Efficacy of Antimicrobial Treatment in Dogs with Atopic Dermatitis: An Observational Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Prospective Study
2.2. Retrospective Study
2.3. Statistical Methods
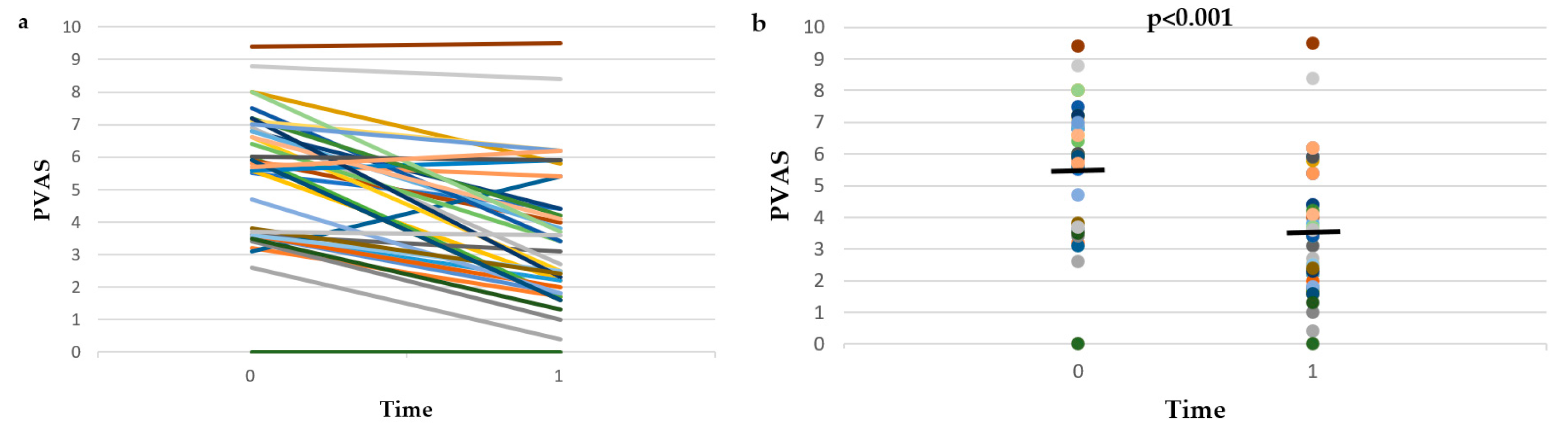
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Halliwell, R. Revised nomenclature for veterinary allergy. Vet. Immunol. Immunopathol. 2006, 114, 207–208. [Google Scholar] [CrossRef] [PubMed]
- Santoro, D.; Marsella, R.; Pucheu-Haston, C.M.; Eisenschenk, M.N.; Nuttall, T.; Bizikova, P. Review: Pathogenesis of canine atopic dermatitis: Skin barrier and host-micro-organism interaction. Vet. Dermatol. 2015, 26, 84-e25. [Google Scholar] [CrossRef] [PubMed]
- Bizikova, P.; Pucheu-Haston, C.M.; Eisenschenk, M.N.; Marsella, R.; Nuttall, T.; Santoro, D. Review: Role of genetics and the environment in the pathogenesis of canine atopic dermatitis. Vet. Dermatol. 2015, 26, 95-e26. [Google Scholar] [CrossRef] [PubMed]
- Pucheu-Haston, C.M.; Bizikova, P.; Eisenschenk, M.N.; Santoro, D.; Nuttall, T.; Marsella, R. Review: The role of antibodies, autoantigens and food allergens in canine atopic dermatitis. Vet. Dermatol. 2015, 26, 115-e130. [Google Scholar] [CrossRef] [PubMed]
- Pucheu-Haston, C.M.; Bizikova, P.; Marsella, R.; Santoro, D.; Nuttall, T.; Eisenschenk, M.N. Review: Lymphocytes, cytokines, chemokines and the T-helper 1-T-helper 2 balance in canine atopic dermatitis. Vet. Dermatol. 2015, 26, 124-e132. [Google Scholar] [CrossRef] [Green Version]
- Nuttall, T.J.; Marsella, R.; Rosenbaum, M.R.; Gonzales, A.J.; Fadok, V.A. Update on pathogenesis, diagnosis, and treatment of atopic dermatitis in dogs. J. Am. Vet. Med. Assoc. 2019, 254, 1291–1300. [Google Scholar] [CrossRef] [PubMed]
- Marsella, R. Advances in our understanding of canine atopic dermatitis. Vet. Dermatol. 2021, 32, 547-e151. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues Hoffmann, A. The cutaneous ecosystem: The roles of the skin microbiome in health and its association with inflammatory skin conditions in humans and animals. Vet. Dermatol. 2017, 28, 60-e15. [Google Scholar] [CrossRef] [PubMed]
- Yue, H.; Umehara, Y.; Trujillo-Paez, J.V.; Peng, G.; Nguyen, H.L.T.; Chieosilapatham, P.; Kiatsurayanon, C.; Song, P.; Okumura, K.; Ogawa, H.; et al. Exogenous factors in the pathogenesis of atopic dermatitis: Irritants and cutaneous infections. Clin. Exp. Allergy 2021, 51, 382–392. [Google Scholar] [CrossRef]
- Meason-Smith, C.; Diesel, A.; Patterson, A.P.; Older, C.E.; Mansell, J.M.; Suchodolski, J.S.; Rodrigues Hoffmann, A. What is living on your dog’s skin? Characterization of the canine cutaneous mycobiota and fungal dysbiosis in canine allergic dermatitis. FEMS Microbiol. Ecol. 2015, 91, fiv139. [Google Scholar] [CrossRef] [Green Version]
