Canine Fecal Microbiota Transplantation: Current Application and Possible Mechanisms
Abstract
:Simple Summary
Abstract
1. Introduction
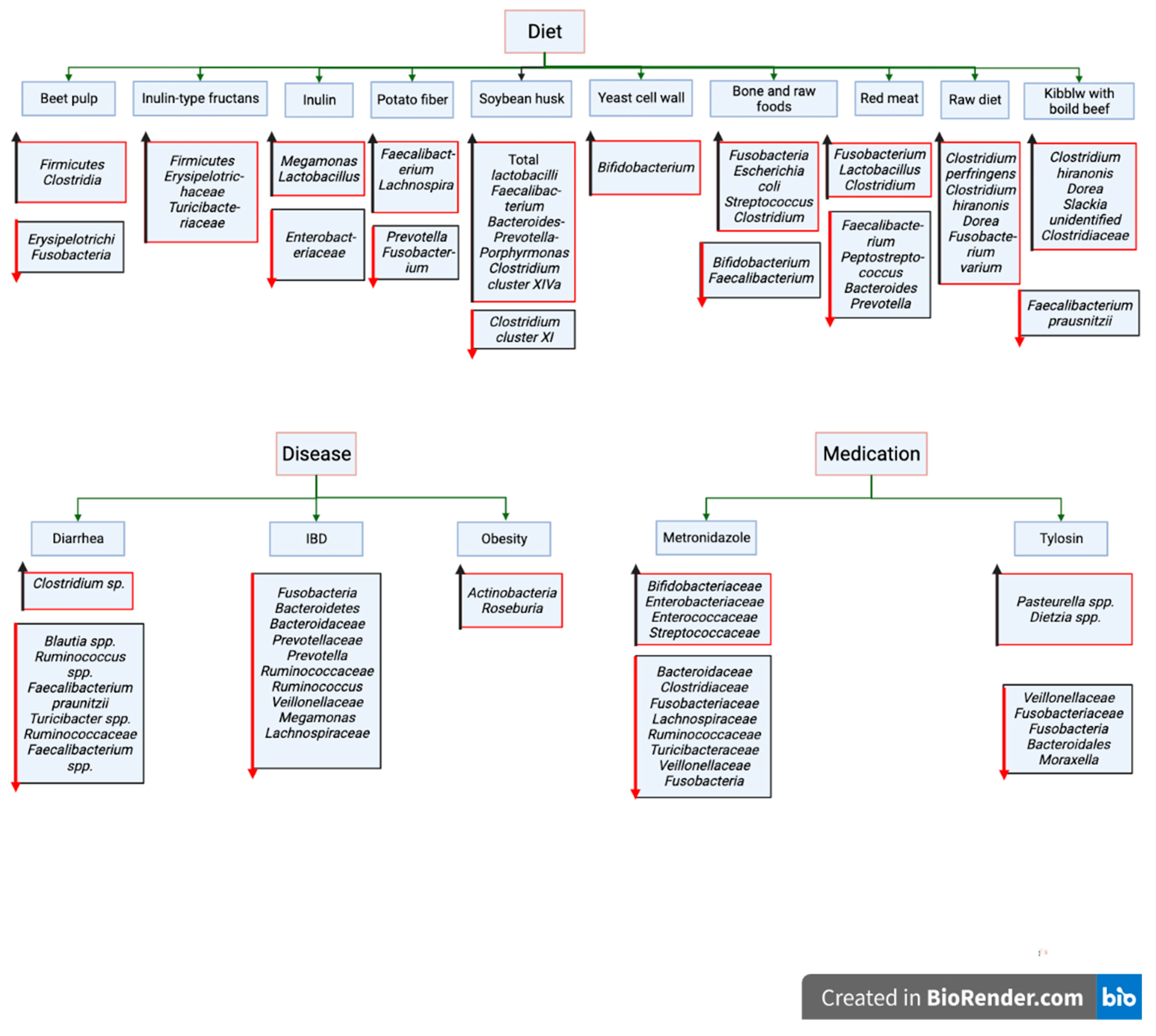
2. Major Factors Influencing Canine Gut Microbiota
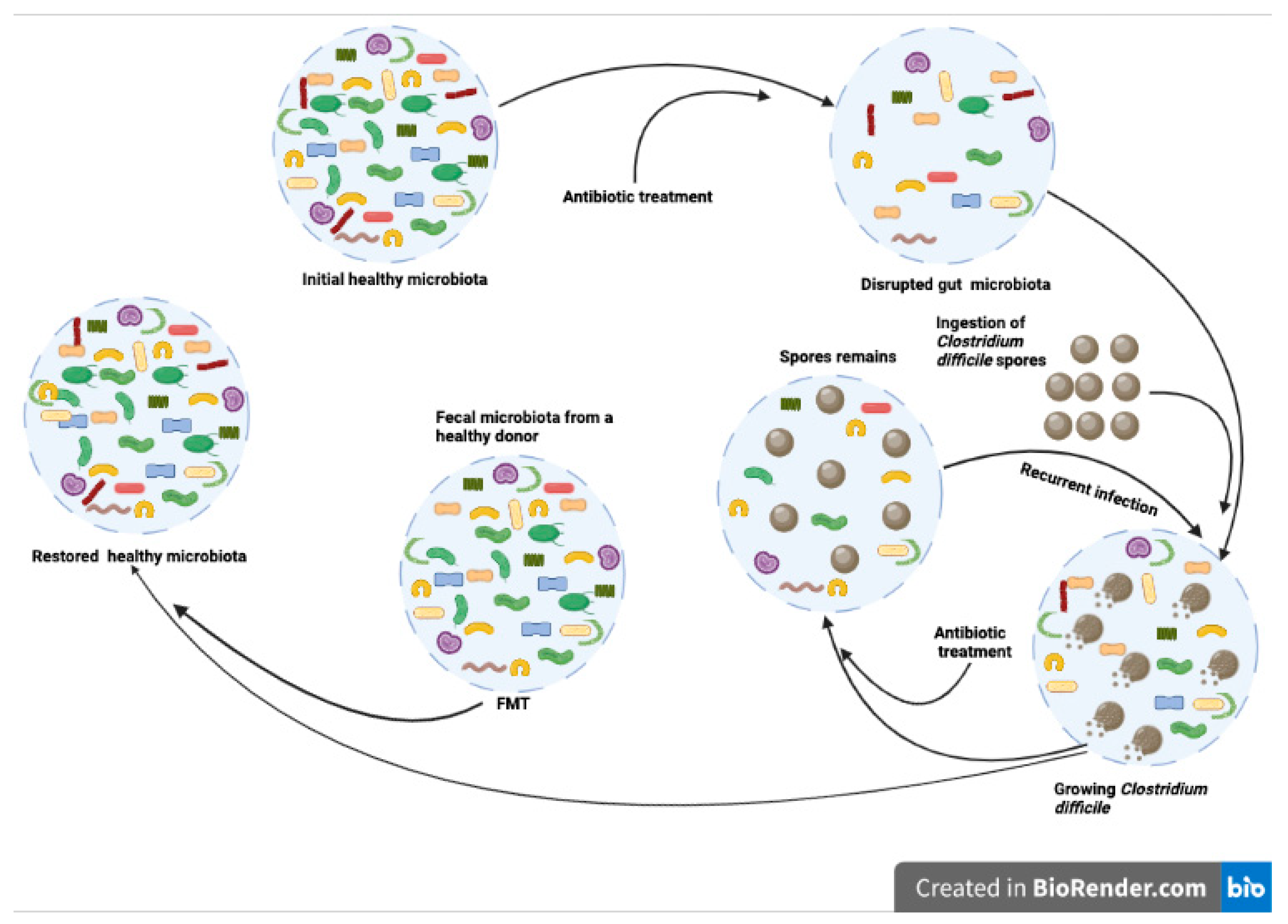
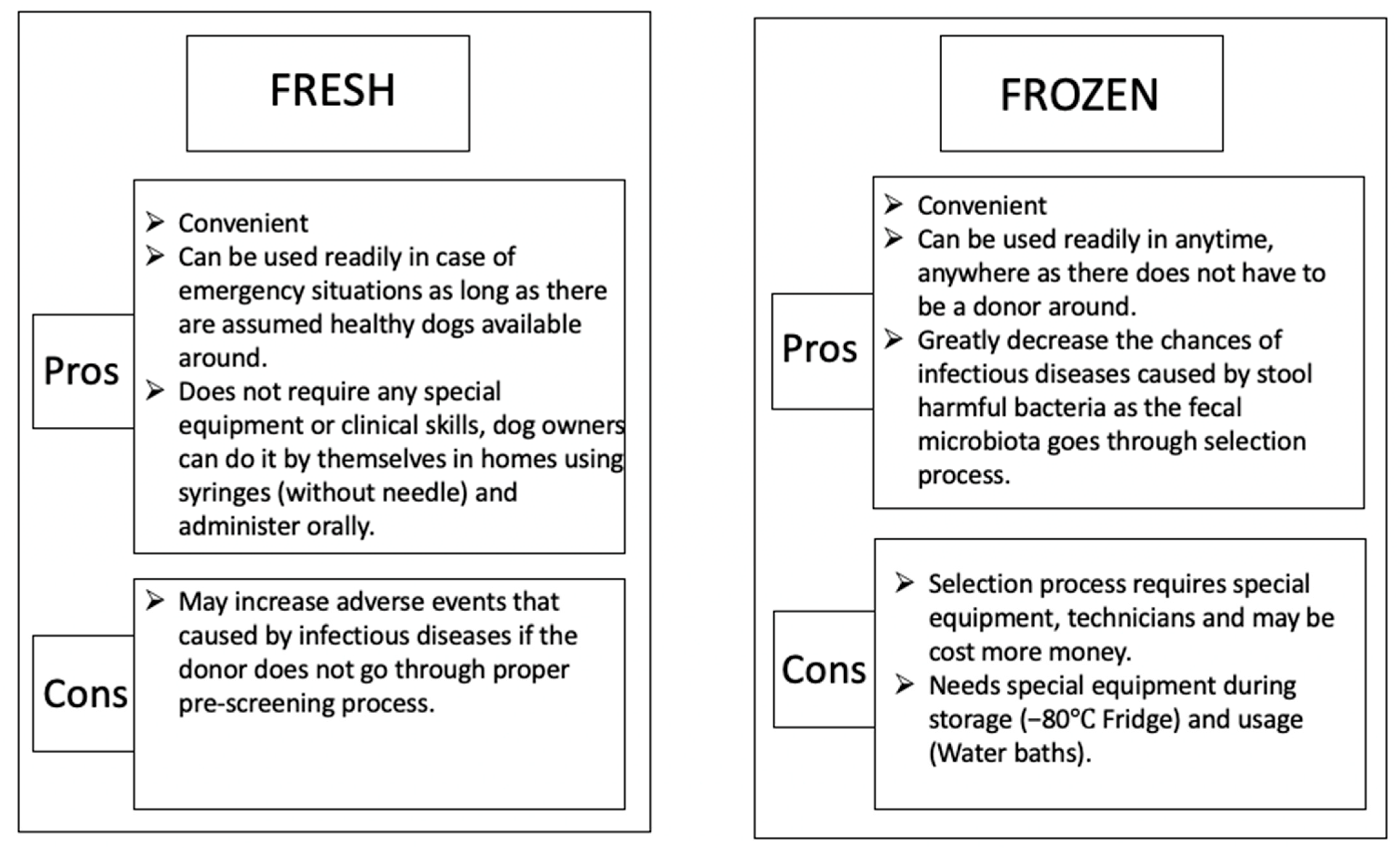
3. Fecal Microbiota Transplantation in Dogs
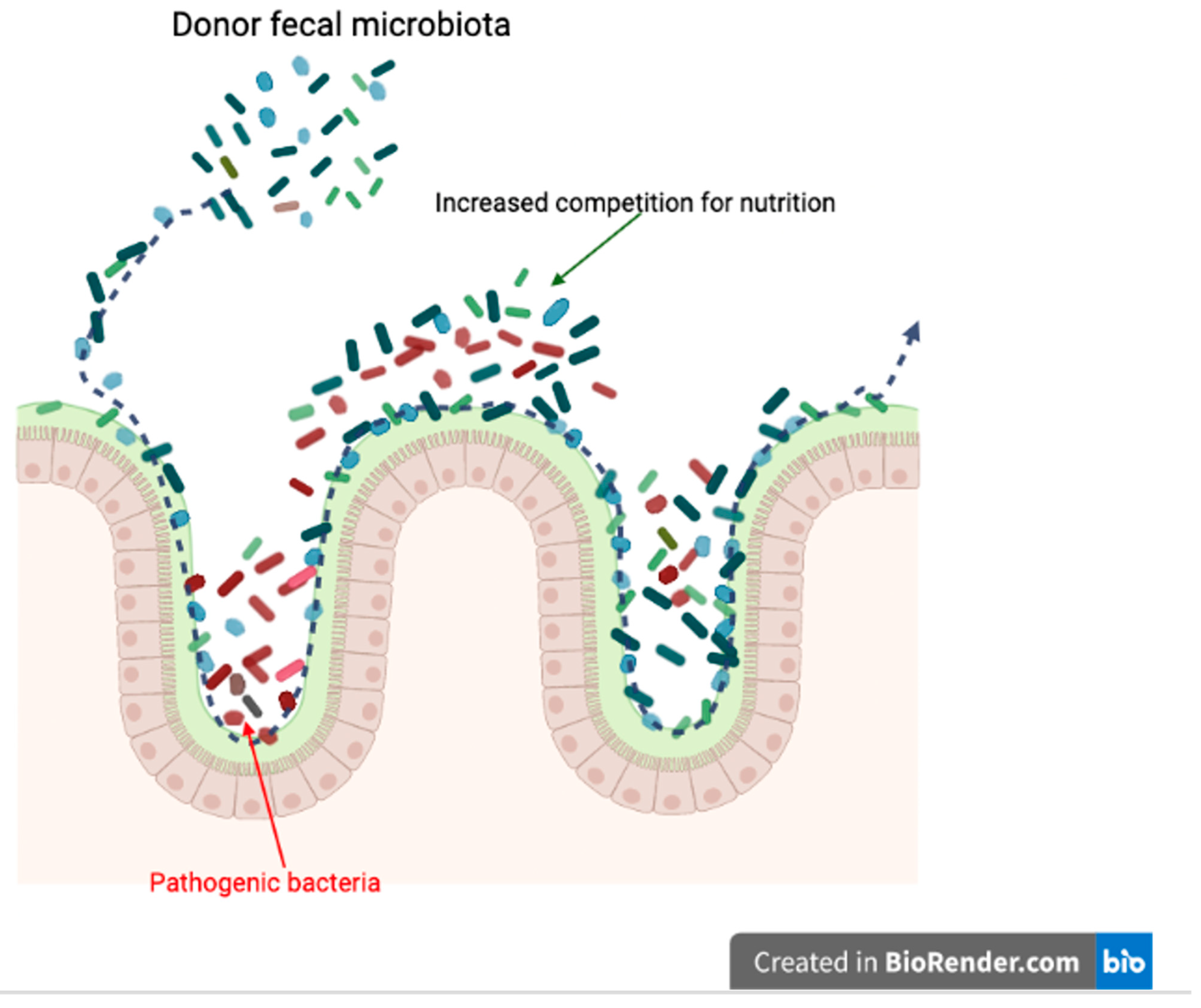
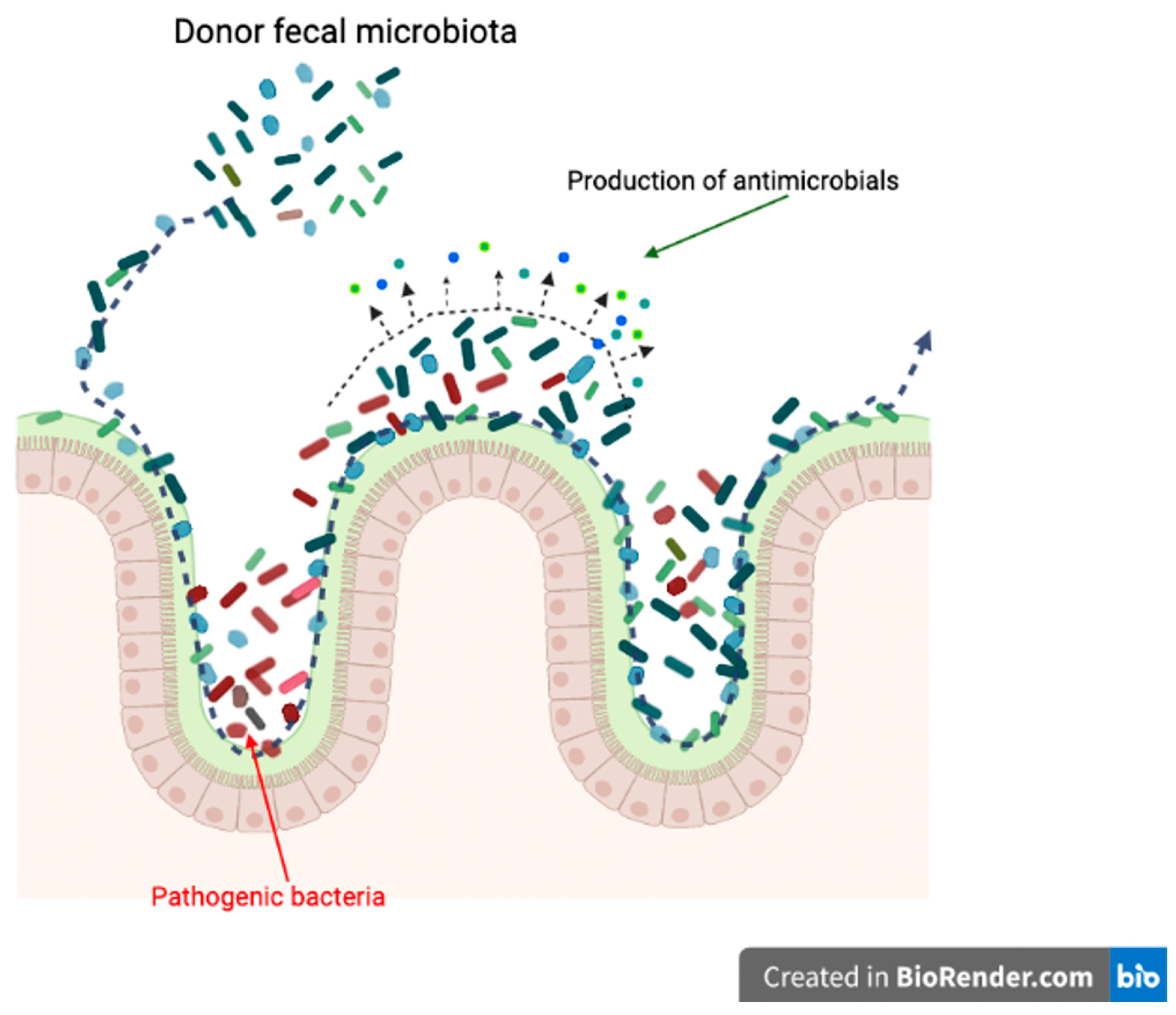
4. Mechanisms of Fecal Microbiota Transplantation in Dogs
5. Risks and Limitations of Canine FMT
6. Future Perspective of Canine FMT
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bowden, G.H.W. Actinomyces, Propionibacterium propionicus, and Streptomyces. In Medical Microbiology; Baron, S., Ed.; University of Texas Medical Branch at Galveston: Galveston, TX, USA, 1996; ISBN 978-0-9631172-1-2. [Google Scholar]
- Thursby, E.; Juge, N. Introduction to the human gut microbiota. Biochem. J. 2017, 474, 1823–1836. [Google Scholar] [CrossRef] [PubMed]
- Pluznick, J.L. Gut Microbes and Host Physiology: What Happens When You Host Billions of Guests? Front. Endocrinol. 2014, 5, 91. [Google Scholar] [CrossRef] [PubMed]
- Mondo, E.; Marliani, G.; Accorsi, P.A.; Cocchi, M.; Di Leone, A. Role of gut microbiota in dog and cat’s health and diseases. Open Vet. J. 2019, 9, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Dewhirst, F.E.; Klein, E.A.; Thompson, E.C.; Blanton, J.M.; Chen, T.; Milella, L.; Buckley, C.M.F.; Davis, I.J.; Bennett, M.-L.; Marshall-Jones, Z.V. The Canine Oral Microbiome. PLoS ONE 2012, 7, e36067. [Google Scholar] [CrossRef]
- Tress, B.; Dorn, E.S.; Suchodolski, J.S.; Nisar, T.; Ravindran, P.; Weber, K.; Hartmann, K.; Schulz, B.S. Bacterial microbiome of the nose of healthy dogs and dogs with nasal disease. PLoS ONE 2017, 12, e0176736. [Google Scholar] [CrossRef]
- García-Fonticoba, R.; Ferrer, L.; Francino, O.; Cuscó, A. The microbiota of the surface, dermis and subcutaneous tissue of dog skin. Anim. Microbiome 2020, 2, 34. [Google Scholar] [CrossRef]
- Cuscó, A.; Sánchez, A.; Altet, L.; Ferrer, L.; Francino, O. Individual Signatures Define Canine Skin Microbiota Composition and Variability. Front. Vet. Sci. 2017, 4, 6. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Pan, Z.; Yang, R.; Bi, Y.; Xiong, X. The canine gastrointestinal microbiota: Early studies and research frontiers. Gut. Microbes 2020, 11, 635–654. [Google Scholar] [CrossRef]
- Pilla, R.; Suchodolski, J.S. The Role of the Canine Gut Microbiome and Metabolome in Health and Gastrointestinal Disease. Front. Vet. Sci. 2020, 6, 498. [Google Scholar] [CrossRef] [PubMed]
- Ericsson, A.C.; Personett, A.R.; Grobman, M.E.; Rindt, H.; Reinero, C.R. Composition and Predicted Metabolic Capacity of Upper and Lower Airway Microbiota of Healthy Dogs in Relation to the Fecal Microbiota. PLoS ONE 2016, 11, e0154646. [Google Scholar] [CrossRef] [PubMed]
- Fastrès, A.; Canonne, M.A.; Taminiau, B.; Billen, F.; Garigliany, M.-M.; Daube, G.; Clercx, C. Analysis of the lung microbiota in dogs with Bordetella bronchiseptica infection and correlation with culture and quantitative polymerase chain reaction. Vet. Res. 2020, 51, 46. [Google Scholar] [CrossRef] [PubMed]
- Fastrès, A.; Roels, E.; Taminiau, B.; Rajamäki, M.; Daube, G.; Guiot, J.; Clercx, C. Analysis of the lung microbiota in healthy dogs and in canine idiopathic pulmonary fibrosis, a possible spontaneous model for human IPF. Eur. Respir. J. 2018, 52, PA999. [Google Scholar] [CrossRef]
