jiang, H.; Liang, S.; Yao, X.R.; Jin, Y.X.; Shen, X.H.; Yuan, B.; Zhang, J.B.; Kim, N.H. Laminarin improves developmental competence of porcine early stage embryos by inhibiting oxidative stress. Theriogenology 2018, 115, 38–44. [Google Scholar] [CrossRef]
- Guérin, P.; El Mouatassim, S.; Ménézo, Y. Oxidative stress and protection against reactive oxygen species in the pre-implantation embryo and its surroundings. Hum. Reprod. Update 2001, 7, 175–189. [Google Scholar] [CrossRef]
- Khazaei, M.; Aghaz, F. Reactive oxygen species generation and use of antioxidants during in vitro maturation of oocytes. Int. J. Fertil. Steril. 2017, 11, 63–70. [Google Scholar]
- Hansen, P.J. Effects of heat stress on mammalian reproduction. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2009, 364, 3341–3350. [Google Scholar] [CrossRef]
- Sasaki, H.; Hamatani, T.; Kamijo, S.; Iwai, M.; Kobanawa, M.; Ogawa, S.; Miyado, K.; Tanaka, M. Impact of oxidative stress on age-associated decline in oocyte developmental competence. Front. Endocrinol. 2019, 10, 811. [Google Scholar] [CrossRef] [PubMed]
- McGinnis, L.K.; Pelech, S.; Kinsey, W.H. Post-ovulatory aging of oocytes disrupts kinase signaling pathways and lysosome biogenesis. Mol. Reprod. Dev. 2014, 81, 928–945. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.; Tiwari, M.; Pandey, A.N.; Shrivastav, T.G.; Chaube, S.K. Impact of stress on oocyte quality and reproductive outcome. J. Biomed. Sci. 2016, 23, 36. [Google Scholar] [CrossRef]
- Takahashi, M. Oxidative stress and redox regulation on in vitro development of mammalian embryos. J. Reprod. Dev. 2012, 58, 1–9. [Google Scholar] [CrossRef]
- Silva, E.; Greene, A.F.; Strauss, K.; Herrick, J.R.; Schoolcraft, W.B.; Krisher, R.L. Antioxidant supplementation during in vitro culture improves mitochondrial function and development of embryos from aged female mice. Reprod. Fertil. Dev. 2015, 27, 975–983. [Google Scholar] [CrossRef]
- Ferreira, E.; Vireque, A.; Adona, P.; Meirelles, F.; Ferriani, R.; Navarro, P. Cytoplasmic maturation of bovine oocytes: Structural and biochemical modifications and acquisition of developmental competence. Theriogenology 2009, 71, 836–848. [Google Scholar] [CrossRef]
- Rao, B.; Naidu, K.; Amarnath, D.; Vagdevi, R.; Rao, A.; Brahmaiah, K.; Rao, V. In vitro maturation of sheep oocytes in different media during breeding and non-breeding seasons. Small Rumin. Res. 2002, 43, 31–36. [Google Scholar] [CrossRef]
- Gupta, P.; Ravindra, J.; Kumar, V.G.; Raghu, H.; Nandi, S. Stimulation of in vitro ovine oocyte maturation with a novel peptide isolated from follicular fluid of the buffalo (Bubalus bubalis). Small Rumin. Res. 2005, 59, 33–40. [Google Scholar] [CrossRef]
- Miclea, I.; Hettig, A.; Zahan, M.; Roman, I.; Miclea, V. The effect of several α-tocopherol concentrations on swine oocyte maturation and embryo culture. Bull. UASVM Anim. Sci. Biotechnol. 2009, 66, 385–392. [Google Scholar]
- Watson, A. Oocyte cytoplasmic maturation: A key mediator of oocyte and embryo developmental competence. J. Anim. Sci. 2007, 85, E1–E3. [Google Scholar] [CrossRef]
- Mao, L.; Lou, H.; Lou, Y.; Wang, N.; Jin, F. Behaviour of cytoplasmic organelles and cytoskeleton during oocyte maturation. Reprod. BioMed. Online 2014, 28, 284–299. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.-C.; Kim, N.-H. Molecular mechanisms of asymmetric division in oocytes. Microsc. Microanal. 2013, 19, 883–897. [Google Scholar] [CrossRef] [PubMed]
- Gosden, R.; Lee, B. Portrait of an oocyte: Our obscure origin. J. Clin. Investig. 2010, 120, 973–983. [Google Scholar] [CrossRef] [PubMed]
- Almonacid, M.; Al Jord, A.; El-Hayek, S.; Othmani, A.; Coulpier, F.; Lemoine, S.; Miyamoto, K.; Grosse, R.; Klein, C.; Piolot, T. Active fluctuations of the nuclear envelope shape the transcriptional dynamics in oocytes. Dev. Cell 2019, 51, 145–157.e10. [Google Scholar] [CrossRef]
- Sun, S.-C.; Wang, Z.-B.; Xu, Y.-N.; Lee, S.-E.; Cui, X.-S.; Kim, N.-H. Arp2/3 complex regulates asymmetric division and cytokinesis in mouse oocytes. PLoS ONE 2011, 6, e18392. [Google Scholar] [CrossRef]
- Zeng, J.; Jiang, M.; Wu, X.; Diao, F.; Qiu, D.; Hou, X.; Wang, H.; Li, L.; Li, C.; Ge, J. SIRT 4 is essential for metabolic control and meiotic structure during mouse oocyte maturation. Aging Cell 2018, 17, e12789. [Google Scholar] [CrossRef]
- Gilchrist, R.B.; Thompson, J.G. Oocyte maturation: Emerging concepts and technologies to improve developmental potential in vitro. Theriogenology 2007, 67, 6–15. [Google Scholar] [CrossRef]
- Eppig, J.J. Coordination of nuclear and cytoplasmic oocyte maturation in eutherian mammals. Reprod. Fertil. Dev. 1996, 8, 485–489. [Google Scholar] [CrossRef]
- Combelles, C.M.H.; Cekleniak, N.A.; Racowsky, C.; Albertini, D.F. Assessment of nuclear and cytoplasmic maturation in in-vitro matured human oocytes. Hum. Reprod. 2002, 17, 1006–1016. [Google Scholar] [CrossRef]
- Goudet, G.; Bézard, J.; Duchamp, G.; Gérard, N.; Palmer, E. Equine oocyte competence for nuclear and cytoplasmic in vitro maturation: Effect of follicle size and hormonal environment. Biol. Reprod. 1997, 57, 232–245. [Google Scholar] [CrossRef][Green Version]
- Lange Consiglio, A.; Arrighi, S.; Cremonesi, F. Time course of in vitro maturation of compact cumulus horse oocytes after roscovitine-induced meiotic inhibition: Effects on the coordination between nuclear and cytoplasmic maturation. Reprod. Domest. Anim. 2010, 45, e313–e322. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Zhao, S.; Xu, S.; Zhang, D.; Zhu, M.; Pan, Q.; Huang, J. Granulosa Cells Improved Mare Oocyte Cytoplasmic Maturation by Providing Collagens. Front. Cell Dev. Biol. 2022, 10, 914735. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B.; Gutteridge, J.M. Free Radicals in Biology and Medicine; Oxford University Press: Oxford, UK, 2015. [Google Scholar]
- Oborna, I.; Wojewodka, G.; de Sanctis, J.B.; Fingerova, H.; Svobodova, M.; Brezinova, J.; Hajduch, M.; Novotny, J.; Radova, L.; Radzioch, D. Increased lipid peroxidation and abnormal fatty acid profiles in seminal and blood plasma of normozoospermic males from infertile couples. Hum. Reprod. 2010, 25, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Yalçınkaya, E.; Çakıroğlu, Y.; Doğer, E.; Budak, Ö.; Çekmen, M.; Çalışkan, E. Effect of follicular fluid NO, MDA and GSH levels on in vitro fertilization outcomes. J. Turk. Ger. Gynecol. Assoc. 2013, 14, 136–141. [Google Scholar] [CrossRef]
