Therapeutic Effect and Immune Changes after Treatment of Hymenolepis nana-Infected BALB/c Mice with Compounds Isolated from Leucaena leucocephala
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Extraction and Isolation of L. leucocephala
2.3. Experimental Procedures for L. leucocephala Components
2.4. Materials, Kits, and Chemicals
2.5. Ethics Statement
2.6. Preparation of Adult H. nana Worms
2.7. Mortality and Motility Assessment of H. nana by Compounds Isolated from L. leucocephala
2.8. Morphological Observation of the Segments of H. nana
2.9. In Vivo Study and Infected Evaluation in H. nana-Infected Mice
2.10. Primary Culture of Spleen Cells, Cultivation, and Cytokine Assay
2.11. Statistical Analysis of Data
3. Results
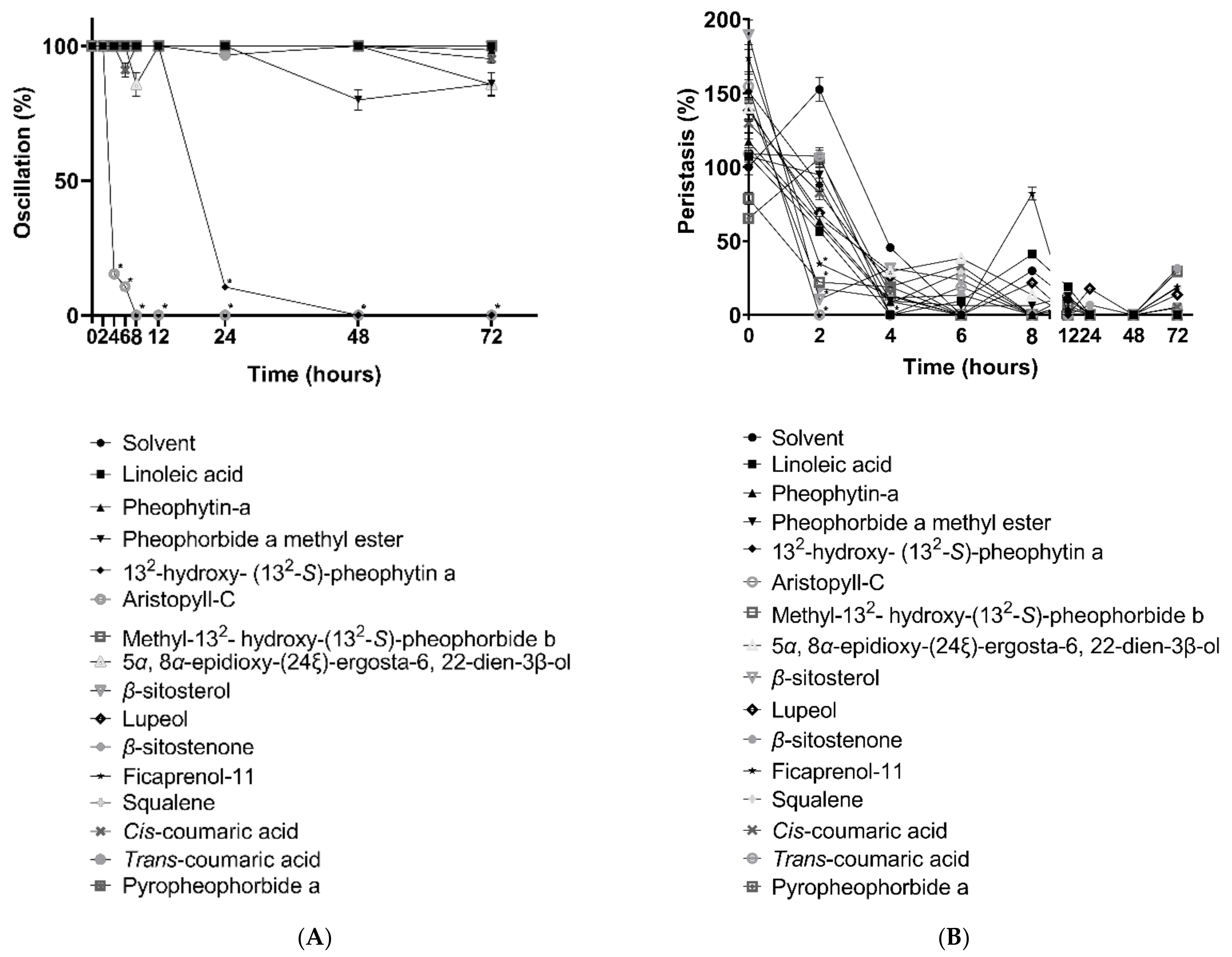
3.1. In Vitro Mobility of L. leucocephala-Purified Compound
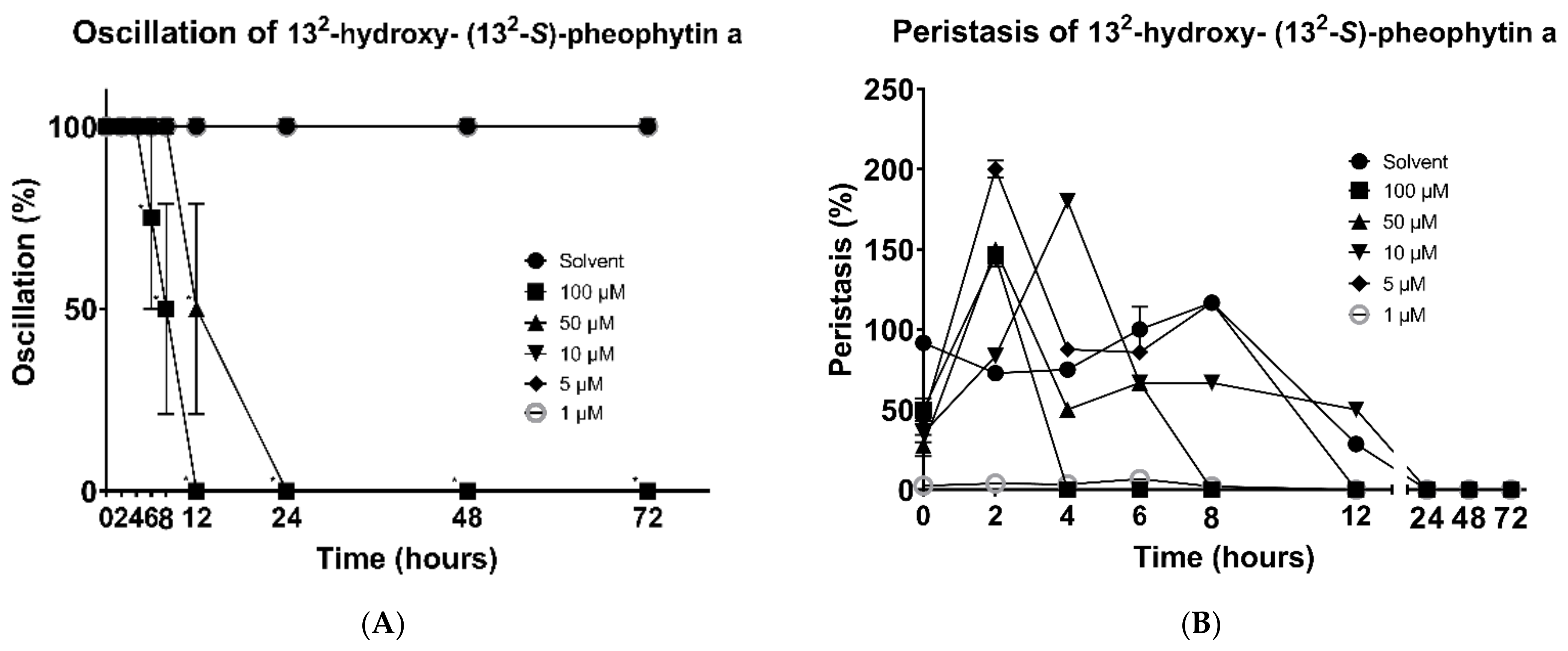
3.2. 132-Hydroxy-(132-S)-Pheophytin a and Aristophyll-C Induced Change of Death Rate and Mobility of Oscillation and Peristalsis Activities
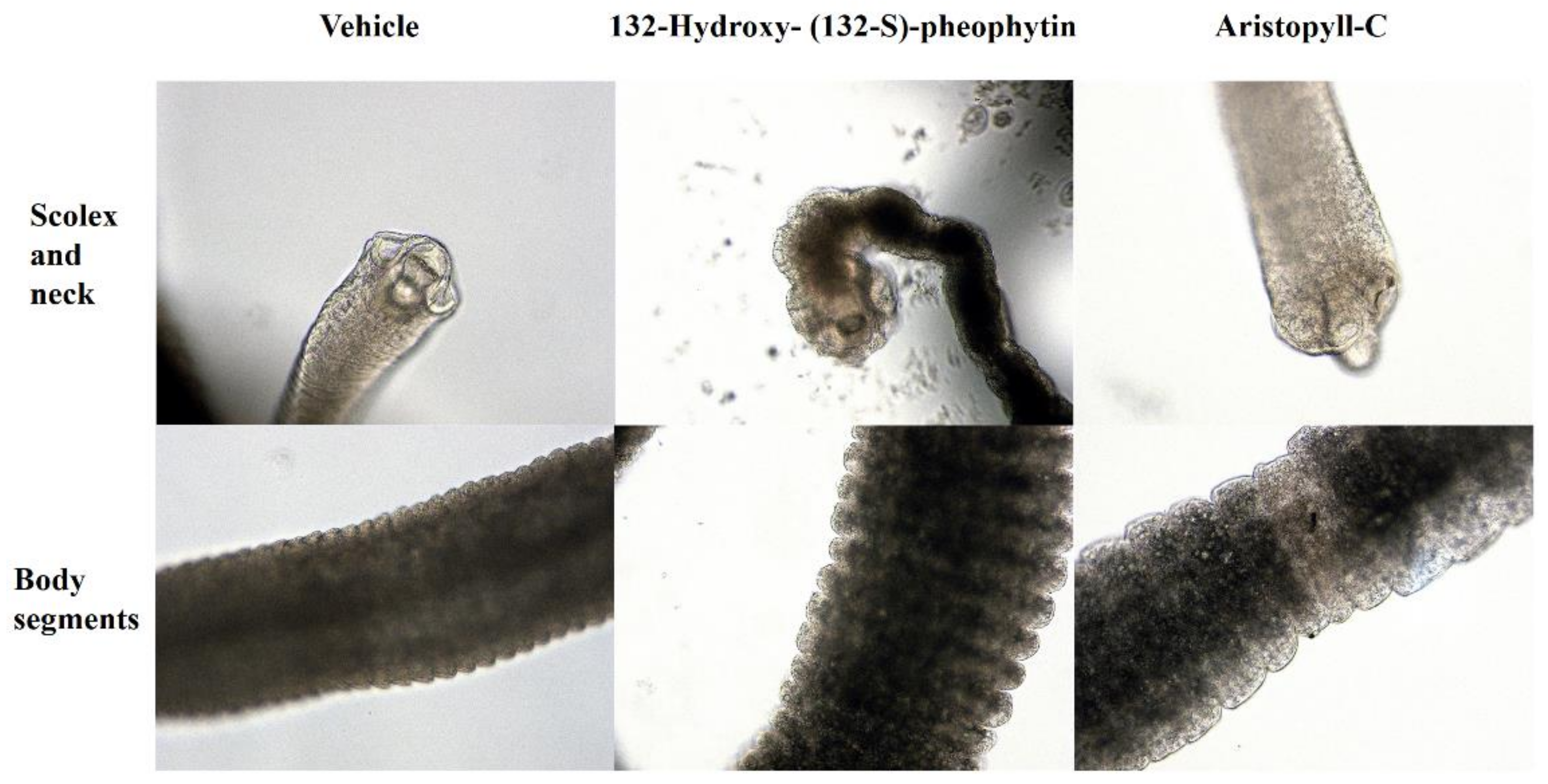
3.3. Morphology
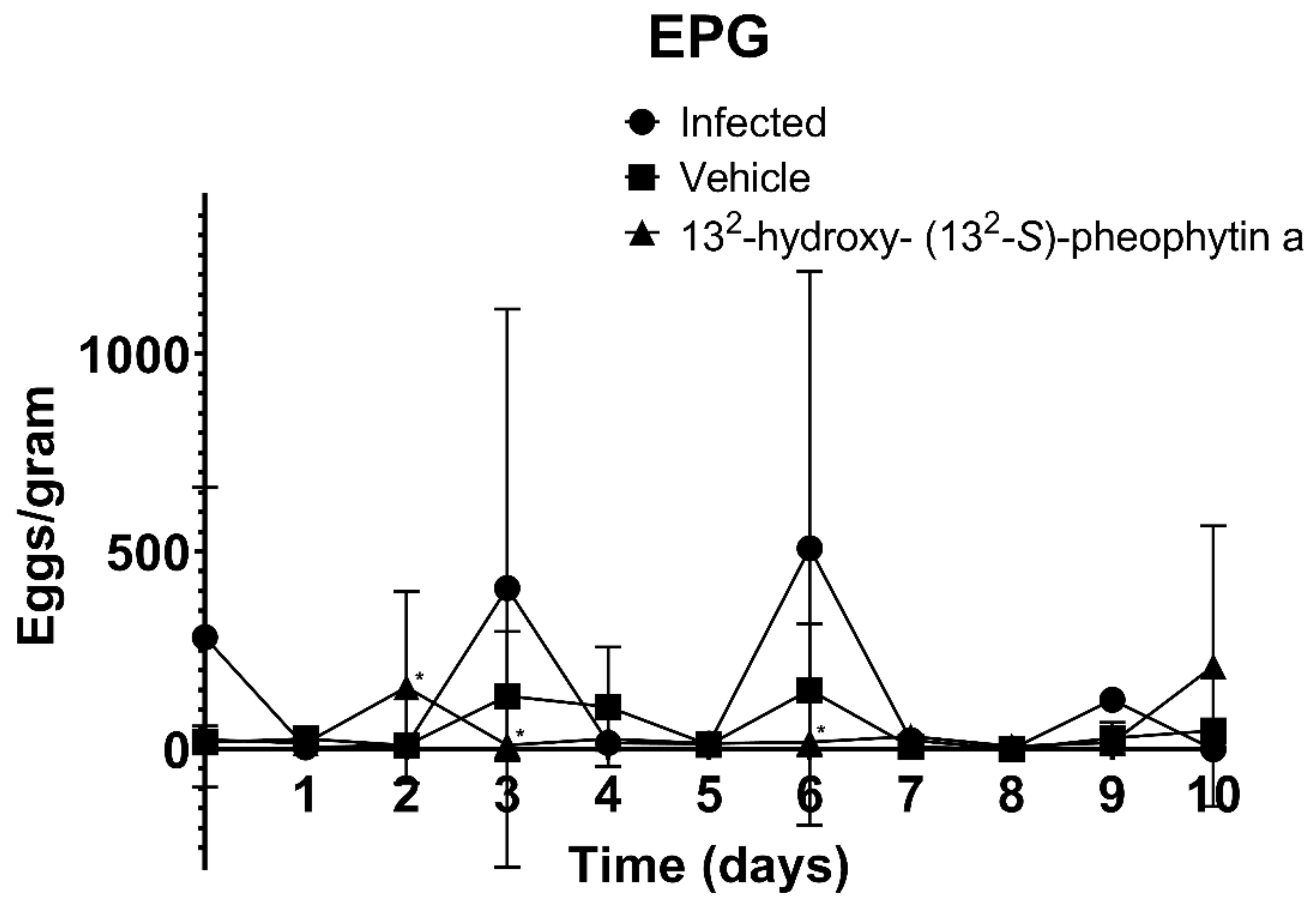
3.4. Mouse Spleen Weight, Body Weight, and EPG
3.5. In Vivo Cestocidal Activity of 132-Hydroxy-(132-S)-Pheophytin a
3.6. Cultivation of Spleen Cells for Cytokine Assay
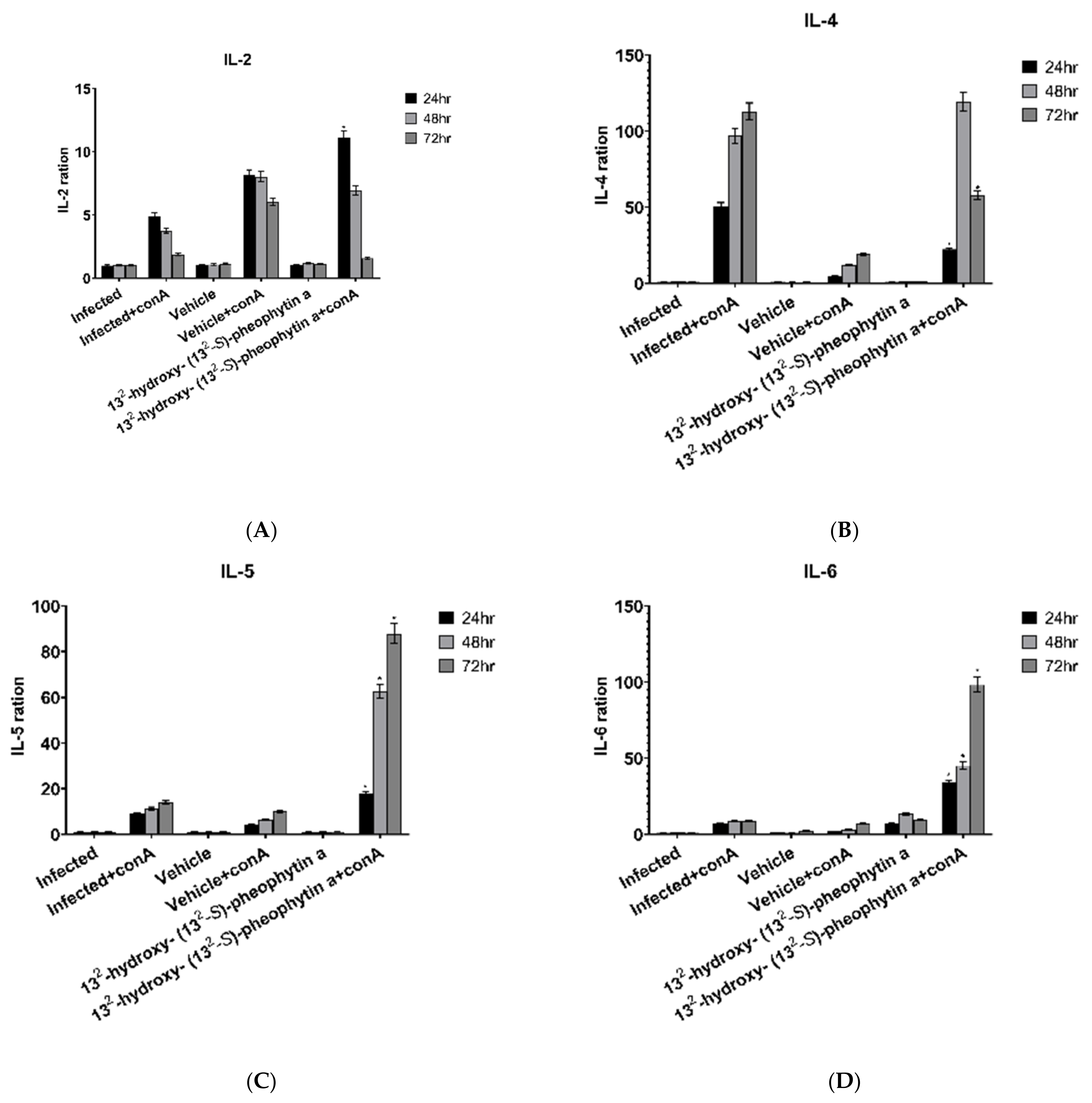
3.6.1. IL-2
3.6.2. IL-4
3.6.3. IL-5
3.6.4. IL-6
3.6.5. IL-10
3.6.6. IL-13,17
3.6.7. IFN-γ
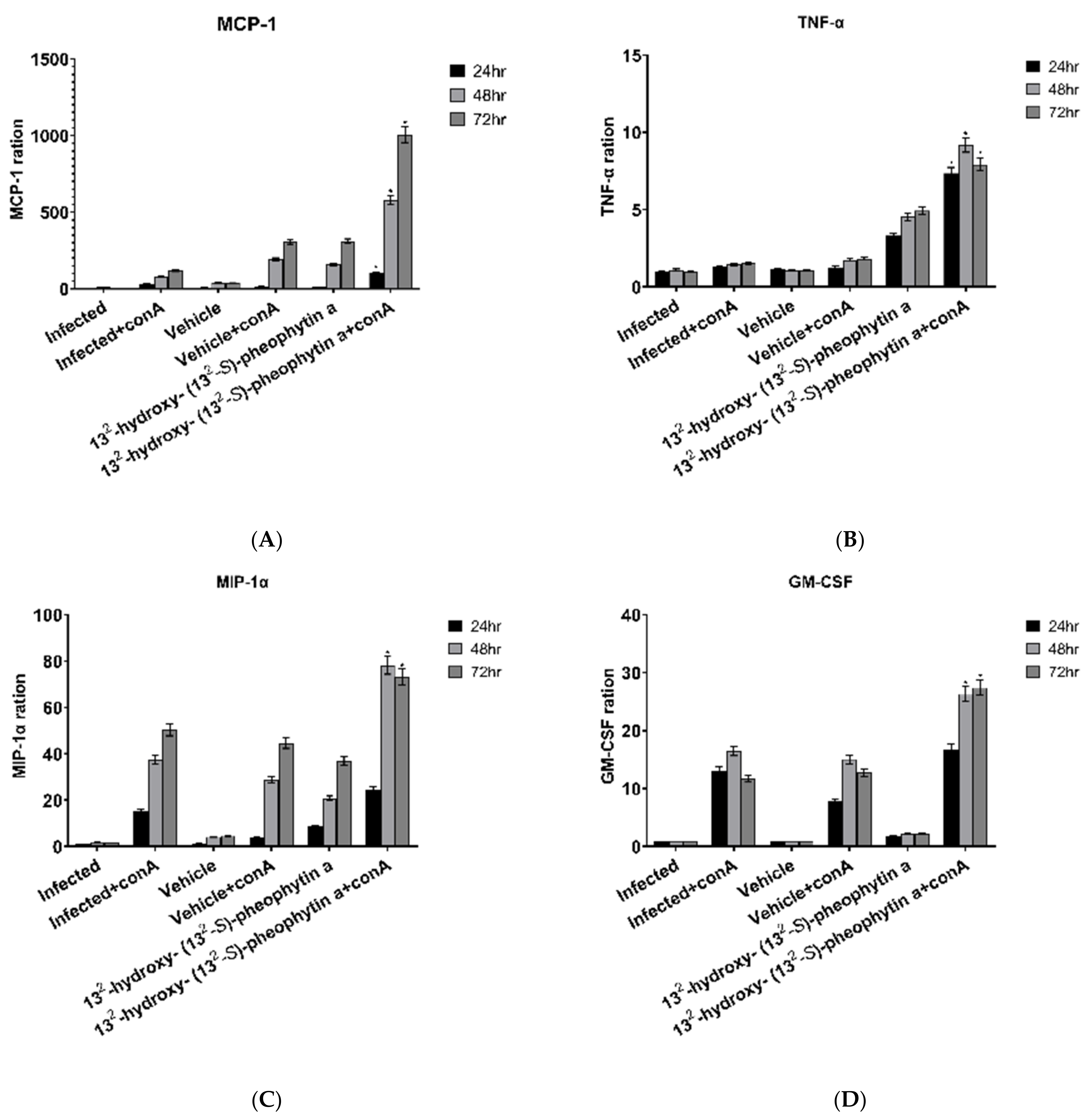