- Bradley, C.W.; Morris, D.O.; Rankin, S.C.; Cain, C.L.; Misic, A.M.; Houser, T.; Mauldin, E.A.; Grice, E.A. Longitudinal evaluation of the skin microbiome and association with microenvironment and treatment in canine atopic dermatitis. J. Investig. Dermatol. 2016, 136, 1182–1190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pierezan, F.; Olivry, T.; Paps, J.S.; Lawhon, S.D.; Wu, J.; Steiner, J.M.; Suchodolski, J.S.; Rodrigues Hoffmann, A. The skin microbiome in allergen-induced canine atopic dermatitis. Vet. Dermatol. 2016, 27, 332-e382. [Google Scholar] [CrossRef] [PubMed]
- Chermprapai, S.; Ederveen, T.H.A.; Broere, F.; Broens, E.M.; Schlotter, Y.M.; van Schalkwijk, S.; Boekhorst, J.; van Hijum, S.A.F.T.; Rutten, V.P.M.G. The bacterial and fungal microbiome of the skin of healthy dogs and dogs with atopic dermatitis and the impact of topical antimicrobial therapy, an exploratory study. Vet. Microbiol. 2019, 229, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Asahina, R.; Ueda, K.; Oshima, Y.; Kanei, T.; Kato, M.; Furue, M.; Tsukui, T.; Nagata, M.; Maeda, S. Serum canine thymus and activation-regulated chemokine (TARC/CCL17) concentrations correlate with disease severity and therapeutic responses in dogs with atopic dermatitis. Vet. Dermatol. 2020, 31, 446-e118. [Google Scholar] [CrossRef] [PubMed]
- Meason-Smith, C.; Olivry, T.; Lawhon, S.D.; Rodrigues Hoffmann, A. Malassezia species dysbiosis in natural and allergen-induced atopic dermatitis in dogs. Med. Mycol. 2020, 58, 756–765. [Google Scholar] [CrossRef]
- DeBoer, D.J.; Marsella, R. The ACVD task force on canine atopic dermatitis (XII): The relationship of cutaneous infections to the pathogenesis and clinical course of canine atopic dermatitis. Vet. Immunol. Immunopathol. 2001, 81, 239–249. [Google Scholar] [CrossRef]
- Older, C.E.; Rodrigues Hoffmann, A.; Hoover, K.; Banovic, F. Characterization of cutaneous bacterial microbiota from superficial pyoderma forms in atopic dogs. Pathogens 2020, 9, 638. [Google Scholar] [CrossRef]
- Olivry, T.; Sousa, C.A. The ACVD task force on canine atopic dermatitis (XIX): General principles of therapy. Vet. Immunol. Immunopathol. 2001, 81, 311–316. [Google Scholar] [CrossRef]
- Nuttall, T.J.; Halliwell, R.E. Serum antibodies to Malassezia yeasts in canine atopic dermatitis. Vet. Dermatol. 2001, 12, 327–332. [Google Scholar] [CrossRef] [Green Version]
- DeBoer, D.J. Guidelines for symptomatic medical treatment of canine atopic dermatitis. In Veterinary Allergy, 1st ed.; Noli, C., Foster, A., Rosenkrantz, W., Eds.; Wiley Blackwell: Oxford, UK, 2014; pp. 90–95. [Google Scholar]
- Kawarai, S.; Fujimoto, A.; Nozawa, G.; Kanemaki, N.; Madarame, H.; Shida, T.; Kiuchi, A. Evaluation of weekly bathing in allergic dogs with methicillin-resistant Staphylococcal colonization. Jpn. J. Vet. Res. 2016, 64, 153–158. [Google Scholar] [PubMed]