- Wiener-Megnazi, Z.; Vardi, L.; Lissak, A.; Shnizer, S.; Reznick, A.Z.; Ishai, D.; Lahav-Baratz, S.; Shiloh, H.; Koifman, M.; Dirnfeld, M. Oxidative stress indices in follicular fluid as measured by the thermochemiluminescence assay correlate with outcome parameters in in vitro fertilization. Fertil. Steril. 2004, 82, 1171–1176. [Google Scholar] [CrossRef] [PubMed]
- Sugino, N. Roles of reactive oxygen species in the corpus luteum. Anim. Sci. J. 2006, 77, 556–565. [Google Scholar] [CrossRef]
- Combelles, C.M.; Gupta, S.; Agarwal, A. Could oxidative stress influence the in-vitro maturation of oocytes? Reprod. Biomed. Online 2009, 18, 864–880. [Google Scholar] [CrossRef]
- Kala, M.; Shaikh, M.V.; Nivsarkar, M. Equilibrium between anti-oxidants and reactive oxygen species: A requisite for oocyte development and maturation. Reprod. Med. Biol. 2017, 16, 28–35. [Google Scholar] [CrossRef]
- Chelikani, P.; Fita, I.; Loewen, P.C. Diversity of structures and properties among catalases. Cell. Mol. Life Sci. 2004, 61, 192–208. [Google Scholar] [CrossRef]
- Brigelius-Flohé, R. Tissue-specific functions of individual glutathione peroxidases. Free. Radic. Biol. Med. 1999, 27, 951–965. [Google Scholar] [CrossRef]
- Johnson, F.; Giulivi, C. Superoxide dismutases and their impact upon human health. Mol. Asp. Med. 2005, 26, 340–352. [Google Scholar] [CrossRef] [PubMed]
- Changyong, C.; Yong-Won, S.; Eun-Jin, K.; Sang-Rae, C.; Hyun-Jong, K.; Man-Hye, H.; Jaehee, H.; Dong-Soo, S.; Dawon, K. Synergistic effects of glutathione and β-mercaptoethanol treatment during in vitro maturation of porcine oocytes on early embryonic development in a culture system supplemented with L-cysteine. J. Reprod. Dev. 2010, 56, 575–582. [Google Scholar]
- Nishikimi, M.; Yagi, K. Biochemistry and molecular biology of ascorbic acid biosynthesis. Subcell. Biochem. Ascorbic Acid Biochem. Biomed. Cell Biol. 1996, 25, 17–39. [Google Scholar] [CrossRef]
- Schneider, C. Chemistry and biology of vitamin E. Mol. Nutr. Food Res. 2005, 49, 7–30. [Google Scholar] [CrossRef]
- Zhao, X.M.; Hao, H.S.; Du, W.H.; Zhao, S.J.; Wang, H.Y.; Wang, N.; Wang, D.; Liu, Y.; Qin, T.; Zhu, H.B. Melatonin inhibits apoptosis and improves the developmental potential of vitrified bovine oocytes. J. Pineal Res. 2016, 60, 132–141. [Google Scholar] [CrossRef]
- Wu, G.-Q.; Jia, B.-Y.; Li, J.-J.; Fu, X.-W.; Zhou, G.-B.; Hou, Y.-P.; Zhu, S.-E. L-carnitine enhances oocyte maturation and development of parthenogenetic embryos in pigs. Theriogenology 2011, 76, 785–793. [Google Scholar] [CrossRef]
- You, J.; Lee, J.; Hyun, S.-H.; Lee, E. L-carnitine treatment during oocyte maturation improves in vitro development of cloned pig embryos by influencing intracellular glutathione synthesis and embryonic gene expression. Theriogenology 2012, 78, 235–243. [Google Scholar] [CrossRef]
- Chowdhury, M.; Mesalam, A.; Khan, I.; Joo, M.D.; Lee, K.L.; Xu, L.; Afrin, F.; Kong, I.K. Improved developmental competence in embryos treated with lycopene during in vitro culture system. Mol. Reprod. Dev. 2018, 85, 46–61. [Google Scholar] [CrossRef]
- Agarwal, A.; Said, T.M.; Bedaiwy, M.A.; Banerjee, J.; Alvarez, J.G. Oxidative stress in an assisted reproductive techniques setting. Fertil. Steril. 2006, 86, 503–512. [Google Scholar] [CrossRef]
- Brunet, S.; Maro, B. Cytoskeleton and cell cycle control during meiotic maturation of the mouse oocyte: Integrating time and space. Reproduction 2005, 130, 801–811. [Google Scholar] [CrossRef]
- Leoni, G.G.; Rosati, I.; Succu, S.; Bogliolo, L.; Bebbere, D.; Berlinguer, F.; Ledda, S.; Naitana, S. A low oxygen atmosphere during IVF accelerates the kinetic of formation of in vitro produced ovine blastocysts. Reprod. Domest. Anim. 2007, 42, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Liebel, F.; Kaur, S.; Ruvolo, E.; Kollias, N.; Southall, M.D. Irradiation of skin with visible light induces reactive oxygen species and matrix-degrading enzymes. J. Investig. Dermatol. 2012, 132, 1901–1907. [Google Scholar] [CrossRef] [PubMed]
- Khoudja, R.Y.; Xu, Y.; Li, T.; Zhou, C. Better IVF outcomes following improvements in laboratory air quality. J. Assist. Reprod. Genet. 2013, 30, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, M.; Prasad, S.; Tripathi, A.; Pandey, A.N.; Singh, A.K.; Shrivastav, T.G.; Chaube, S.K. Involvement of reactive oxygen species in meiotic cell cycle regulation and apoptosis in mammalian oocytes. React. Oxyg. Species 2016, 1, 110–116. [Google Scholar] [CrossRef]
- Johnson, M.H.; Nasresfahani, M.H. Radical solutions and cultural problems: Could free oxygen radicals be responsible for the impaired development of preimplantation mammalian embryos in vitro? Bioessays 1994, 16, 31–38. [Google Scholar] [CrossRef]
- Velez-Pardo, C.; Morales, A.T.; del Rio, M.J.; Olivera-Angel, M. Endogenously generated hydrogen peroxide induces apoptosis via mitochondrial damage independent of NF-κB and p53 activation in bovine embryos. Theriogenology 2007, 67, 1285–1296. [Google Scholar] [CrossRef]
- Fujitani, Y.; Kasai, K.; Ohtani, S.; Nishimura, K.; Yamada, M.; Utsumi, K. Effect of oxygen concentration and free radicals on in vitro development of in vitro-produced bovine embryos. J. Anim. Sci. 1997, 75, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Olson, S.; Seidel, G. Culture of in vitro-produced bovine embryos with vitamin E improves development in vitro and after transfer to recipients. Biol. Reprod. 2000, 62, 248–252. [Google Scholar] [CrossRef]
- Fischer, B.; Bavister, B. Oxygen tension in the oviduct and uterus of rhesus monkeys, hamsters and rabbits. J. Reprod. Fertil. 1993, 99, 673–679. [Google Scholar] [CrossRef]
- Kaufman, D.L.; Mitchell, J.A. Intrauterine oxygen tension during the oestrous cycle in the hamster: Patterns of change. Comp. Biochem. Physiol. Part A Physiol. 1994, 107, 673–678. [Google Scholar] [CrossRef]
- Thompson, J.; Simpson, A.; Pugh, P.; Donnelly, P.; Tervit, H. Effect of oxygen concentration on in-vitro development of preimplantation sheep and cattle embryos. J. Reprod. Fertil. 1990, 89, 573–578. [Google Scholar] [CrossRef] [PubMed]
- Batt, P.; Gardner, D.; Cameron, A. Oxygen concentration and protein source affect the development of preimplantation goat embryos in vitro. Reprod. Fertil. Dev. 1991, 3, 601–607. [Google Scholar] [CrossRef] [PubMed]
- Booth, P.J.; Holm, P.; Callesen, H. The effect of oxygen tension on porcine embryonic development is dependent on embryo type. Theriogenology 2005, 63, 2040–2052. [Google Scholar] [CrossRef] [PubMed]
- Rinaudo, P.F.; Giritharan, G.; Talbi, S.; Dobson, A.T.; Schultz, R.M. Effects of oxygen tension on gene expression in preimplantation mouse embryos. Fertil. Steril. 2006, 86, 1252–1265. [Google Scholar] [CrossRef]