3.6.8. MCP-1
3.6.9. TNF-α
3.6.10. MIP-1α and GM-CSF
3.6.11. Others
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Strand, T.M.; Lundkvist, Å. Rat-borne diseases at the horizon. A systematic review on infectious agents carried by rats in Europe 1995–2016. Infect. Ecol. Epidemiol. 2019, 9, 1553461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crompton, D.W. How Much Human Helminthiasis Is There in the World? J. Parasitol. 1999, 85, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Craig, P.; Ito, A. Intestinal cestodes. Curr. Opin. Infect. Dis. 2007, 20, 524–532. [Google Scholar] [CrossRef] [PubMed]
- Macnish, M.G.; Morgan-Ryan, U.M.; Monis, P.T.; Behnke, J.M.; Thompson, R.C.A. A molecular phylogeny of and mitochondrial sequences in Hymenolepis nana (Cestoda) supports the existance of a cryptic species. Parasitology 2002, 125, 567–575. [Google Scholar] [CrossRef]
- Al-Mekhlaf, H.M. The neglected cestode infection: Epidemiology of Hymenolepis nana infection among children in rural Yemen. Helminthologia 2020, 57, 293–305. [Google Scholar] [CrossRef]
- Cabeza, M.I.; Cabezas, M.T.; Cobo, F.; Salas, J.; Vázquez, J. Hymenolepis nana infection: Associated factors with this parasitism in a health area of Southern Spain. Rev. Chil. Infectol. 2015, 32, 593–595. [Google Scholar] [CrossRef] [Green Version]
- Kusel, J.; Hagan, P. Praziquantel—Its use, cost and possible development of resistance. Parasitol. Today 1999, 15, 352–354. [Google Scholar] [CrossRef]
