Chitosan/Calcium-Coated Ginsenoside Rb1 Phosphate Flower-like Microparticles as an Adjuvant to Enhance Immune Responses
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Preparation of CS/CaP Flower-like Microparticles and GRb1/IL-4@CS/CaP Microparticles
2.3. Measurement of Particle Size of GRb1/IL-4@CS/CaP Microparticles
2.4. Animal Immunization
2.5. Viability of DCs
2.6. Investigation of DC Activation and Maturation, Cytokine Secretion in Bone Marrow Cells
2.7. ELISA Analysis of Antibodies
2.8. Determination of Cytokine Levels
2.9. Statistical Analysis
3. Results
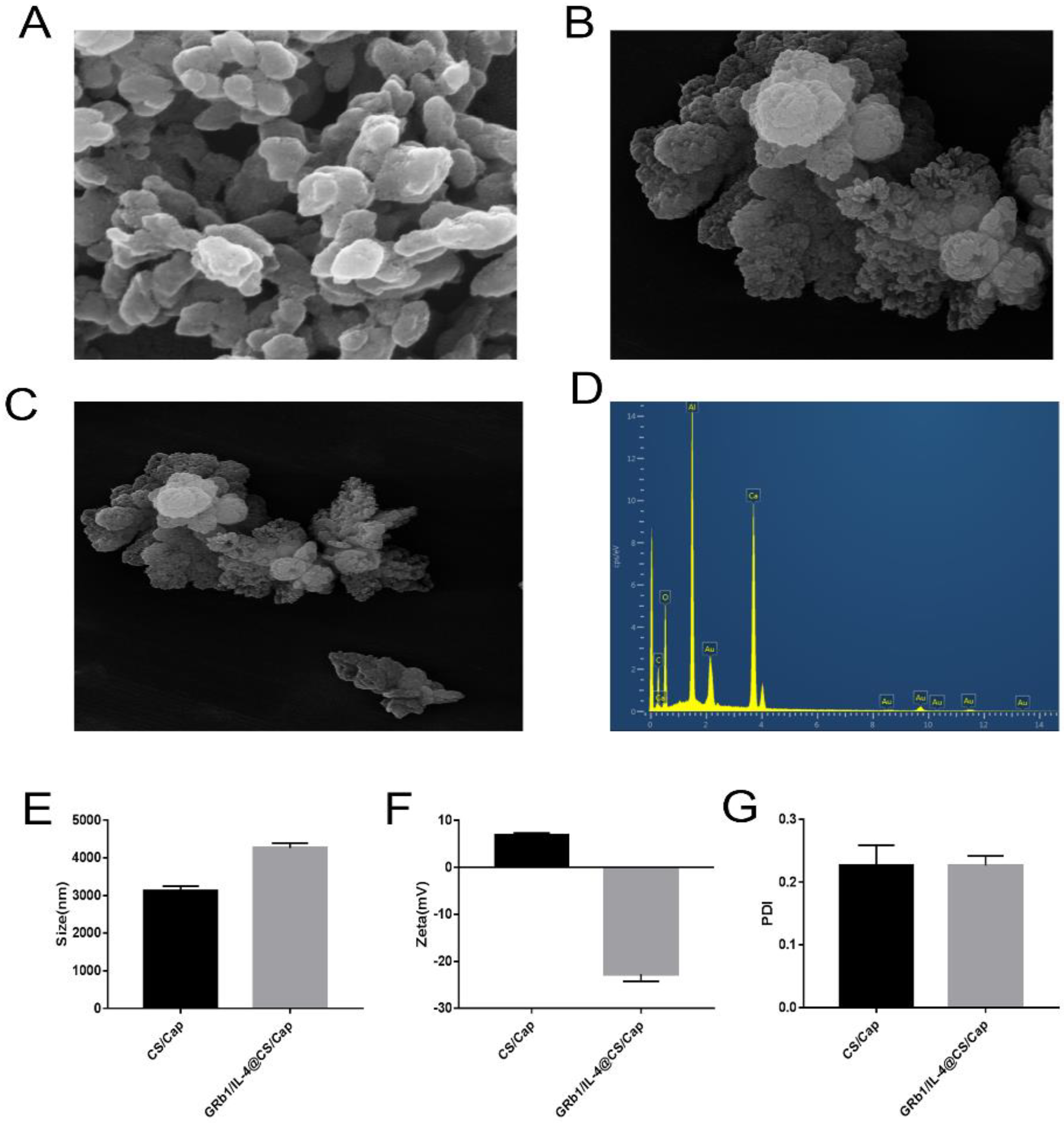
3.1. Characterization of CS/CaP and GRb1/IL-4@CS/CaP Microparticles