- Ciray, H.N.; Aksoy, T.; Yaramanci, K.; Karayaka, I.; Bahceci, M. In vitro culture under physiologic oxygen concentration improves blastocyst yield and quality: A prospective randomized survey on sibling oocytes. Fertil. Steril. 2009, 91, 1459–1461. [Google Scholar] [CrossRef]
- Nakayama, T.; Noda, Y.; Goto, Y.; Mori, T. Effects of visible light and other environmental factors on the production of oxygen radicals by hamster embryos. Theriogenology 1994, 41, 499–510. [Google Scholar] [CrossRef]
- Hashimoto, S.; Minami, N.; Yamada, M.; Imai, H. Excessive concentration of glucose during in vitro maturation impairs the developmental competence of bovine oocytes after in vitro fertilization: Relevance to intracellular reactive oxygen species and glutathione contents. Mol. Reprod. Dev. 2000, 56, 520–526. [Google Scholar] [CrossRef]
- Vandaele, L.; Thys, M.; Bijttebier, J.; van Langendonckt, A.; Donnay, I.; Maes, D.; Meyer, E.; van Soom, A. Short-term exposure to hydrogen peroxide during oocyte maturation improves bovine embryo development. Reproduction 2010, 139, 505–511. [Google Scholar] [CrossRef]
- Tatemoto, H.; Sakurai, N.; Muto, N. Protection of porcine oocytes against apoptotic cell death caused by oxidative stress during in vitro maturation: Role of cumulus cells. Biol. Reprod. 2000, 63, 805–810. [Google Scholar] [CrossRef]
- Luciano, A.M.; Goudet, G.; Perazzoli, F.; Lahuec, C.; Gérard, N. Glutathione content and glutathione peroxidase expression in in vivo and in vitro matured equine oocytes. Mol. Reprod. Dev. 2006, 73, 658–666. [Google Scholar] [CrossRef] [PubMed]
- Cetica, P.; Pintos, L.; Dalvit, G.; Beconi, M. Antioxidant enzyme activity and oxidative stress in bovine oocyte in vitro maturation. IUBMB Life 2001, 51, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Jiang, S.; Wozniak, P.J.; Yang, X.; Godke, R.A. Cumulus cell function during bovine oocyte maturation, fertilization, and embryo development in vitro. Mol. Reprod. Dev. 1995, 40, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Tamura, H.; Takasaki, A.; Miwa, I.; Taniguchi, K.; Maekawa, R.; Asada, H.; Taketani, T.; Matsuoka, A.; Yamagata, Y.; Shimamura, K. Oxidative stress impairs oocyte quality and melatonin protects oocytes from free radical damage and improves fertilization rate. J. Pineal Res. 2008, 44, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Tatemoto, H.; Muto, N.; Sunagawa, I.; Shinjo, A.; Nakada, T. Protection of porcine oocytes against cell damage caused by oxidative stress during in vitro maturation: Role of superoxide dismutase activity in porcine follicular fluid. Biol. Reprod. 2004, 71, 1150–1157. [Google Scholar] [CrossRef]
- Takami, M.; Preston, S.; Toyloy, V.; Behrman, H.R. Antioxidants reversibly inhibit the spontaneous resumption of meiosis. Am. J. Physiol.-Endocrinol. Metab. 1999, 276, E684–E688. [Google Scholar] [CrossRef]
- Tarin, J.J. Potential effects of age-associated oxidative stress on mammalian oocytes/embryos. Mol. Hum. Reprod. 1996, 2, 717–724. [Google Scholar] [CrossRef]
- Hassold, T.; Hunt, P. To err (meiotically) is human: The genesis of human aneuploidy. Nat. Rev. Genet. 2001, 2, 280–291. [Google Scholar] [CrossRef] [PubMed]
- Tarin, J.; Vendrell, F.J.; Ten, J.; Cano, A. Antioxidant therapy counteracts the disturbing effects of diamide and maternal ageing on meiotic division and chromosomal segregation in mouse oocytes. Mol. Hum. Reprod. 1998, 4, 281–288. [Google Scholar] [CrossRef]
- Sanfins, A.; Plancha, C.E.; Overstrom, E.W.; Albertini, D.F. Meiotic spindle morphogenesis in in vivo and in vitro matured mouse oocytes: Insights into the relationship between nuclear and cytoplasmic quality. Hum. Reprod. 2004, 19, 2889–2899. [Google Scholar] [CrossRef]
- Li, Y.; Feng, H.-L.; Cao, Y.-J.; Zheng, G.-J.; Yang, Y.; Mullen, S.; Critser, J.K.; Chen, Z.-J. Confocal microscopic analysis of the spindle and chromosome configurations of human oocytes matured in vitro. Fertil. Steril. 2006, 85, 827–832. [Google Scholar] [CrossRef]
- Sanfins, A.; Lee, G.Y.; Plancha, C.E.; Overstrom, E.W.; Albertini, D.F. Distinctions in Meiotic Spindle Structure and Assembly During In Vitro and In Vivo Maturation of Mouse Oocytes. Biol. Reprod. 2003, 69, 2059–2067. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.-J.; Banerjee, J.; Falcone, T.; Bena, J.; Agarwal, A.; Sharma, R.K. Oxidative stress and tumor necrosis factor–α–induced alterations in metaphase II mouse oocyte spindle structure. Fertil. Steril. 2007, 88, 1220–1231. [Google Scholar] [CrossRef] [PubMed]
- Morado, S.A.; Cetica, P.D.; Beconi, M.T.; Dalvit, G.C. Reactive oxygen species in bovine oocyte maturation in vitro. Reprod. Fertil. Dev. 2009, 21, 608–614. [Google Scholar] [CrossRef] [PubMed]
- Romero, S.; Pella, R.; Zorrilla, I.; Berrío, P.; Escudero, F.; Pérez, Y.; García, M.; Gonzalez, C.; Orihuela, P. Coenzyme Q10 improves the in vitro maturation of oocytes exposed to the intrafollicular environment of patients on fertility treatment. JBRA Assist. Reprod. 2020, 24, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Sovernigo, T.; Adona, P.; Monzani, P.; Guemra, S.; Barros, F.; Lopes, F.; Leal, C. Effects of supplementation of medium with different antioxidants during in vitro maturation of bovine oocytes on subsequent embryo production. Reprod. Domest. Anim. 2017, 52, 561–569. [Google Scholar] [CrossRef] [PubMed]
- Budani, M.C.; Tiboni, G.M. Effects of Supplementation with Natural Antioxidants on Oocytes and Preimplantation Embryos. Antioxidants 2020, 9, 612. [Google Scholar] [CrossRef]
- Kang, J.T.; Koo, O.J.; Kwon, D.K.; Park, H.J.; Jang, G.; Kang, S.K.; Lee, B.C. Effects of melatonin on in vitro maturation of porcine oocyte and expression of melatonin receptor RNA in cumulus and granulosa cells. J. Pineal Res. 2009, 46, 22–28. [Google Scholar] [CrossRef]
- Zhao, X.-M.; Min, J.-T.; Du, W.-H.; Hao, H.-S.; Liu, Y.; Qin, T.; Wang, D.; Zhu, H.-B. Melatonin enhances the in vitro maturation and developmental potential of bovine oocytes denuded of the cumulus oophorus. Zygote 2015, 23, 525–536. [Google Scholar] [CrossRef]
- Nagina, G.; Asima, A.; Nemat, U.; Shamim, A. Effect of melatonin on maturation capacity and fertilization of Nili-Ravi buffalo (Bubalus bubalis) oocytes. Open Vet. J. 2016, 6, 128–134. [Google Scholar] [CrossRef]
- Tian, X.; Wang, F.; Zhang, L.; He, C.; Ji, P.; Wang, J.; Zhang, Z.; Lv, D.; Abulizi, W.; Wang, X.; et al. Beneficial Effects of Melatonin on the In Vitro Maturation of Sheep Oocytes and Its Relation to Melatonin Receptors. Int. J. Mol. Sci. 2017, 18, 834. [Google Scholar] [CrossRef]