- Meyer, T.; Sekljic, H.; Fuchs, S.; Bothe, H.; Schollmeyer, D.; Miculka, C. Taste, a new incentive to switch to (R)-praziquantel in schistosomiasis treatment. PLoS Negl. Trop. Dis. 2009, 3, e357. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.H.; Qian, M.X.; Wang, X.G.; Jia, J.; Wang, Q.N.; Jiang, Y.F.; Wang, R.Q.; Yan, S.H.; Chen, B.Y.; Li, J.S.; et al. Comparative efficacy of praziquantel and its optic isomers in experimental therapy of schistosomiasis japonica in rabbits. Chin. Med. J. 1986, 99, 935–940. [Google Scholar]
- Xiao, S.H.; Catto, B.A. Comparative in vitro and in vivo activity of racemic praziquantel and its levorotated isomer on Schistosoma mansoni. J. Infect. Dis. 1989, 159, 589–592. [Google Scholar]
- Mwangi, I.N.; Sanchez, M.C.; Mkoji, G.M.; Agola, L.E.; Runo, S.M.; Cupit, P.M.; Cunningham, C. Praziquantel sensitivity of Kenyan Schistosoma mansoni isolates and the generation of a laboratory strain with reduced susceptibility to the drug. Int. J. Parasitol. Drugs Drug Resist. 2014, 4, 296–300. [Google Scholar] [CrossRef] [Green Version]
- Woo, E.; Yu, Y.L.; Huang, C.Y. Cerebral infarction precipitated by praziquantel in neurocysticercosis: A cautionary note. Trop. Geogr. Med. 1988, 40, 143–146. [Google Scholar]
- Esatgil, M.U.; Gülanber, A.; Aydın, H. Efficacy of praziquantel (injection formula) in the treatment of Hymenolepis diminuta infection in laboratory rats by oral application. Trop. Med. Health 2009, 37, 13–16. [Google Scholar] [CrossRef] [Green Version]