- Burton, E.N.; Cohn, L.A.; Reinero, C.N.; Rindt, H.; Moore, S.; Ericsson, A.C. Characterization of the urinary microbiome in healthy dogs. PLoS ONE 2017, 12, e0177783. [Google Scholar] [CrossRef]
- Hutchins, R.; Vaden, S.; Jacob, M.; Harris, T.; Bowles, K.; Wood, M.; Bailey, C. Vaginal Microbiota of Spayed Dogs with or without Recurrent Urinary Tract Infections. J. Vet. Intern. Med. 2014, 28, 300–304. [Google Scholar] [CrossRef]
- Lyman, C.C.; Holyoak, G.R.; Meinkoth, K.; Wieneke, X.; Chillemi, K.A.; DeSilva, U. Canine endometrial and vaginal microbiomes reveal distinct and complex ecosystems. PLoS ONE 2019, 14, e0210157. [Google Scholar] [CrossRef]
- Golińska, E.; Sowińska, N.; Tomusiak-Plebanek, A.; Szydło, M.; Witka, N.; Lenarczyk, J.; Strus, M. The vaginal microflora changes in various stages of the estrous cycle of healthy female dogs and the ones with genital tract infections. BMC Vet. Res. 2021, 17, 8. [Google Scholar] [CrossRef] [PubMed]
- Suchodolski, J.S.; Camacho, J.; Steiner, J.M. Analysis of bacterial diversity in the canine duodenum, jejunum, ileum, and colon by comparative 16S rRNA gene analysis. FEMS Microbiol. Ecol. 2008, 66, 567–578. [Google Scholar] [CrossRef] [PubMed]
- Pipan, M.Z.; Kajdič, L.; Kalin, A.; Plavec, T.; Zdovc, I. Do newborn puppies have their own microbiota at birth? Influence of type of birth on newborn puppy microbiota. Theriogenology 2020, 152, 18–28. [Google Scholar] [CrossRef]
- Jeffery, N.D.; Barker, A.K.; Alcott, C.J.; Levine, J.M.; Meren, I.; Wengert, J.; Jergens, A.E.; Suchodolski, J.S. The Association of Specific Constituents of the Fecal Microbiota with Immune-Mediated Brain Disease in Dogs. PLoS ONE 2017, 12, e0170589. [Google Scholar] [CrossRef]
- Tizard, I.R.; Jones, S.W. The Microbiota Regulates Immunity and Immunologic Diseases in Dogs and Cats. Veter Clin. N. Am. Small Anim. Pract. 2018, 48, 307–322. [Google Scholar] [CrossRef]
- Allen, K.; Blascovich, J.; Mendes, W.B. Cardiovascular reactivity and the presence of pets, friends, and spouses: The truth about cats and dogs. Psychosom. Med. 2002, 64, 727–739. [Google Scholar] [CrossRef] [PubMed]
- Honneffer, J.; Minamoto, Y.; Suchodolski, J.S. Microbiota alterations in acute and chronic gastrointestinal inflammation of cats and dogs. World J. Gastroenterol. 2014, 20, 16489–16497. [Google Scholar] [CrossRef] [PubMed]
- Suchodolski, J.S.; Markel, M.E.; Garcia-Mazcorro, J.F.; Unterer, S.; Heilmann, R.M.; Dowd, S.E.; Kachroo, P.; Ivanov, I.; Minamoto, Y.; Dillman, E.M.; et al. The Fecal Microbiome in Dogs with Acute Diarrhea and Idiopathic Inflammatory Bowel Disease. PLoS ONE 2012, 7, e51907. [Google Scholar] [CrossRef] [PubMed]
- Thanissery, R.; McLaren, M.; Rivera, A.; Reed, A.; Betrapally, N.; Burdette, T.; Winston, J.; Jacob, M.; Callahan, B.; Theriot, C. Clostridioides difficile carriage in animals and the associated changes in the host fecal microbiota. Anaerobe 2020, 66, 102279. [Google Scholar] [CrossRef] [PubMed]
- Guard, B.C.; Barr, J.W.; Reddivari, L.; Klemashevich, C.; Jayaraman, A.; Steiner, J.M.; Vanamala, J.; Suchodolski, J.S. Characterization of Microbial Dysbiosis and Metabolomic Changes in Dogs with Acute Diarrhea. PLoS ONE 2015, 10, e0127259. [Google Scholar] [CrossRef] [PubMed]
- Suchodolski, J.S. Fecal Microbiome in Dogs with Acute Diarrhea and Idiopathic Inflammatory Bowel Disease. In Encyclopedia of Metagenomics; Springer: New York, NY, USA, 2013. [Google Scholar] [CrossRef]