- McFadden, R.A.; Heinrich, N.A.; Haarstad, A.C.; Tomlinson, D.J. A double-blinded, randomized, controlled, crossover evaluation of a zinc methionine supplement as an adjunctive treatment for canine atopic dermatitis. Vet. Dermatol. 2017, 28, 569-e138. [Google Scholar] [CrossRef]
- Esumi, M.; Kanda, S.; Shimoura, H.; Hsiao, Y.H.; Iyori, K. Preliminary evaluation of two bathing methods for the management of Malassezia overgrowth in dogs with atopic dermatitis. Vet. Dermatol. 2021, 32, 228-e259. [Google Scholar] [CrossRef] [PubMed]
- Olivry, T.; Bensignor, E.; Favrot, C.; Griffin, C.E.; Hill, P.B.; Mueller, R.S.; Plant, J.D.; Williams, H.C.; for the International Committee of Allergic Diseases of Animals (ICADA). Development of a core outcome set for therapeutic clinical trials enrolling dogs with atopic dermatitis (COSCAD’18). BMC Vet. Res. 2018, 14, 238. [Google Scholar] [CrossRef] [PubMed]
- Favrot, C.; Steffan, J.; Seewald, W.; Picco, F. A prospective study on the clinical features of chronic canine atopic dermatitis and its diagnosis. Vet. Dermatol. 2010, 21, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Beco, L.; Guaguère, E.; Lorente Méndez, C.; Noli, C.; Nuttall, T.; Vroom, M. Suggested guidelines for using systemic antimicrobials in bacterial skin infections (1): Diagnosis based on clinical presentation, cytology and culture. Vet. Rec. 2013, 172, 72–78. [Google Scholar] [CrossRef] [Green Version]
- Bond, R.; Morris, D.O.; Guillot, J.; Bensignor, E.J.; Robson, D.; Mason, K.V.; Kano, R.; Hill, P.B. Biology, diagnosis and treatment of Malassezia dermatitis in dogs and cats. Clinical consensus guidelines of the World Association for Veterinary Dermatology. Vet. Dermatol. 2020, 31, 27-e24. [Google Scholar] [CrossRef]
- Tapes, D.; Skampardonis, V.; Chatzis, M.K.; Apostolidis, K.; Athanasiou, L.V.; Kasabalis, D.; Kokkinaki, K.C.G.; Katsogiannou, E.G.; Petanides, T.; Leontides, L.; et al. Repeatability and reproducibility of microscopic examination of adhesive tape strip cytology slides for the quantification of Malassezia spp. in canine skin. Vet. Dermatol. 2022, 33, 305-e71. [Google Scholar] [CrossRef]
- Olivry, T.; Saridomichelakis, M.; for the International Committee on Atopic Diseases of Animals (ICADA). Evidence-based guidelines for anti-allergic drug withdrawal times before allergen-specific intradermal and IgE serological tests in dogs. Vet. Dermatol. 2013, 24, 225–232. [Google Scholar] [CrossRef]
- Saridomichelakis, M.N.; Athanasiou, L.V.; Salame, M.; Chatzis, M.K.; Katsoudas, V.; Pappas, I.S. Serum pharmacokinetics of clindamycin hydrochloride in normal dogs when administered at two dosage regimens. Vet. Dermatol. 2011, 22, 429–435. [Google Scholar] [CrossRef]