- Smith, I.K.; Fowden, L. A Study of Mimosine Toxicity in Plants. J. Exp. Bot. 1966, 17, 750–761. [Google Scholar] [CrossRef]
- Widaad, A.; Zulkipli, I.N.; Petalcorin, M.I.R. Anthelmintic Effect of Leucaena leucocephala Extract and Its Active Compound, Mimosine, on Vital Behavioral Activities in Caenorhabditis elegans. Molecules 2022, 27, 1875. [Google Scholar] [CrossRef]
- Li, X.-J.; Deng, J.-G.; Qin, Z.-L.; Huang, H.-B. Experimental study on antidiabetic effect of the total flavonoids in Leucaena seeds. Zhongguo Zhong Yao Za Zhi 2005, 30, 842–844. [Google Scholar]
- Song, X.-Y.; Wang, H.; Ren, F.; Wang, K.; Dou, G.; Lv, X.; Yan, D.-H.; Strobel, G. An Endophytic Diaporthe apiculatum Produces Monoterpenes with Inhibitory Activity against Phytopathogenic Fungi. Antibiotics 2019, 8, 231. [Google Scholar] [CrossRef] [Green Version]
- Soares, A.M.; de Araújo, S.A.; Lopes, S.G.; Costa Junior, L.M. Anthelmintic activity of Leucaena leucocephala protein extracts on Haemonchus contortus. Rev. Bras. Parasitol. Vet. 2015, 24, 396–401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernex, E.V.S.-D.; Alonso-Díaz, M.; Gives, P.M.-D.; la Mora, B.V.-D.; González-Cortazar, M.; Zamilpa, A.; Gallegos, E.C. Elucidation of Leucaena leucocephala anthelmintic-like phytochemicals and the ultrastructural damage generated to eggs of Cooperia spp. Vet. Parasitol. 