- Kubinyi, E.; Rhali, S.B.; Sándor, S.; Szabó, A.; Felföldi, T. Gut Microbiome Composition is Associated with Age and Memory Performance in Pet Dogs. Animals 2020, 10, 1488. [Google Scholar] [CrossRef] [PubMed]
- Mondo, E.; Barone, M.; Soverini, M.; D’Amico, F.; Cocchi, M.; Petrulli, C.; Mattioli, M.; Marliani, G.; Candela, M.; Accorsi, P. Gut microbiome structure and adrenocortical activity in dogs with aggressive and phobic behavioral disorders. Heliyon 2020, 6, e03311. [Google Scholar] [CrossRef]
- You, I.; Kim, M.J. Comparison of Gut Microbiota of 96 Healthy Dogs by Individual Traits: Breed, Age, and Body Condition Score. Animals 2021, 11, 2432. [Google Scholar] [CrossRef]
- Turroni, S.; Rampelli, S.; Biagi, E.; Consolandi, C.; Severgnini, M.; Peano, C.; Quercia, S.; Soverini, M.; Carbonero, F.G.; Bianconi, G.; et al. Temporal dynamics of the gut microbiota in people sharing a confined environment, a 520-day ground-based space simulation, MARS500. Microbiome 2017, 5, 39. [Google Scholar] [CrossRef] [PubMed]
- Moon, C.D.; Young, W.; Maclean, P.H.; Cookson, A.L.; Bermingham, E.N. Metagenomic insights into the roles of Proteobacteria in the gastrointestinal microbiomes of healthy dogs and cats. Microbiol. Open 2018, 7, e00677. [Google Scholar] [CrossRef]
- Li, Q.; Larouche-Lebel, E.; Loughran, K.A.; Huh, T.P.; Suchodolski, J.S.; Oyama, M.A. Gut Dysbiosis and Its Associations with Gut Microbiota-Derived Metabolites in Dogs with Myxomatous Mitral Valve Disease. mSystems 2021, 6, e00111-21. [Google Scholar] [CrossRef] [PubMed]
- Swanson, K.; Dowd, S.; Suchodolski, J.; Middelbos, I.S.; Vester, B.M.; Barry, A.K.; Nelson, E.K.; Torralba, M.; Henrissat, B.; Coutinho, P.M.; et al. Phylogenetic and gene-centric metagenomics of the canine intestinal microbiome reveals similarities with humans and mice. ISME J. 2010, 5, 639–649. [Google Scholar] [CrossRef] [PubMed]
- Suchodolski, J.S. Companion Animals Symposium: Microbes and gastrointestinal health of dogs and cats1. J. Anim. Sci. 2011, 89, 1520–1530. [Google Scholar] [CrossRef] [PubMed]
- Nicco, C.; Paule, A.; Konturek, P.; Edeas, M. From Donor to Patient: Collection, Preparation and Cryopreservation of Fecal Samples for Fecal Microbiota Transplantation. Diseases 2020, 8, 9. [Google Scholar] [CrossRef] [PubMed]
- Sugita, K.; Yanuma, N.; Ohno, H.; Takahashi, K.; Kawano, K.; Morita, H.; Ohmori, K. Oral faecal microbiota transplantation for the treatment of Clostridium difficile-associated diarrhoea in a dog: A case report. BMC Vet. Res. 2019, 15, 11. [Google Scholar] [CrossRef]
- Pilla, R.; Suchodolski, J.S. The Gut Microbiome of Dogs and Cats, and the Influence of Diet. Veter Clin. N. Am. Small Anim. Pract. 2021, 51, 605–621. [Google Scholar] [CrossRef] [PubMed]
- Pilla, R.; Gaschen, F.; Barr, J.W.; Olson, E.; Honneffer, J.; Guard, B.C.; Blake, A.B.; Villanueva, D.; Khattab, M.R.; Alshawaqfeh, M.K.; et al. Effects of metronidazole on the fecal microbiome and metabolome in healthy dogs. J. Vet. Intern. Med. 2020, 34, 1853–1866. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, H.; Maeda, S.; Ohno, K.; Horigome, A.; Odamaki, T.; Tsujimoto, H. Effect of Oral Administration of Metronidazole or Prednisolone on Fecal Microbiota in Dogs. PLoS ONE 2014, 9, e107909. [Google Scholar] [CrossRef]
- Lucchetti, B.; Lane, S.L.; Koenig, A.; Good, J.; Suchodolski, J.S.; Brainard, B.M. Effects of a Perioperative Antibiotic and Vet-erinary Probiotic on Fecal Dysbiosis Index in Dogs. Can. Vet. J. 2021, 62, 240–246. [Google Scholar] [PubMed]