- Keshavarzi, S.; Salehi, M.; Farifteh-Nobijari, F.; Hosseini, T.; Hosseini, S.; Ghazifard, A.; Novin, M.G.; Fallah-Omrani, V.; Nourozian, M.; Hosseini, A. Melatonin modifies histone acetylation during in vitro maturation of mouse oocytes. Cell J. 2018, 20, 244. [Google Scholar] [PubMed]
- Chowdhury, M.; Choi, B.-H.; Khan, I.; Lee, K.-L.; Mesalam, A.; Song, S.-H.; Xu, L.; Joo, M.-D.; Afrin, F.; Kong, I.-K. Supplementation of lycopene in maturation media improves bovine embryo quality in vitro. Theriogenology 2017, 103, 173–184. [Google Scholar] [CrossRef]
- Rakha, S.I.; Elmetwally, M.A.; Ali, H.E.; Balboula, A.Z.; Mahmoud, A.M.; Zaabel, S.M. Lycopene improves maturation rate and antioxidant status of in vitro matured mouse oocytes. Int. J. Vet. Sci. 2022; unpublished manuscript. [Google Scholar]
- Xiang, D.-C.; Jia, B.-Y.; Fu, X.-W.; Guo, J.-X.; Hong, Q.-H.; Quan, G.-B.; Wu, G.-Q. Role of astaxanthin as an efficient antioxidant on the in vitro maturation and vitrification of porcine oocytes. Theriogenology 2021, 167, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.A.; Chaudhary, S.S.; Puri, G.; Singh, V.K.; Odedara, A.B. Effects of β-mercaptoethanol on in vitro maturation and glutathione level of buffalo oocytes. Vet. World 2015, 8, 213–216. [Google Scholar] [CrossRef]
- Merlo, B.; Iacono, E.; Bucci, D.; Spinaci, M.; Galeati, G.; Mari, G. Beta-mercaptoethanol supplementation of in vitro maturation medium does not influence nuclear and cytoplasmic maturation of equine oocytes. Reprod. Domest. Anim. 2016, 51, 992–996. [Google Scholar] [CrossRef]
- Chen, N.; Liow, S.-L.; Yip, W.-Y.; Tan, L.-G.; Ng, S.-C. Influence of cysteamine supplementation and culture in portable dry-incubator on the in vitro maturation, fertilization and subsequent development of mouse oocytes. Theriogenology 2005, 63, 2300–2310. [Google Scholar] [CrossRef]
- Nadri, B.; Zeinoaldini, S.; Kohram, H. Ascorbic acid effects on in vitro maturation of mouse oocyte with or without cumulus cell. Afr. J. Biotechnol. 2009, 8, 5627–5631. [Google Scholar]
- Dalvit, G.; Llanes, S.; Descalzo, A.; Insani, M.; Beconi, M.; Cetica, P. Effect of alpha-tocopherol and ascorbic acid on bovine oocyte in vitro maturation. Reprod. Domest. Anim. 2005, 40, 93–97. [Google Scholar] [CrossRef]
- Tareq, K.; Akter, Q.S.; Mam, Y.; Tsujii, H. Selenium and vitamin E improve the in vitro maturation, fertilization and culture to blastocyst of porcine oocytes. J. Reprod. Dev. 2012, 58, 621–628. [Google Scholar] [CrossRef]
- Wang, F.; Tian, X.; Zhang, L.; He, C.; Ji, P.; Li, Y.; Tan, D.; Liu, G. Beneficial effect of resveratrol on bovine oocyte maturation and subsequent embryonic development after in vitro fertilization. Fertil. Steril. 2014, 101, 577–586.e1. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhu, X.; Liang, X.; Xu, H.; Liao, Y.; Lu, K.; Lu, S. Effects of resveratrol on in vitro maturation of porcine oocytes and subsequent early embryonic development following somatic cell nuclear transfer. Reprod. Domest. Anim. 2019, 54, 1195–1205. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Zhao, H.; Wang, Z.; Zhang, C.; Bian, Y.; Liu, X.; Zhang, C.; Zhang, X.; Zhao, Y. Quercetin promotes in vitro maturation of oocytes from humans and aged mice. Cell Death Dis. 2020, 11, 965. [Google Scholar] [CrossRef] [PubMed]
- Fathi, M.; El-Shahat, K. L-carnitine enhances oocyte maturation and improves in vitro development of embryos in dromedary camels (Camelus dromedaries). Theriogenology 2017, 104, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Moawad, A.R.; Salama, A.; Badr, M.R.; Fathi, M. Beneficial Effects of L-Carnitine Supplementation during IVM of Canine Oocytes on Their Nuclear Maturation and Development In Vitro. Animals 2021, 11, 581. [Google Scholar] [CrossRef]
- Pu, Y.; Wang, Z.; Bian, Y.; Zhang, F.; Yang, P.; Li, Y.; Zhang, Y.; Liu, Y.; Fang, F.; Cao, H. All-trans retinoic acid improves goat oocyte nuclear maturation and reduces apoptotic cumulus cells during in vitro maturation. Anim. Sci. J. 2014, 85, 833–839. [Google Scholar] [CrossRef]
- Saadeldin, I.M.; Swelum, A.A.; Elsafadi, M.; Mahmood, A.; Yaqoob, S.H.; Alfayez, M.; Alowaimer, A.N. Effects of all-trans retinoic acid on the in vitro maturation of camel (Camelus dromedarius) cumulus-oocyte complexes. J. Reprod. Dev. 2019, 65, 215–221. [Google Scholar] [CrossRef]
- Maside, C.; Martinez, C.A.; Cambra, J.M.; Lucas, X.; Martinez, E.A.; Gil, M.A.; Rodriguez-Martinez, H.; Parrilla, I.; Cuello, C. Supplementation with exogenous coenzyme Q10 to media for in vitro maturation and embryo culture fails to promote the developmental competence of porcine embryos. Reprod. Domest. Anim. 2019, 54, 72–77. [Google Scholar] [CrossRef]
- Ma, L.; Cai, L.; Hu, M.; Wang, J.; Xie, J.; Xing, Y.; Shen, J.; Cui, Y.; Liu, X.J.; Liu, J. Coenzyme Q10 supplementation of human oocyte in vitro maturation reduces postmeiotic aneuploidies. Fertil. Steril. 2020, 114, 331–337. [Google Scholar] [CrossRef]
- Click, R.E. A review: Alteration of in vitro reproduction processes by thiols—Emphasis on 2-mercaptoethanol. J. Reprod. Dev. 2014, 60, 399–405. [Google Scholar] [CrossRef]
- Deponte, M. The Incomplete Glutathione Puzzle, Just Guessing at Numbers and Figures? Antioxid. Redox Signal. 2017, 27, 1130–1161. [Google Scholar] [CrossRef] [PubMed]
- Gardiner, C.S.; Salmen, J.J.; Brandt, C.J.; Stover, S.K. Glutathione is present in reproductive tract secretions and improves development of mouse embryos after chemically induced glutathione depletion. Biol. Reprod. 1998, 59, 431–436. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Bilodeau, J.; Sirard, M. Antioxidant requirements for bovine oocytes varies during in vitro maturation, fertilization and development. Theriogenology 2003, 59, 939–949. [Google Scholar] [CrossRef]
- Janjic, D.; Wollheim, C.B. Effect of 2-mercaptoethanol on glutathione levels, cystine uptake and insulin secretion in insulin-secreting cells. Eur. J. Biochem. 1992, 210, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Ishii, T.; Bannai, S.; Sugita, Y. Mechanism of growth stimulation of L1210 cells by 2-mercaptoethanol in vitro. Role of the mixed disulfide of 2-mercaptoethanol and cysteine. J. Biol. Chem. 1981, 256, 12387–12392. [Google Scholar]
- de Matos, D.G.; Furnus, C.C.; Moses, D.F.; Baldassarre, H. Effect of cysteamine on glutathione level and developmental capacity of bovine oocyte matured in vitro. Mol. Reprod. Dev. 1995, 42, 432–436. [Google Scholar] [CrossRef]