2015, 214, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Jamous, R.M.; Ali-Shtayeh, M.S.; Abu-Zaitoun, S.Y.; Markovics, A.; Azaizeh, H. Effects of selected Palestinian plants on the in vitro exsheathment of the third stage larvae of gastrointestinal nematodes. BMC Vet. Res. 2017, 13, 308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castañeda-Ramírez, G.S.; Torres-Acosta, J.F.J.; Sandoval-Castro, C.A.; González-Pech, P.G.; Parra-Tabla, V.P.; Mathieu, C. Is there a negative association between the content of condensed tannins, total phenols, and total tannins of tropical plant extracts and in vitro anthelmintic activity against Haemonchus contortus eggs? Parasitol. Res. 2017, 116, 3341–3348. [Google Scholar] [CrossRef]
- Castañeda-Ramírez, G.S.; Rodríguez-Labastida, M.; Ortiz-Ocampo, G.I.; González-Pech, P.G.; Ventura-Cordero, J.; Borges-Argáez, R.; Torres-Acosta, J.F.J.; Sandoval-Castro, C.A.; Mathieu, C. An in vitro approach to evaluate the nutraceutical value of plant foliage against Haemonchus contortus. Parasitol. Res. 2018, 117, 3979–3991. [Google Scholar] [CrossRef] [Green Version]
- Piñeiro-Vázquez, A.T.; Canul-Solis, J.R.; Jiménez-Ferrer, G.O.; Alayón-Gamboa, J.A.; Chay-Canul, A.J.; Ayala-Burgos, A.J.; Aguilar-Pérez, C.F.; Ku-Vera, J.C. Effect of condensed tannins from Leucaena leucocephala on rumen fermentation, methane production and population of rumen protozoa in heifers fed low-quality forage. Asian-Australas. J. Anim. Sci. 2018, 31, 1738–1746. [Google Scholar] [CrossRef] [Green Version]