- Manchester, A.C.; Webb, C.B.; Blake, A.B.; Sarwar, F.; Lidbury, J.A.; Steiner, J.M.; Suchodolski, J.S. Long-term impact of tylosin on fecal microbiota and fecal bile acids of healthy dogs. J. Vet. Intern. Med. 2019, 33, 2605–2617. [Google Scholar] [CrossRef] [PubMed]
- Handl, S.; German, A.; Holden, S.L.; Dowd, S.; Steiner, J.M.; Heilmann, R.M.; Grant, R.; Swanson, K.; Suchodolski, J. Faecal microbiota in lean and obese dogs. FEMS Microbiol. Ecol. 2013, 84, 332–343. [Google Scholar] [CrossRef] [PubMed]
- Jergens, A.E.; Guard, B.C.; Redfern, A.; Rossi, G.; Mochel, J.; Pilla, R.; Chandra, L.; Seo, Y.-J.; Steiner, J.M.; Lidbury, J.; et al. Microbiota-Related Changes in Unconjugated Fecal Bile Acids Are Associated with Naturally Occurring, Insulin-Dependent Diabetes Mellitus in Dogs. Front. Vet. Sci. 2019, 6, 199. [Google Scholar] [CrossRef]
- García-Belenguer, S.; Grasa, L.; Valero, O.; Palacio, J.; Luño, I.; Rosado, B. Gut Microbiota in Canine Idiopathic Epilepsy: Effects of Disease and Treatment. Animals 2021, 11, 3121. [Google Scholar] [CrossRef]
- Montoya-Alonso, J.A.; Bautista-Castaño, I.; Peña, C.; Suárez, L.; Juste, M.C.; Tvarijonaviciute, A. Prevalence of Canine Obesity, Obesity-Related Metabolic Dysfunction, and Relationship with Owner Obesity in an Obesogenic Region of Spain. Front. Vet. Sci. 2017, 4, 59. [Google Scholar] [CrossRef]
- Lehtimäki, J.; Sinkko, H.; Hielm-Björkman, A.; Laatikainen, T.; Ruokolainen, L.; Lohi, H. Simultaneous allergic traits in dogs and their owners are associated with living environment, lifestyle and microbial exposures. Sci. Rep. 2020, 10, 21954. [Google Scholar] [CrossRef]
- Chaitman, J.; Gaschen, F. Fecal Microbiota Transplantation in Dogs. Vet. Clin. N. Am. Small Anim. Pract. 2020, 51, 219–233. [Google Scholar] [CrossRef]
- Chaitman, J.; Ziese, A.-L.; Pilla, R.; Minamoto, Y.; Blake, A.B.; Guard, B.C.; Isaiah, A.; Lidbury, J.A.; Steiner, J.M.; Unterer, S.; et al. Fecal Microbial and Metabolic Profiles in Dogs with Acute Diarrhea Receiving Either Fecal Microbiota Transplantation or Oral Metronidazole. Front. Vet. Sci. 2020, 7, 192. [Google Scholar] [CrossRef]
- Alshawaqfeh, M.K.; Wajid, B.; Minamoto, Y.; Markel, M.; Lidbury, J.A.; Steiner, J.M.; Serpedin, E.; Suchodolski, J.S. A dysbiosis index to assess microbial changes in fecal samples of dogs with chronic inflammatory enteropathy. FEMS Microbiol. Ecol. 2017, 93, fix136. [Google Scholar] [CrossRef] [PubMed]
- Gal, A.; Barko, P.C.; Biggs, P.J.; Gedye, K.R.; Midwinter, A.C.; Williams, D.A.; Burchell, R.K.; Pazzi, P. One dog’s waste is another dog’s wealth: A pilot study of fecal microbiota transplantation in dogs with acute hemorrhagic diarrhea syndrome. PLoS ONE 2021, 16, e0250344. [Google Scholar] [CrossRef] [PubMed]
- Pereira, G.Q.; Gomes, L.A.; Santos, I.S.; Alfieri, A.; Weese, J.S.; Costa, M.C. Fecal microbiota transplantation in puppies with canine parvovirus infection. J. Vet. Intern. Med. 2018, 32, 707–711. [Google Scholar] [CrossRef]
- Niina, A.; Kibe, R.; Suzuki, R.; Yuchi, Y.; Teshima, T.; Matsumoto, H.; Kataoka, Y.; Koyama, H. Improvement in Clinical Symptoms and Fecal Microbiome After Fecal Microbiota Transplantation in a Dog with Inflammatory Bowel Disease. Vet. Med. Res. Rep. 2019, ume 10, 197–201. [Google Scholar] [CrossRef]