- de Matos, D.G.; Gasparrini, B.; Pasqualini, S.R.; Thompson, J.G. Effect of glutathione synthesis stimulation during in vitro maturation of ovine oocytes on embryo development and intracellular peroxide content. Theriogenology 2002, 57, 1443–1451. [Google Scholar] [CrossRef]
- Kitagawa, Y.; Suzuki, K.; Yoneda, A.; Watanabe, T. Effects of oxygen concentration and antioxidants on the in vitro developmental ability, production of reactive oxygen species (ROS), and DNA fragmentation in porcine embryos. Theriogenology 2004, 62, 1186–1197. [Google Scholar] [CrossRef]
- Kim, M.K.; Fibrianto, Y.H.; Oh, H.J.; Jang, G.; Kim, H.J.; Lee, K.S.; Kang, S.K.; Lee, B.C.; Hwang, W.S. Effect of beta-mercaptoethanol or epidermal growth factor supplementation on in vitro maturation of canine oocytes collected from dogs with different stages of the estrus cycle. J. Vet. Sci. 2004, 5, 253–258. [Google Scholar] [CrossRef][Green Version]
- Takahashi, M.; Nagai, T.; Okamura, N.; Takahashi, H.; Okano, A. Promoting Effect of β-Mercaptoethanol on In Vitro Development under Oxidative Stress and Cystine Uptake of Bovine Embryos. Biol. Reprod. 2002, 66, 562–567. [Google Scholar] [CrossRef]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef] [PubMed]
- An, F.; Wang, S.; Yuan, D.; Gong, Y.; Wang, S. Attenuation of oxidative stress of erythrocytes by plant-derived flavonoids, orientin and luteolin. Evid.-Based Complementary Altern. Med. 2016, 2016, 3401269. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Jeong, P.-S.; Joo, Y.E.; Kang, H.-G.; Kim, M.J.; Lee, S.; Song, B.-S.; Kim, S.-U.; Cho, S.-K.; Sim, B.-W. Luteolin orchestrates porcine oocyte meiotic progression by maintaining organelle dynamics under oxidative stress. Front. Cell Dev. Biol. 2021, 9, 1510. [Google Scholar] [CrossRef] [PubMed]
- El-Raey, M.; Geshi, M.; Somfai, T.; Kaneda, M.; Hirako, M.; Abdel-Ghaffar, A.E.; Sosa, G.A.; El-Roos, M.E.; Nagai, T. Evidence of melatonin synthesis in the cumulus oocyte complexes and its role in enhancing oocyte maturation in vitro in cattle. Mol. Reprod. Dev. 2011, 78, 250–262. [Google Scholar] [CrossRef] [PubMed]
- Sampaio, R.V.; Conceição, D.S.B.; Miranda, M.S.; de Fatima, S.S.L.; Ohashi, O.M. MT3 melatonin binding site, MT1 and MT2 melatonin receptors are present in oocyte, but only MT1 is present in bovine blastocyst produced in vitro. Reprod. Biol. Endocrinol. 2012, 10, 103. [Google Scholar] [CrossRef]
- Wang, J.; Zhuo, Z.; Ma, X.; Liu, Y.; Xu, J.; He, C.; Fu, Y.; Wang, F.; Ji, P.; Zhang, L.; et al. Melatonin Alleviates the Suppressive Effect of Hypoxanthine on Oocyte Nuclear Maturation and Restores Meiosis via the Melatonin Receptor 1 (MT1)-Mediated Pathway. Front. Cell Dev. Biol. 2021, 9, 648148. [Google Scholar] [CrossRef]
- Cebrian-Serrano, A.; Salvador, I.; Raga, E.; Dinnyes, A.; Silvestre, M. Beneficial Effect of Melatonin on Blastocyst In Vitro Production from Heat-Stressed Bovine Oocytes. Reprod. Domest. Anim. 2013, 48, 738–746. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Z.; He, C.; Zhu, K.; Xu, Z.; Ma, T.; Tao, J.; Liu, G. Melatonin protects porcine oocyte in vitro maturation from heat stress. J. Pineal Res. 2015, 59, 365–375. [Google Scholar] [CrossRef]
- Tao, Y.; Zhou, B.; Xia, G.; Wang, F.; Wu, Z.; Fu, M. Exposure to L-Ascorbic Acid or α-Tocopherol Facilitates the Development of Porcine Denuded Oocytes from Metaphase I to Metaphase II and Prevents Cumulus Cells from Fragmentation. Reprod. Domest. Anim. 2004, 39, 52–57. [Google Scholar] [CrossRef]
- Goodwin, T. The Biochemistry of the Carotenoids: Volume I Plants; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Yabuzaki, J. Carotenoids Database: Structures, chemical fingerprints and distribution among organisms. Database 2017, 2017, bax004. [Google Scholar] [CrossRef]
- Nagao, A. Metabolism of Carotenoids in Mammals. In Carotenoids, Biosynthetic and Biofunctional Approaches; Springer: Berlin/Heidelberg, Germany, 2021; pp. 67–78. [Google Scholar]
- Stahl, W.; Sies, H. Antioxidant activity of carotenoids. Mol. Asp. Med. 2003, 24, 345–351. [Google Scholar] [CrossRef]
- Jackson, H.; Braun, C.L.; Ernst, H. The chemistry of novel xanthophyll carotenoids. Am. J. Cardiol. 2008, 101, S50–S57. [Google Scholar] [CrossRef] [PubMed]
- Langi, P.; Kiokias, S.; Varzakas, T.; Proestos, C. Carotenoids, From Plants to Food and Feed Industries. Methods Mol. Biol. 2018, 1852, 57–71. [Google Scholar] [PubMed]
- Yu, S.; Zhao, Y.; Feng, Y.; Zhang, H.; Li, L.; Shen, W.; Zhao, M.; Min, L. β-carotene improves oocyte development and maturation under oxidative stress in vitro. In Vitro Cell. Dev. Biol. Anim. 2019, 55, 548–558. [Google Scholar] [CrossRef] [PubMed]
- Surai, P. The antioxidant properties of canthaxanthin and its potential effects in the poultry eggs and on embryonic development of the chick. Part 1. World’s Poult. Sci. J. 2012, 68, 465–476. [Google Scholar] [CrossRef]
- Baker, R.; Günther, C. The role of carotenoids in consumer choice and the likely benefits from their inclusion into products for human consumption. Trends Food Sci. Technol. 2004, 15, 484–488. [Google Scholar] [CrossRef]
- Taweechaipaisankul, A.; Jin, J.X.; Lee, S.; Kim, G.A.; Lee, B.C. The effects of canthaxanthin on porcine oocyte maturation and embryo development in vitro after parthenogenetic activation and somatic cell nuclear transfer. Reprod. Domest. Anim. 2016, 51, 870–876. [Google Scholar] [CrossRef]
- Do, L.T.K.; Luu, V.V.; Morita, Y.; Taniguchi, M.; Nii, M.; Peter, A.T.; Otoi, T. Astaxanthin present in the maturation medium reduces negative effects of heat shock on the developmental competence of porcine oocytes. Reprod. Biol. 2015, 15, 86–93. [Google Scholar] [CrossRef]
- Bose, B.K. Global warming, Energy, environmental pollution, and the impact of power electronics. IEEE Ind. Electron. Mag. 2010, 4, 6–17. [Google Scholar] [CrossRef]
- Boni, R. Heat stress, a serious threat to reproductive function in animals and humans. Mol. Reprod. Dev. 2019, 86, 1307–1323. [Google Scholar] [CrossRef]
- Roth, Z. Effect of Heat Stress on Reproduction in Dairy Cows, Insights into the Cellular and Molecular Responses of the Oocyte. Annu. Rev. Anim. Biosci. 2017, 5, 151–170. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, J.A.; Nashiruddullah, N.; Dutta, D.; Biswas, R.K.; Borah, P. Cumulus cell expansion and ultrastructural changes in in vitro matured bovine oocytes under heat stress. Iran. J. Vet. Res. 2017, 18, 203–207. [Google Scholar]