- Wanderley, L.F.; Batista, K.L.R.; De Carvalho, J.F.; Lima, A.D.S.; Landulfo, G.A.; Soares, A.M.D.S.; Costa-Junior, L. The first assessment of the stress inducible defense of Leucaena leucocephala with acaricidal potential effect against Rhipicephalus (Boophilus) microplus (Acari: Ixodidae). Rev. Bras. Parasitol. Vet. 2017, 26, 171–176. [Google Scholar] [CrossRef]
- Chen, C.Y.; Wang, Y.D. Secondary metabolites from Leucaena leucocephala. Chem. Nat. Compd. 2011, 47, 145–146, (SCI). [Google Scholar] [CrossRef]
- She, L.C.; Liu, C.M.; Chen, C.T.; Li, H.T.; Li, W.J.; Chen, C.Y. The anti-cancer and anti-metastasis effects of phytochemical constituents from Leucaena leucocephala. Biomed. Res. 2017, 28, 2893–2897, (SCI). [Google Scholar]
- Lin, R.-J.; Chen, C.-Y.; Lu, C.-M.; Ma, Y.-H.; Chung, L.-Y.; Wang, J.-J.; Lee, J.-D.; Yen, C.-M. Anthelmintic constituents from ginger (Zingiber officinale) against Hymenolepis nana. Acta Trop. 2014, 140, 50–60. [Google Scholar] [CrossRef]
- Willis, H.H. A Simple Levitation Method for the Detection of Hookworm Ova. Med. J. Aust. 1921, 2, 375–376. [Google Scholar] [CrossRef]
- Lin, C.Y.; Lee, C.H.; Chang, Y.W.; Wang, H.M.; Chen, C.Y.; Chen, Y.H. Pheophytin a inhibits inflammation via suppression of LPS-induced nitric oxide synthase-2, prostaglandin E2, and interleukin-1beta of macrophages. Int. J. Mol. Sci. 2014, 15, 22819–22834. [Google Scholar] [CrossRef] [Green Version]