- Niina, A.; Kibe, R.; Suzuki, R.; Yuchi, Y.; Teshima, T.; Matsumoto, H.; Kataoka, Y.; Koyama, H. Fecal microbiota transplantation as a new treatment for canine inflammatory bowel disease. Biosci. Microbiota Food Health 2021, 40, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Bottero, E.; Benvenuti, E.; Ruggiero, P. Faecal Microbiota Transplantation in 16 Dogs with Idiopathic Inflammatory Bowel Disease. Veterinaria 2017, 31, 1–12. [Google Scholar]
- Diniz, A.N.; Souza, A.D.C.F.D.; Nepomuceno, A.C.; Marcelino, S.A.C.; Pierezan, F.; Lobato, F.C.F.; Silva, R.O.S. Fecal microbiota transplantation via colonoscopy in a dog with Clostridioides (Clostridium) difficile infection. Ciência Rural 2021, 51, 783. [Google Scholar] [CrossRef]
- Burton, E.N.; O’Connor, E.; Ericsson, A.C.; Franklin, C.L. Evaluation of Fecal Microbiota Transfer as Treatment for Post-weaning Diarrhea in Research-Colony Puppies. J. Am. Assoc. Lab. Anim. Sci. 2016, 55, 582–587. [Google Scholar] [PubMed]
- Kelly, C.R.; Khoruts, A.; Staley, C.; Sadowsky, M.J.; Abd, M.; Alani, M.; Bakow, B.B.; Curran, P.; McKenney, M.J.; Tisch, N.A.; et al. Effect of Fecal Microbiota Transplantation on Recurrence in Multiply Recurrent Clostridium difficile Infection. Ann. Intern. Med. 2016, 165, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Mullish, B.; Quraishi, M.N.; Segal, J.P.; McCune, V.L.; Baxter, M.; Marsden, G.; Moore, D.; Colville, A.; Bhala, N.; Iqbal, T.H.; et al. The use of faecal microbiota transplant as treatment for recurrent or refractory Clostridium difficile infection and other potential indications: Joint British Society of Gastroenterology (BSG) and Healthcare Infection Society (HIS) guidelines. J. Hosp. Infect. 2018, 100, S1–S31. [Google Scholar] [CrossRef]
- Baktash, A.; Terveer, E.M.; Zwittink, R.D.; Hornung, B.V.H.; Corver, J.; Kuijper, E.J.; Smits, W.K. Mechanistic Insights in the Success of Fecal Microbiota Transplants for the Treatment of Clostridium difficile Infections. Front. Microbiol. 2018, 9, 1242. [Google Scholar] [CrossRef]
- Krajicek, E.; Fischer, M.; Allegretti, J.R.; Kelly, C.R. Nuts and Bolts of Fecal Microbiota Transplantation. Clin. Gastroenterol. Hepatol. 2019, 17, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Weingarden, A.R.; Dosa, P.I.; DeWinter, E.; Steer, C.J.; Shaughnessy, M.K.; Johnson, J.R.; Khoruts, A.; Sadowsky, M.J. Changes in Colonic Bile Acid Composition following Fecal Microbiota Transplantation Are Sufficient to Control Clostridium difficile Germination and Growth. PLoS ONE 2016, 11, e0147210. [Google Scholar] [CrossRef] [PubMed]
- Staley, C.; Weingarden, A.R.; Khoruts, A.; Sadowsky, M.J. Interaction of gut microbiota with bile acid metabolism and its influence on disease states. Appl. Microbiol. Biotechnol. 2017, 101, 47–64. [Google Scholar] [CrossRef] [PubMed]
- Moayyedi, P.; Surette, M.G.; Kim, P.T.; Libertucci, J.; Wolfe, M.; Onischi, C.; Armstrong, D.; Marshall, J.K.; Kassam, Z.; Reinisch, W.; et al. Fecal Microbiota Transplantation Induces Remission in Patients with Active Ulcerative Colitis in a Randomized Controlled Trial. Gastroenterology 2015, 149, 102–109.e6. [Google Scholar] [CrossRef] [PubMed]
- Andrés-Lasheras, S.; Martín-Burriel, I.; Mainar-Jaime, R.C.; Morales, M.; Kuijper, E.; Blanco, J.L.; Chirino-Trejo, M.; Bolea, R. Preliminary studies on isolates of Clostridium difficile from dogs and exotic pets. BMC Vet. Res. 2018, 14, 77. [Google Scholar] [CrossRef] [PubMed]