- Beco, L.; Guaguère, E.; Lorente Méndez, C.; Noli, C.; Nuttall, T.; Vroom, M. Suggested guidelines for using systemic antimicrobials in bacterial skin infections: Part 2-antimicrobial choice, treatment regimens and compliance. Vet. Rec. 2013, 172, 156–160. [Google Scholar] [CrossRef] [Green Version]
- Hillier, A.; Lloyd, D.H.; Weese, J.S.; Blondeau, J.M.; Boothe, D.; Breitschwerd, E.; Guardabassi, L.; Papich, M.G.; Rankin, S.; Turnidge, J.D.; et al. Guidelines for the diagnosis and antimicrobial therapy of canine superficial bacterial folliculitis (Antimicrobial Guidelines Working Group of the International Society for Companion Animal Infectious Diseases). Vet. Dermatol. 2014, 25, 163-e143. [Google Scholar] [CrossRef] [PubMed]
- Olivry, T.; Saridomichelakis, M.; Nuttall, T.; Bensignor, E.; Griffin, C.E.; Hill, P.B.; for the International Committee on Allergic Diseases of Animals (ICADA). Validation of the Canine Atopic Dermatitis Extent and Severity Index (CADESI)-4, a simplified severity scale for assessing skin lesions of atopic dermatitis in dogs. Vet. Dermatol. 2014, 25, 77-e25. [Google Scholar] [CrossRef]
- Olivry, T. Could we use the erythema grading of the CADESI4 as a simple instrument for future short-duration clinical trials of dogs with atopic dermatitis? Vet. Dermatol. 2019, 30, 80–81. [Google Scholar] [CrossRef] [PubMed]
- High, E.J.; Olivry, T. Development and validation of a graphic 2D investigator’s global assessment instrument for grading the overall severity of atopic dermatitis in dogs. Vet. Dermatol. 2020, 31, 207-e243. [Google Scholar] [CrossRef] [PubMed]
- Hill, P.B.; Lau, P.; Rybnicek, J. Development of an owner-assesed scale to measure the severity of pruritus in dogs. Vet. Dermatol. 2007, 18, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Rybnícek, J.; Lau-Gillard, P.J.; Harvey, R.; Hill, P.B. Further validation of a pruritus severity scale for use in dogs. Vet. Dermatol. 2009, 20, 115–122. [Google Scholar] [CrossRef]
- Pucheu-Haston, C.M.; Mougeot, I. Serum IgE and IgG responses to dietary antigens in dogs with and without cutaneous adverse food reactions. Vet. Dermatol. 2020, 31, 116-e120. [Google Scholar] [CrossRef]
- Hensel, P.; Santoro, D.; Favrot, C.; Hill, P.; Griffin, C. Canine atopic dermatitis: Detailed guidelines for diagnosis and allergen identification. BMC Vet. Res. 2015, 11, 196. [Google Scholar] [CrossRef] [Green Version]
- Olivry, T. New diagnostic criteria for canine atopic dermatitis. Vet. Dermatol. 2010, 21, 124–127. [Google Scholar] [CrossRef]
- Wilhem, S.; Kovalik, M.; Favrot, C. Breed-associated phenotypes in canine atopic dermatitis. Vet. Dermatol. 2011, 22, 143–149. [Google Scholar] [CrossRef]