- Conchedda, M.; Bortoletti, G.; Gabriele, F.; Wakelin, D.; Palmas, C. Immune response to the cestode Hymenolepis nana: Cytokine production during infection with eggs or cysts. Int. J. Parasitol. 1997, 27, 321–327. [Google Scholar] [CrossRef]
- Ajami, A.; Rafiei, A. Cytokine production in Hymenolepis nana infection. Iran J. Immunol. 2007, 4, 236–240. [Google Scholar]
- Marillier, R.G.; Michels, C.; Smith, E.M.; Fick, L.C.; Leeto, M.; Dewals, B.; Horsnell, W.G.; Brombacher, F. IL-4/IL-13 independent goblet cell hyperplasia in experimental helminth infections. BMC Immunol. 2008, 9, 11. [Google Scholar] [CrossRef] [Green Version]
- Turner, J.E.; Stockinger, B.; Helmby, H. IL-22 Mediates Goblet Cell Hyperplasia and Worm Expulsion in Intestinal Helminth Infection. PLoS Pathog. 2013, 9, e1003698. [Google Scholar] [CrossRef]




| Compounds | Parts |
|---|---|
| Linoleic acid (1) | Seeds, mature pods, immature pods |
| Pheophytin-a (2) | Leaves |
| Pheophorbide a methyl ester (3) | Leaves, immature pods |
| 132-hydroxy- (132-S)-pheophytin a (4) | Leaves |
| Aristophyll-C (5) | Leaves |
| Methyl-132- hydroxy-(132-S)-pheophorbide b (6) | Leaves |
| 5α, 8α-epidioxy-(24ξ)-ergosta-6, 22-dien-3β-ol (7) | Mature pods |
| β-sitosterol (8) | Seeds |
| Lupeol (9) | Mature pods |
| β-sitostenone (10) | Mature pods |
| Ficaprenol-11 (11) | Immature pods |
| Squalene (12) | Immature pods |