- Niederwerder, M.C. Fecal microbiota transplantation as a tool to treat and reduce susceptibility to disease in animals. Vet. Immunol. Immunopathol. 2018, 206, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.C.; Kamm, A.M.; Yeoh, Y.K.; Chan, P.K.S.; Zuo, T.; Tang, W.; Sood, A.; Andoh, A.; Ohmiya, N.; Zhou, Y.; et al. Scientific frontiers in faecal microbiota transplantation: Joint document of Asia-Pacific Association of Gastroenterology (APAGE) and Asia-Pacific Society for Digestive Endoscopy (APSDE). Gut 2019, 69, 83–91. [Google Scholar] [CrossRef]
- Kirchoff, N.S.; Udell, M.A.; Sharpton, T.J. The gut microbiome correlates with conspecific aggression in a small population of rescued dogs (Canis familiaris). PeerJ 2019, 7, e6103. [Google Scholar] [CrossRef]
- Kelly, J.R.; Borre, Y.; O’Brien, C.; Patterson, E.; El Aidy, S.; Deane, J.; Kennedy, P.J.; Beers, S.; Scott, K.; Moloney, G.; et al. Transferring the blues: Depression-associated gut microbiota induces neurobehavioural changes in the rat. J. Psychiatr. Res. 2016, 82, 109–118. [Google Scholar] [CrossRef]
- Bharwani, A.; Mian, M.F.; Surette, M.G.; Bienenstock, J.; Forsythe, P. Oral treatment with Lactobacillus rhamnosus attenuates behavioural deficits and immune changes in chronic social stress. BMC Med. 2017, 15, 7. [Google Scholar] [CrossRef]
- Hsiao, E.Y.; McBride, S.W.; Hsien, S.; Sharon, G.; Hyde, E.R.; McCue, T.; Codelli, J.A.; Chow, J.; Reisman, S.E.; Petrosino, J.F.; et al. Microbiota Modulate Behavioral and Physiological Abnormalities Associated with Neurodevelopmental Disorders. Cell 2013, 155, 1451–1463. [Google Scholar] [CrossRef] [PubMed]
- Staley, C.; Hamilton, M.J.; Vaughn, B.; Graiziger, C.T.; Newman, K.M.; Kabage, A.J.; Sadowsky, M.; Khoruts, A. Successful Resolution of Recurrent Clostridium difficile Infection using Freeze-Dried, Encapsulated Fecal Microbiota; Pragmatic Cohort Study. Am. J. Gastroenterol. 2017, 112, 940–947. [Google Scholar] [CrossRef]
- Terveer, E.; Van Beurden, Y.; Goorhuis, A.; Seegers, J.; Bauer, M.; Van Nood, E.; Dijkgraaf, M.; Mulder, C.; Vandenbroucke-Grauls, C.; Verspaget, H.; et al. How to: Establish and run a stool bank. Clin. Microbiol. Infect. 2017, 23, 924–930. [Google Scholar] [CrossRef]
- Kieler, I.N.; Kamal, S.S.; Vitger, A.D.; Nielsen, D.S.; Lauridsen, C.; Bjornvad, C. Gut microbiota composition may relate to weight loss rate in obese pet dogs. Vet. Med. Sci. 2017, 3, 252–262. [Google Scholar] [CrossRef]
- Sanchez, S.B.; Pilla, R.; Sarawichitr, B.; Gramenzi, A.; Marsilio, F.; Steiner, J.M.; Lidbury, J.A.; Woods, G.R.; German, A.J.; Suchodolski, J.S. Fecal microbiota in client-owned obese dogs changes after weight loss with a high-fiber-high-protein diet. PeerJ 2020, 8, e9706. [Google Scholar] [CrossRef]
- Kootte, R.S.; Levin, E.; Salojärvi, J.; Smits, L.P.; Hartstra, A.V.; Udayappan, S.D.; Hermes, G.; Bouter, K.E.; Koopen, A.M.; Holst, J.J.; et al. Improvement of Insulin Sensitivity after Lean Donor Feces in Metabolic Syndrome Is Driven by Baseline Intestinal Microbiota Composition. Cell Metab. 2017, 26, 611–619.e6. [Google Scholar] [CrossRef]
- Vrieze, A.; Van Nood, E.; Holleman, F.; Salojärvi, J.; Kootte, R.S.; Bartelsman, J.F.; Dallinga-Thie, G.M.; Ackermans, M.T.; Serlie, M.J.; Oozeer, R.; et al. Transfer of Intestinal Microbiota From Lean Donors Increases Insulin Sensitivity in Individuals With Metabolic Syndrome. Gastroenterology 2012, 143, 913–916.e7. [Google Scholar] [CrossRef]
- Keshteli, A.H.; Millan, B.; Madsen, K.L. Pretreatment with antibiotics may enhance the efficacy of fecal microbiota transplantation in ulcerative colitis: A meta-analysis. Mucosal Immunol. 2017, 10, 565–566. [Google Scholar] [CrossRef] [PubMed]