- Fleck, T.J.; Norris, L.R.; Mahabir, S.; Walters, R.R.; Martinon, O.; Dunham, S.A.; Gonzales, A.J. Onset and duration of action of lokivetmab in a canine model of IL-31 induced pruritus. Vet. Dermatol. 2021, 32, 681-e182. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.C.; Tsai, T.F. Itraconazole in the treatment of nonfungal cutaneous diseases: A review. Dermatol. Ther. 2019, 9, 271–280. [Google Scholar] [CrossRef] [Green Version]
- Assar, S.; Nosratabadi, R.; Azad, H.K.; Masoumi, J.; Mohamadi, M.; Hassanshahi, G. A review of immunomodulatory effects of fluoroquinolones. Immunol. Investig. 2021, 50, 1007–1026. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.; Jaros, J.; Shi, V.Y. Staphylococcus aureus in atopic dermatitis: Past, present, and future. Dermatitis 2020, 31, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Khantavee, N.; Chanthick, C.; Sookrung, N.; Prapasarakul, N. Antibody levels to Malassezia pachydermatis and Staphylococcus pseudintermedius in atopic dogs and their relationship with lesion scores. Vet. Dermatol. 2019, 31, 111-e118. [Google Scholar] [CrossRef]
- Morris, D.O.; Loeffler, A.; Davis, M.F.; Guardabassi, L.; Weese, J.S. Recommendations for approaches to meticillin-resistant staphylococcal infections of small animals: Diagnosis, therapeutic considerations and preventative measures: Clinical Consensus Guidelines of the World Association for Veterinary Dermatology. Vet. Dermatol. 2017, 28, 304-e369. [Google Scholar] [CrossRef] [Green Version]
- Sousa, C.A.; Chatfield, J.; File, T.M.; Koch, S.N.; Leu, D.B.; Loeffler, A.; Loft, K.E.; Souza, C.; Weese, J.S. Hostage to history-questioning the duration of systemic antimicrobial therapy for the treatment of canine superficial bacterial folliculitis. J. Am. Vet. Med. Assoc. 2022, 1–4. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sofou, E.I.; Aleksandrova, S.; Badulescu, E.; Chatzis, M.; Saridomichelakis, M. Efficacy of Antimicrobial Treatment in Dogs with Atopic Dermatitis: An Observational Study. Vet. Sci. 2022, 9, 385. https://doi.org/10.3390/vetsci9080385
Sofou EI, Aleksandrova S, Badulescu E, Chatzis M, Saridomichelakis M. Efficacy of Antimicrobial Treatment in Dogs with Atopic Dermatitis: An Observational Study. Veterinary Sciences. 2022; 9(8):385. https://doi.org/10.3390/vetsci9080385
Chicago/Turabian StyleSofou, Evi I., Svetlina Aleksandrova, Elisa Badulescu, Manolis Chatzis, and Manolis Saridomichelakis. 2022. "Efficacy of Antimicrobial Treatment in Dogs with Atopic Dermatitis: An Observational Study" Veterinary Sciences 9, no. 8: 385. https://doi.org/10.3390/vetsci9080385
APA StyleSofou, E. I., Aleksandrova, S., Badulescu, E., Chatzis, M., & Saridomichelakis, M. (2022). Efficacy of Antimicrobial Treatment in Dogs with Atopic Dermatitis: An Observational Study. Veterinary Sciences, 9(8), 385. https://doi.org/10.3390/vetsci9080385