| Cis-coumaric acid (13) | Immature pods |
| Trans-coumaric acid (14) | Immature pods |
| Pyropheophorbide a (15) | Immature pods |
| 1, 3-Dipalmitoyl-2-oleoylglycerol (16) | Mature pods, immature pods |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Y.-H.; Chen, C.-Y.; Chung, L.-Y.; Yen, C.-M.; Juan, Y.-S.; Lin, R.-J. Therapeutic Effect and Immune Changes after Treatment of Hymenolepis nana-Infected BALB/c Mice with Compounds Isolated from Leucaena leucocephala. Vet. Sci. 2022, 9, 368. https://doi.org/10.3390/vetsci9070368
Ma Y-H, Chen C-Y, Chung L-Y, Yen C-M, Juan Y-S, Lin R-J. Therapeutic Effect and Immune Changes after Treatment of Hymenolepis nana-Infected BALB/c Mice with Compounds Isolated from Leucaena leucocephala. Veterinary Sciences. 2022; 9(7):368. https://doi.org/10.3390/vetsci9070368
Chicago/Turabian StyleMa, Yi-Hsuan, Chung-Yi Chen, Li-Yu Chung, Chuan-Min Yen, Yung-Shun Juan, and Rong-Jyh Lin. 2022. "Therapeutic Effect and Immune Changes after Treatment of Hymenolepis nana-Infected BALB/c Mice with Compounds Isolated from Leucaena leucocephala" Veterinary Sciences 9, no. 7: 368. https://doi.org/10.3390/vetsci9070368
APA StyleMa, Y.-H., Chen, C.-Y., Chung, L.-Y., Yen, C.-M., Juan, Y.-S., & Lin, R.-J. (2022). Therapeutic Effect and Immune Changes after Treatment of Hymenolepis nana-Infected BALB/c Mice with Compounds Isolated from Leucaena leucocephala. Veterinary Sciences, 9(7), 368. https://doi.org/10.3390/vetsci9070368