- Ji, S.K.; Yan, H.; Jiang, T.; Guo, C.Y.; Liu, J.J.; Dong, S.Z.; Yang, K.L.; Wang, Y.J.; Cao, Z.J.; Li, S.L. Preparing the Gut with Antibiotics Enhances Gut Microbiota Reprogramming Efficiency by Promoting Xenomicrobiota Colonization. Front. Microbiol. 2017, 8, 1208. [Google Scholar] [CrossRef]
- Fang, S.; Song, Y.; Liu, Y.; Wang, L. Randomized clinical trial: Efficacy and tolerability of two different split dose of low-volume polyethylene glycol electrolytes for bowel preparation before colonoscopy in hospitalized children. Pediatr. Res. 2020, 90, 171–175. [Google Scholar] [CrossRef] [PubMed]
- Wrzosek, L.; Ciocan, D.; Borentain, P.; Spatz, M.; Puchois, V.; Hugot, C.; Ferrere, G.; Mayeur, C.; Perlemuter, G.; Cassard, A.-M. Transplantation of human microbiota into conventional mice durably reshapes the gut microbiota. Sci. Rep. 2018, 8, 6854. [Google Scholar] [CrossRef] [PubMed]
- Jergens, A.E.; Crandell, J.; Morrison, J.A.; Deitz, K.; Pressel, M.; Ackermann, M.; Suchodolski, J.; Steiner, J.M.; Evans, R. Comparison of Oral Prednisone and Prednisone Combined with Metronidazole for Induction Therapy of Canine Inflammatory Bowel Disease: A Randomized-Controlled Trial. J. Vet. Intern. Med. 2010, 24, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Ural, K. Fecal microbiota transplantation capsule therapy via oral route for combatting atopic dermatitis in dogs. Ank. Üniversitesi Vet. Fakültesi Derg. 2021, 69, 211–219. [Google Scholar] [CrossRef]
- Tian, H.; Ding, C.; Gong, J.; Wei, Y.; McFarland, L.V.; Li, N. Freeze-dried, Capsulized Fecal Microbiota Transplantation for Relapsing Clostridium difficile Infection. J. Clin. Gastroenterol. 2015, 49, 537–538. [Google Scholar] [CrossRef]
- Hecker, M.T.; Obrenovich, M.E.; Cadnum, J.L.; Jencson, A.L.; Jain, A.K.; Ho, E.; Donskey, C.J. Fecal Microbiota Transplantation by Freeze-Dried Oral Capsules for Recurrent Clostridium difficile Infection. Open Forum Infect. Dis. 2016, 3, ofw091. [Google Scholar] [CrossRef] [PubMed]


| Author, Year | Recipient Feature | Number of Dogs | Frequency of FMT | Delivery Route | Clinical Effects | Effects on Fecal Microbiota | Method for Fecal Preparation |
|---|---|---|---|---|---|---|---|
| Burton et al., 2016 [57] | Weaning puppies, postweaning diarrhea | 11 FMT 12 controls | 5 days, once per day | Oral | No difference in fecal consistency between FMT and control puppies | Wide variability of microbiome in puppies, no clustering with donor microbiome observed | 10 mL fecal suspension (100 g pooled dam feces mixed with 200 mL 2% fat cow’s milk after filtration) |
| Bottero et al., 2017 [55] | IBD refractory to conventional treatment | 16 adult dogs with severe, refractory IBD of >1 year duration | Oral treatment group received FMT q 48–72 h | 9 dogs endoscopy + oral, 7 dogs oral | Overall, mean CCECAI seemed to decrease in most dogs following FMT. Heterogeneous clinical presentation and concurrent treatments complicate evaluation | Not applicable | 60–80 g feces for dogs <20 kg BW, 100–150 g for dogs > 20 kg BW. 1:1 dilution with 0.9% saline, filtered and mixed with low-fat yogurt as enrichment solution |
| Pereia et al., 2018 [52] | Parvovirus infection | 33 received standard treatment, 33 received FMT in addition | FMT administered within 5–12 h of admission and q 48 h thereafter | Endoscopy | No difference in mortality rate, FMT dogs had quicker resolution of diarrhea, and shorter hospitalization | Not applicable | 10 g feces administered per puppy. 1:1 dilution with saline |