- Andreu-Vázquez, C.; López-Gatius, F.; García-Ispierto, I.; Maya-Soriano, M.J.; Hunter, R.H.; López-Béjar, M. Does heat stress provoke the loss of a continuous layer of cortical granules beneath the plasma membrane during oocyte maturation? Zygote 2010, 18, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Sakatani, M. Effects of heat stress on bovine preimplantation embryos produced in vitro. J. Reprod. Dev. 2017, 63, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Diaz, F.A.; Gutierrez-Castillo, E.J.; Foster, B.A.; Hardin, P.T.; Bondioli, K.R.; Jiang, Z. Evaluation of Seasonal Heat Stress on Transcriptomic Profiles and Global DNA Methylation of Bovine Oocytes. Front. Genet. 2021, 12, 699920. [Google Scholar] [CrossRef] [PubMed]
- Waiz, S.A.; Raies-Ul-Haq, M.; Dhanda, S.; Kumar, A.; Goud, T.S.; Chauhan, M.S.; Upadhyay, R.C. Heat stress and antioxidant enzyme activity in bubaline (Bubalus bubalis) oocytes during in vitro maturation. Int. J. Biometeorol. 2016, 60, 1357–1366. [Google Scholar] [CrossRef]
- Wang, J.Z.; Sui, H.S.; Miao, D.Q.; Liu, N.; Zhou, P.; Ge, L.; Tan, J.H. Effects of heat stress during in vitro maturation on cytoplasmic versus nuclear components of mouse oocytes. Reproduction 2009, 137, 181–189. [Google Scholar] [CrossRef]
- Hale, B.J.; Li, Y.; Adur, M.K.; Keating, A.F.; Baumgard, L.H.; Ross, J.W. Characterization of the effects of heat stress on autophagy induction in the pig oocyte. Reprod. Biol. Endocrinol. 2021, 19, 107. [Google Scholar] [CrossRef]
- Ju, J.C.; Tseng, J.K. Nuclear and cytoskeletal alterations of in vitro matured porcine oocytes under hyperthermia. Mol. Reprod. Dev. 2004, 68, 125–133. [Google Scholar] [CrossRef]
- Payton, R.R.; Romar, R.; Coy, P.; Saxton, A.M.; Lawrence, J.L.; Edwards, J.L. Susceptibility of bovine germinal vesicle-stage oocytes from antral follicles to direct effects of heat stress in vitro. Biol. Reprod. 2004, 71, 1303–1308. [Google Scholar] [CrossRef]
- Hansen, P.J. Physiological and cellular adaptations of zebu cattle to thermal stress. Anim. Reprod. Sci. 2004, 82–83, 349–360. [Google Scholar] [CrossRef] [PubMed]
- Sakatani, M.; Yamanaka, K.; Kobayashi, S.; Takahashi, M. Heat shock-derived reactive oxygen species induce embryonic mortality in in vitro early stage bovine embryos. J. Reprod. Dev. 2008, 54, 496–501. [Google Scholar] [CrossRef] [PubMed]
- Ozawa, M.; Hirabayashi, M.; Kanai, Y. Developmental competence and oxidative state of mouse zygotes heat-stressed maternally or in vitro. Reproduction 2002, 124, 683–689. [Google Scholar] [CrossRef] [PubMed]
- Nabenishi, H.; Ohta, H.; Nishimoto, T.; Morita, T.; Ashizawa, K.; Tsuzuki, Y. The effects of cysteine addition during in vitro maturation on the developmental competence, ROS, GSH and apoptosis level of bovine oocytes exposed to heat stress. Zygote 2012, 20, 249–259. [Google Scholar] [CrossRef]
- Sakatani, M.; Kobayashi, S.; Takahashi, M. Effects of heat shock on in vitro development and intracellular oxidative state of bovine preimplantation embryos. Mol. Reprod. Dev. 2004, 67, 77–82. [Google Scholar] [CrossRef]
- Paes, V.M.; Vieira, L.A.; Correia, H.H.V.; Sa, N.A.R.; Moura, A.A.A.; Sales, A.D.; Rodrigues, A.P.R.; Magalhães-Padilha, D.M.; Santos, F.W.; Apgar, G.A.; et al. Effect of heat stress on the survival and development of in vitro cultured bovine preantral follicles and on in vitro maturation of cumulus-oocyte complex. Theriogenology 2016, 86, 994–1003. [Google Scholar] [CrossRef]
- Yin, C.; Liu, J.; He, B.; Jia, L.; Gong, Y.; Guo, H.; Zhao, R. Heat stress induces distinct responses in porcine cumulus cells and oocytes associated with disrupted gap junction and trans-zonal projection colocalization. J. Cell. Physiol. 2019, 234, 4787–4798. [Google Scholar] [CrossRef]
- Gendelman, M.; Aroyo, A.; Yavin, S.; Roth, Z. Seasonal effects on gene expression, cleavage timing, and developmental competence of bovine preimplantation embryos. Reproduction 2010, 140, 73–82. [Google Scholar] [CrossRef]
- Edwards, J.L.; Bogart, A.N.; Rispoli, L.A.; Saxton, A.M.; Schrick, F.N. Developmental competence of bovine embryos from heat-stressed ova. J. Dairy Sci. 2009, 92, 563–570. [Google Scholar] [CrossRef]
- Ju, J.C.; Jiang, S.; Tseng, J.K.; Parks, J.E.; Yang, X. Heat shock reduces developmental competence and alters spindle configuration of bovine oocytes. Theriogenology 2005, 64, 1677–1689. [Google Scholar] [CrossRef]
- Nabenishi, H.; Takagi, S.; Kamata, H.; Nishimoto, T.; Morita, T.; Ashizawa, K.; Tsuzuki, Y. The role of mitochondrial transition pores on bovine oocyte competence after heat stress, as determined by effects of cyclosporin A. Mol. Reprod. Dev. 2012, 79, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, T.A.; Ispada, J.; Risolia, P.H.; Rodrigues, M.T.; Lima, R.S.; Assumpção, M.E.; Visintin, J.A.; Paula-Lopes, F.F. Thermoprotective effect of insulin-like growth factor 1 on in vitro matured bovine oocyte exposed to heat shock. Theriogenology 2016, 86, 2028–2039. [Google Scholar] [CrossRef] [PubMed]
- Maya-Soriano, M.J.; Taberner, E.; López-Béjar, M. Retinol improves in vitro oocyte nuclear maturation under heat stress in heifers. Zygote 2013, 21, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Kidder, B.L. In vitro maturation and in vitro fertilization of mouse oocytes and preimplantation embryo culture. Methods Mol. Biol. 2014, 1150, 191–199. [Google Scholar] [PubMed]
- Paula-Lopes, F.F.; Lima, R.S.; Satrapa, R.A.; Barros, C.M. Physiology and Endocrinology Symposium: Influence of cattle genotype (Bos indicus vs. Bos taurus) on oocyte and preimplantation embryo resistance to increased temperature. J. Anim. Sci. 2013, 91, 1143–1153. [Google Scholar] [PubMed]
- El-Sayed, A.; Nagy, R.; El-Asheeri, A.K.; Eid, L.N. Developmental and molecular responses of buffalo (Bubalus bubalis) cumulus-oocyte complex matured in vitro under heat shock conditions. Zygote 2018, 26, 177–190. [Google Scholar] [CrossRef]
- Tseng, J.K.; Tang, P.C.; Ju, J.C. In vitro thermal stress induces apoptosis and reduces development of porcine parthenotes. Theriogenology 2006, 66, 1073–1082. [Google Scholar] [CrossRef]
- Abdelnour, S.A.; Yang, C.Y.; Swelum, A.A.; El-Hack, M.E.A.; Khafaga, A.F.; Abdo, M.; Shang, J.H.; Lu, Y.Q. Molecular functional, and cellular alterations of oocytes and cumulus cells induced by heat stress and shock in animals. Environ. Sci. Pollut. Res. Int. 2020, 27, 38472–38490. [Google Scholar] [CrossRef]
- Lawrence, J.L.; Payton, R.R.; Godkin, J.D.; Saxton, A.M.; Schrick, F.N.; Edwards, J.L. Retinol improves development of bovine oocytes compromised by heat stress during maturation. J. Dairy Sci. 2004, 87, 2449–2454. [Google Scholar] [CrossRef]
- Roth, Z.; Aroyo, A.; Yavin, S.; Arav, A. The antioxidant epigallocatechin gallate (EGCG) moderates the deleterious effects of maternal hyperthermia on follicle-enclosed oocytes in mice. Theriogenology 2008, 70, 887–897. [Google Scholar] [CrossRef]
- Li, Y.; Wang, J.; Zhang, Z.; Yi, J.; He, C.; Wang, F.; Tian, X.; Yang, M.; Song, Y.; He, P.; et al. Resveratrol compares with melatonin in improving in vitro porcine oocyte maturation under heat stress. J. Anim. Sci. Biotechnol. 2016, 7, 33. [Google Scholar] [CrossRef] [PubMed]
- Ispada, J.; Rodrigues, T.A.; Risolia, P.H.B.; Lima, R.S.; Gonçalves, D.R.; Rettori, D.; Nichi, M.; Feitosa, W.B.; Paula-Lopes, F.F. Astaxanthin counteracts the effects of heat shock on the maturation of bovine oocytes. Reprod. Fertil. Dev. 2018, 30, 1169–1179. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Tian, X.; Zhang, L.; Gao, C.; He, C.; Fu, Y.; Ji, P.; Li, Y.; Li, N.; Liu, G. Beneficial effects of melatonin on in vitro bovine embryonic development are mediated by melatonin receptor 1. J. Pineal Res. 2014, 56, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, H.; Castro, F.; Schefer, L.; Paschoal, D.; Leal, C. 163 Effect of melatonin and its receptors on bovine oocyte maturation and cumulus cell gene expression after heat shock in vitro: Preliminary results. Reprod. Fertil. Dev. 2019, 31, 206–207. [Google Scholar] [CrossRef]
- Garcia-Ispierto, I.; Abdelfatah, A.; López-Gatius, F. Melatonin treatment at dry-off improves reproductive performance postpartum in high-producing dairy cows under heat stress conditions. Reprod. Domest. Anim. 2013, 48, 577–583. [Google Scholar] [CrossRef]
- Yaacobi-Artzi, S.; Shimoni, C.; Kalo, D.; Hansen, P.J.; Roth, Z. Melatonin slightly alleviates the effect of heat shock on bovine oocytes and resulting blastocysts. Theriogenology 2020, 158, 477–489. [Google Scholar] [CrossRef]
- Gendelman, M.; Roth, Z. Incorporation of coenzyme Q10 into bovine oocytes improves mitochondrial features and alleviates the effects of summer thermal stress on developmental competence. Biol. Reprod. 2012, 87, 118. [Google Scholar]
- Templeton, A.; Morris, J.K.; Parslow, W. Factors that affect outcome of in-vitro fertilisation treatment. Lancet 1996, 348, 1402–1406. [Google Scholar] [CrossRef]
- Li, Q.; Geng, X.; Zheng, W.; Tang, J.; Xu, B.; Shi, Q. Current understanding of ovarian aging. Sci. China Life Sci. 2012, 55, 659–669. [Google Scholar] [CrossRef]
- Igarashi, H.; Takahashi, T.; Nagase, S. Oocyte aging underlies female reproductive aging: Biological mechanisms and therapeutic strategies. Reprod. Med. Biol. 2015, 14, 159–169. [Google Scholar] [CrossRef]
- Li, Y.-J.; Han, Z.; Ge, L.; Zhou, C.-J.; Zhao, Y.-F.; Wang, D.-H.; Ren, J.; Niu, X.-X.; Liang, C.-G. C-phycocyanin protects against low fertility by inhibiting reactive oxygen species in aging mice. Oncotarget 2016, 7, 17393. [Google Scholar] [CrossRef] [PubMed]
- Yamada-Fukunaga, T.; Yamada, M.; Hamatani, T.; Chikazawa, N.; Ogawa, S.; Akutsu, H.; Miura, T.; Miyado, K.; Tarín, J.J.; Kuji, N. Age-associated telomere shortening in mouse oocytes. Reprod. Biol. Endocrinol. 2013, 11, 108. [Google Scholar] [CrossRef] [PubMed]
- Salmon, A.B.; Richardson, A.; Pérez, V.I. Update on the oxidative stress theory of aging: Does oxidative stress play a role in aging or healthy aging? Free. Radic. Biol. Med. 2010, 48, 642–655. [Google Scholar] [CrossRef]
- Behrman, H.R.; Kodaman, P.H.; Preston, S.L.; Gao, S. Oxidative stress and the ovary. J. Soc. Gynecol. Investig. 2001, 8, S40–S42. [Google Scholar] [PubMed]
- Dunning, K.R.; Russell, D.L.; Robker, R.L. Lipids and oocyte developmental competence: The role of fatty acids and β-oxidation. Reproduction 2014, 148, R15–R27. [Google Scholar] [CrossRef] [PubMed]
- Mihalas, B.P.; de Iuliis, G.N.; Redgrove, K.A.; McLaughlin, E.A.; Nixon, B. The lipid peroxidation product 4-hydroxynonenal contributes to oxidative stress-mediated deterioration of the ageing oocyte. Sci. Rep. 2017, 7, 6247. [Google Scholar] [CrossRef]
- Simsek-Duran, F.; Li, F.; Ford, W.; Swanson, R.J.; Jones, H.W., Jr.; Castora, F.J. Age-associated metabolic and morphologic changes in mitochondria of individual mouse and hamster oocytes. PLoS ONE 2013, 8, e64955. [Google Scholar] [CrossRef]
- Thouas, G.A.; Trounson, A.O.; Jones, G.M. Effect of female age on mouse oocyte developmental competence following mitochondrial injury. Biol. Reprod. 2005, 73, 366–373. [Google Scholar] [CrossRef]
- Tarín, J.J.; Pérez-Albalá, S.; Cano, A. Oral antioxidants counteract the negative effects of female aging on oocyte quantity and quality in the mouse. Mol. Reprod. Dev. 2002, 61, 385–397. [Google Scholar] [CrossRef]
- Tamura, H.; Kawamoto, M.; Sato, S.; Tamura, I.; Maekawa, R.; Taketani, T.; Aasada, H.; Takaki, E.; Nakai, A.; Reiter, R.J.; et al. Long-term melatonin treatment delays ovarian aging. J. Pineal Res. 2017, 62, e12381. [Google Scholar] [CrossRef]
- Ben-Meir, A.; Burstein, E.; Borrego-Alvarez, A.; Chong, J.; Wong, E.; Yavorska, T.; Naranian, T.; Chi, M.; Wang, Y.; Bentov, Y.; et al. Coenzyme Q10 restores oocyte mitochondrial function and fertility during reproductive aging. Aging Cell 2015, 14, 887–895. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, Z.; Wang, J.; Lv, D.; Zhu, T.; Wang, F.; Tian, X.; Yao, Y.; Ji, P.; Liu, G. Melatonin regulates the activities of ovary and delays the fertility decline in female animals via MT1/AMPK pathway. J. Pineal Res. 2019, 66, e12550. [Google Scholar] [CrossRef] [PubMed]
- Irianni, F.; Hodgen, G.D. Mechanism of ovulation. Endocrinol. Metab. Clin. N. Am. 1992, 21, 19–38. [Google Scholar] [CrossRef]
- Florman, H.M.; Ducibella, T. Fertilization in mammals. Knobil Neill’s Physiol. Reprod. 2006, 3, 55–112. [Google Scholar]
- Okabe, M. The cell biology of mammalian fertilization. Development 2013, 140, 4471–4479. [Google Scholar] [CrossRef]
- Marston, J.H.; Chang, M.C. The fertilizable life of ova and their morphology following delayed insemination in mature and immature mice. J. Exp. Zool. 1964, 155, 237–251. [Google Scholar] [CrossRef]
- Miao, Y.L.; Kikuchi, K.; Sun, Q.Y.; Schatten, H. Oocyte aging: Cellular and molecular changes, developmental potential and reversal possibility. Hum. Reprod. Update 2009, 15, 573–585. [Google Scholar] [CrossRef]
- Takahashi, T.; Takahashi, E.; Igarashi, H.; Tezuka, N.; Kurachi, H. Impact of oxidative stress in aged mouse oocytes on calcium oscillations at fertilization. Mol. Reprod. Dev. 2003, 66, 143–152. [Google Scholar] [CrossRef]
- Lord, T.; Aitken, R.J. Oxidative stress and ageing of the post-ovulatory oocyte. Reproduction 2013, 146, R217–R227. [Google Scholar] [CrossRef]
- Tatone, C.; Carbone, M.C.; Gallo, R.; Monache, S.D.; di Cola, M.; Alesse, E.; Amicarelli, F. Age-associated changes in mouse oocytes during postovulatory in vitro culture: Possible role for meiotic kinases and survival factor BCL2. Biol. Reprod. 2006, 74, 395–402. [Google Scholar] [CrossRef]