| Nina et al., 2019 [53] | IBD refractory to antibiotic and immunosuppressive treatment over time | 10-year-old toy poodle | 9 treatments within 6 months | Endoscopy | Improved CIBDAI. | Increased in Fusobacteria, Firmicutes and Bacteroidetes, decreased in Proteobacteria. Clustered phylogenetically with donor | Feces diluted 1:3 with ringer lactate. The dog received approximately 3 g feces/kg body weight |
| Sugita et al., 2019 [37] | Intermittent large bowel diarrhea, 4 months of duration, feces positive for CD (PCR and toxins A & B) | 8-month-old French bulldog | Oral | Normalization of fecal consistency and defecation frequency within 2–3 days, without recurrence of CD or diarrhea over 190 days | Not applicable | 30 mL fecal suspension (60 g feces diluted in 50 mL tap water after filtration) given orally. Equivalent to approximately 2.5–3 g feces/kg BW | |
| Chitman et al., 2020 [49] | Uncomplicated acute diarrhea of <14 days duration | 11 dogs received a single FMT, 7 dogs received metronidazole 15 mg/kg q 12 h for 7 days | Endoscopy | Lower (better) fecal score at days 7 and 28 for both treatments, FMT fecal score lower than metronidazole at day 28 | Fecal dysbiosis indexes better with FMT than metronidazole at days 7 and 28. FMT dogs tended to cluster healthy dogs at day 28, unlike metronidazole dogs | Fresh feces mixed with 60 mL 0.9% NaCl in a blender. Blend on high until the stool is liquefied and no larger pieces are seen. For very large dogs a larger volume of saline may be needed to obtain sufficiently liquefied fecal solution | |
| Diniz et al., 2021 [56] | Chronic-recurring pasty large bowel diarrhea | 4-year-old female golden retriever | Received FMT via colonoscopy | Colonoscopy | Clostridium difficile no longer present in the dog’s stool | Not applicable | Approximately 65 g of feces were diluted in 250 mL of sterilized PBS |
| Gal et al., 2021 [51] | Canine acute hemorrhagic diarrhea syndrome | 8 dogs aged 3–12 years old | Received FMT via colonoscopy | Colonoscopy | There were no significant differences in median AHDS clinical scores between FMT-recipients and sham-treated controls | Increased microbiota diversity. Short-chain fatty acid producers including Eubacterium biforme, Faecalibacterium prausnitzii, and Prevotella copri were significantly decreased | Stool was homogenized at room temperature in a sterilized blender at a ratio of 1-part stool/4 parts saline. The suspension was passed through a sterilized sieve to remove large particles |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tuniyazi, M.; Hu, X.; Fu, Y.; Zhang, N. Canine Fecal Microbiota Transplantation: Current Application and Possible Mechanisms. Vet. Sci. 2022, 9, 396. https://doi.org/10.3390/vetsci9080396
Tuniyazi M, Hu X, Fu Y, Zhang N. Canine Fecal Microbiota Transplantation: Current Application and Possible Mechanisms. Veterinary Sciences. 2022; 9(8):396. https://doi.org/10.3390/vetsci9080396
Chicago/Turabian StyleTuniyazi, Maimaiti, Xiaoyu Hu, Yunhe Fu, and Naisheng Zhang. 2022. "Canine Fecal Microbiota Transplantation: Current Application and Possible Mechanisms" Veterinary Sciences 9, no. 8: 396. https://doi.org/10.3390/vetsci9080396
APA StyleTuniyazi, M., Hu, X., Fu, Y., & Zhang, N. (2022). Canine Fecal Microbiota Transplantation: Current Application and Possible Mechanisms. Veterinary Sciences, 9(8), 396. https://doi.org/10.3390/vetsci9080396