- Zhang, T.; Zhou, Y.; Li, L.; Wang, H.H.; Ma, X.S.; Qian, W.P.; Shen, W.; Schatten, H.; Sun, Q.Y. SIRT1, 2, 3 protect mouse oocytes from postovulatory aging. Aging 2016, 8, 685–696. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Igarashi, H.; Kawagoe, J.; Amita, M.; Hara, S.; Kurachi, H. Poor embryo development in mouse oocytes aged in vitro is associated with impaired calcium homeostasis. Biol. Reprod. 2009, 80, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Koyama, K.; Kang, S.S.; Huang, W.; Yanagawa, Y.; Takahashi, Y.; Nagano, M. Aging-related changes in in vitro-matured bovine oocytes: Oxidative stress, mitochondrial activity and ATP content after nuclear maturation. J. Reprod. Dev. 2014, 60, 136–142. [Google Scholar] [CrossRef]
- Tang, D.W.; Fang, Y.; Liu, Z.X.; Wu, Y.; Wang, X.L.; Zhao, S.; Han, G.C.; Zeng, S.M. The disturbances of endoplasmic reticulum calcium homeostasis caused by increased intracellular reactive oxygen species contributes to fragmentation in aged porcine oocytes. Biol. Reprod. 2013, 89, 124. [Google Scholar] [CrossRef] [PubMed]
- Asano, Y. Age-related accumulation of non-heme ferric and ferrous iron in mouse ovarian stroma visualized by sensitive non-heme iron histochemistry. J. Histochem. Cytochem. Off. J. Histochem. Soc. 2012, 60, 229–242. [Google Scholar] [CrossRef]
- Tatone, C.; di Emidio, G.; Barbaro, R.; Vento, M.; Ciriminna, R.; Artini, P.G. Effects of reproductive aging and postovulatory aging on the maintenance of biological competence after oocyte vitrification: Insights from the mouse model. Theriogenology 2011, 76, 864–873. [Google Scholar] [CrossRef]
- Lord, T.; Nixon, B.; Jones, K.T.; Aitken, R.J. Melatonin prevents postovulatory oocyte aging in the mouse and extends the window for optimal fertilization in vitro. Biol. Reprod. 2013, 88, 67. [Google Scholar] [CrossRef] [PubMed]
- Goto, Y.; Noda, Y.; Mori, T.; Nakano, M. Increased generation of reactive oxygen species in embryos cultured in vitro. Free Radic. Biol. Med. 1993, 15, 69–75. [Google Scholar] [CrossRef]
- Boerjan, M.L.; de Boer, P. First cell cycle of zygotes of the mouse derived from oocytes aged postovulation in vivo and fertilized in vivo. Mol. Reprod. Dev. 1990, 25, 155–163. [Google Scholar] [CrossRef]
- Yoshida, M.; Ishigaki, K.; Nagai, T.; Chikyu, M.; Pursel, V.G. Glutathione concentration during maturation and after fertilization in pig oocytes: Relevance to the ability of oocytes to form male pronucleus. Biol. Reprod. 1993, 49, 89–94. [Google Scholar] [CrossRef]
- Tarín, J.J.; Ten, J.; Vendrell, F.J.; Cano, A. Dithiothreitol prevents age-associated decrease in oocyte/conceptus viability in vitro. Hum. Reprod. 1998, 13, 381–386. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Oikawa, S.; Hiraku, Y.; Fujiwara, T.; Saito, I.; Kawanishi, S. Site-specific hydroxylation at polyguanosine in double-stranded DNA by nickel(II) in the presence of SH compounds: Comparison with singlet oxygen-induced DNA damage. Chem. Res. Toxicol. 2002, 15, 1017–1022. [Google Scholar] [CrossRef] [PubMed]
- Rakha, S.I.; Elmetwally, M.A.; Ali, H.E.; Balboula, A.Z.; Mahmoud, A.M.; Zaabel, S.M. Lycopene Reduces the In Vitro Aging Phenotypes of Mouse Oocytes by Improving Their Oxidative Status. Vet. Sci. 2022, 9, 336. [Google Scholar] [CrossRef] [PubMed]
- BioRender. Available online: https://biorender.com/ (accessed on 29 July 2022).
| Antioxidant | Type | Dose | Species | Maturation Rate vs. (Control) | References |
|---|---|---|---|---|---|
| Melatonin | Hormone | 4.3 × 10−8 M (10 ng/mL) | porcine | 84.6 (75.6) * | [88] |
| 10−9 M | bovine | 82.3 (65.7) * | [89] | ||
| 2.5 × 10−4 M | buffalo | 42.8 (33)ns | [90] | ||
| 10−7 M | sheep | 85.3 (75.3) * | [91] | ||
| 10−6 M | mouse | 85 (64) * | [92] | ||
| Lycopene | Carotenoid | 2 × 10−7 M | bovine | 76 (66.3) * | [93] |
| 2 × 10−7 M | mouse | 89.9 (66.7) * | [94] | ||
| Astaxanthin | Carotenoid | 2.5 × 10−6 M | porcine | 89.5 (87.1)ns | [95] |
| Beta-Mercaptoethanol (β-ME) | Thiol | 2 × 10−5 M | buffalo | 76.2 (66.7)ns | [96] |
| 10−5 M | equine | 55.6 (51.9)ns | [97] | ||
| Cystamine | Thiol | 10−5 M | mouse | 80.1 (57.7) * | [98] |
| Vitamin C | Vitamin | 2.5 × 10−4 M | mouse | 29.7 (70.3) * | [99] |
| 2.3 × 10−3 M (1 mg/mL) | bovine | ~80 (~80)ns | [100] | ||
| Vitamin E | Vitamin | 2.3 × 10−3 M (1 mg/mL) | bovine | ~80 (~80)ns | [100] |
| 10−3 M | porcine | 72.2 (67.6)ns | [101] | ||
| Selenium (SeMet) | Trace element | 2.5 × 10−8 M | porcine | 80.2 (67.6) * | [101] |
| Vitamin E; Selenium (SeMet) | Vitamin; trace element | 10−3 M; 2.5 × 10−8 M | porcine | 85.1 (67.6) * | [101] |
| Resveratrol | Polyphenolic compound | 10−6 M | bovine | 93.4 (87.9) * | [102] |
| 5 × 10−6 M | porcine | 84.5 (72.6) * | [103] | ||
| Quercetin | Polyphenolic compound | 10−5 M | mouse | 86.6 (79.7) * | [104] |
| human | 92.3 (87.5)ns | ||||
| L-Carnitine | Amino acid derivative | 3.1 × 10−3 M (0.5 mg/mL) | porcine | 60.7 (56.4) * | [47] |
| 3.1 × 10−3 M (0.5 mg/mL) | camel | 74.7 (60.2) * | [105] | ||
| 3.7 × 10−3 M (0.6 mg/mL) | canine | 41.4 (23.4) * | [106] | ||
| Retinoic acid | Vitamin A metabolite | 10−8 M | goat | 78.7 (65.1) * | [107] |
| 2 × 10−5 M | camel | 69.4 (52.9) * | [108] | ||
| Coenzyme Q10 | Coenzyme | 10−5 M | porcine | 76.4 (66)ns | [109] |
| 5 × 10−5 M | human | 82.6 (63.0) * | [110] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rakha, S.I.; Elmetwally, M.A.; El-Sheikh Ali, H.; Balboula, A.; Mahmoud, A.M.; Zaabel, S.M. Importance of Antioxidant Supplementation during In Vitro Maturation of Mammalian Oocytes. Vet. Sci. 2022, 9, 439. https://doi.org/10.3390/vetsci9080439
Rakha SI, Elmetwally MA, El-Sheikh Ali H, Balboula A, Mahmoud AM, Zaabel SM. Importance of Antioxidant Supplementation during In Vitro Maturation of Mammalian Oocytes. Veterinary Sciences. 2022; 9(8):439. https://doi.org/10.3390/vetsci9080439
Chicago/Turabian StyleRakha, Shimaa I., Mohammed A. Elmetwally, Hossam El-Sheikh Ali, Ahmed Balboula, Abdelmonem Montaser Mahmoud, and Samy M. Zaabel. 2022. "Importance of Antioxidant Supplementation during In Vitro Maturation of Mammalian Oocytes" Veterinary Sciences 9, no. 8: 439. https://doi.org/10.3390/vetsci9080439
APA StyleRakha, S. I., Elmetwally, M. A., El-Sheikh Ali, H., Balboula, A., Mahmoud, A. M., & Zaabel, S. M. (2022). Importance of Antioxidant Supplementation during In Vitro Maturation of Mammalian Oocytes. Veterinary Sciences, 9(8), 439. https://doi.org/10.3390/vetsci9080439