3.2. Encapsulation Efficiency of CS/CaP and GRb1/IL-4@CS/CaP Microparticles
3.3. Stability of CS/CaP and GRb1/IL-4@CS/CaP
3.4. Viability of DCs
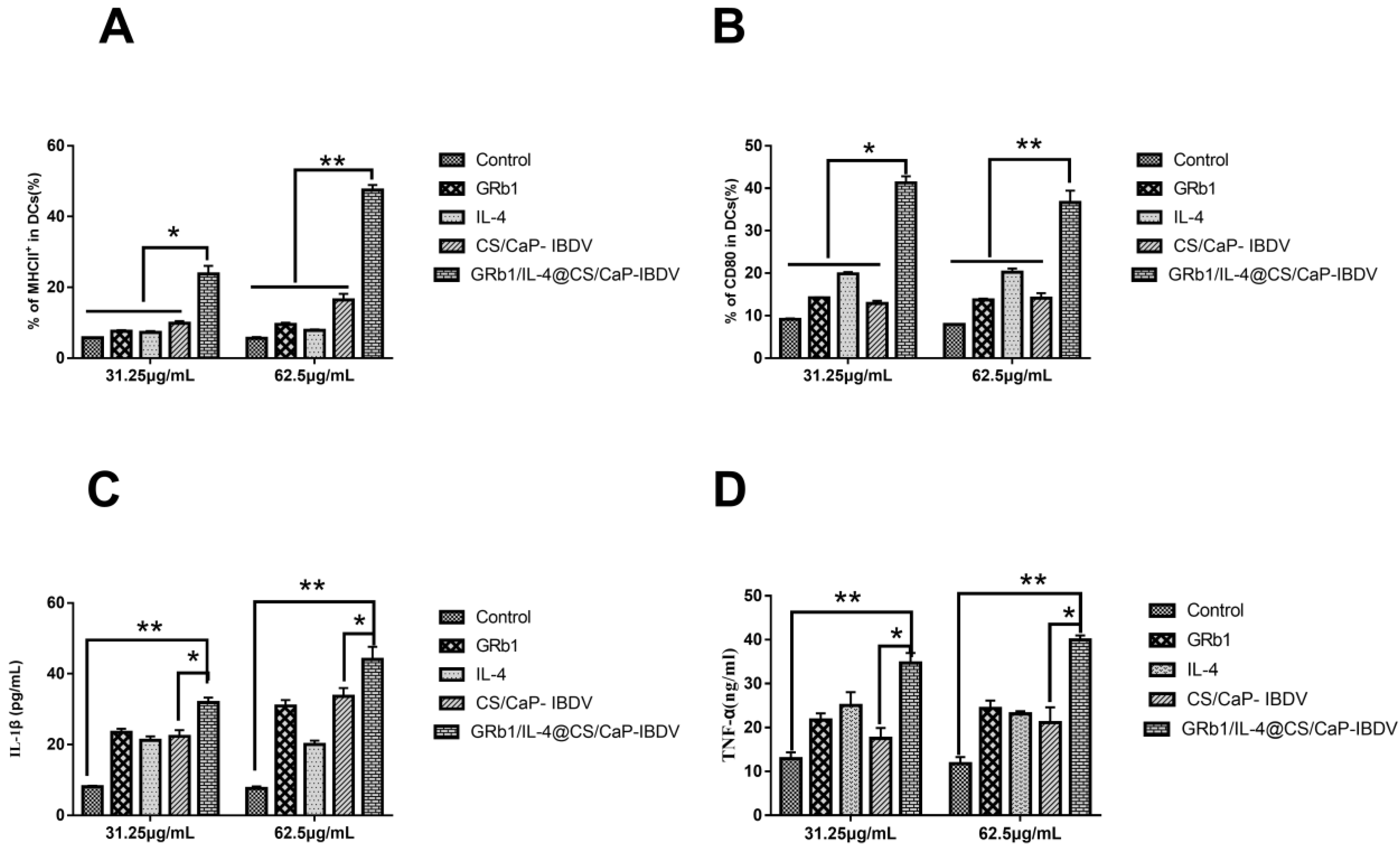
3.5. Surface Molecular Expression Levels and Cytokine Secretion in DCs
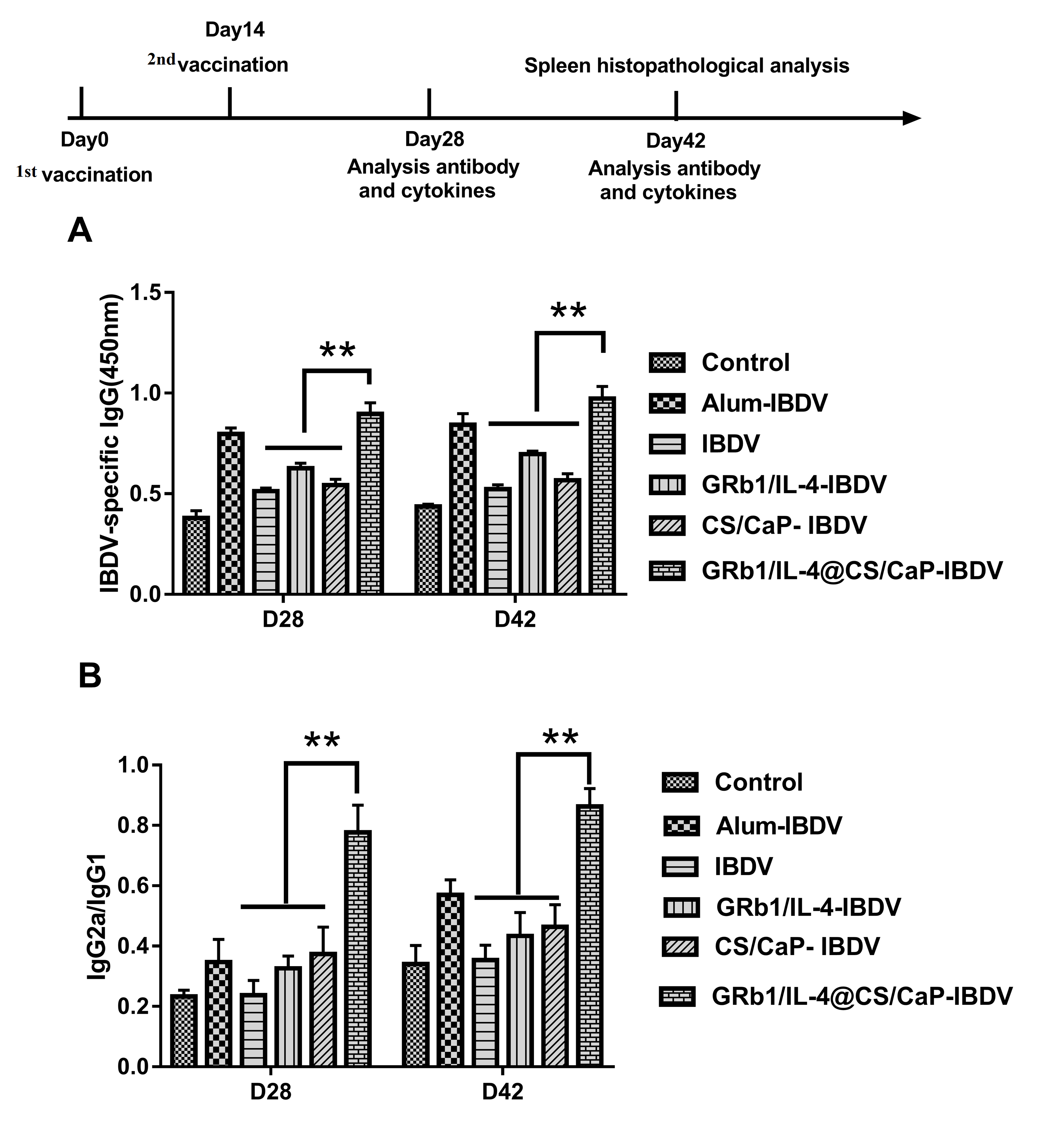
3.6. IBDV-Specific Antibody Response in Vaccinated Chicken
3.7. Cytokine Levels in Chicken Serum
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mahgoub, H.A.; Bailey, M.; Kaiser, P. An overview of infectious bursal disease. Arch. Virol. 2012, 157, 2047–2057. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Lecompte, J.C.; Niño-Fong, R.; Lopez, A.; Frederick Markham, R.J.; Kibenge, F.S. Infectious bursal disease virus (IBDV) induces apoptosis in chicken B cells. Comp. Immunol. Microbiol. Infect. Dis. 2005, 28, 321–337. [Google Scholar] [CrossRef] [PubMed]
- Ray, S.M.; Ashash, U.; Muthukumar, S. A field study on the evaluation of day-of-hatch and in grow-out application of live infectious bursal disease virus vaccine in broiler chickens. Poult. Sci. 2021, 100, 101252. [Google Scholar] [CrossRef] [PubMed]
- Lucero, M.S.; Richetta, M.; Chimeno Zoth, S.; Jaton, J.; Pinto, S.; Canet, Z.; Berinstein, A.; Gómez, E. Plant-based vaccine candidate against Infectious bursal disease: An alternative to inactivated vaccines for breeder hens. Vaccine 2019, 37, 5203–5210. [Google Scholar] [CrossRef] [PubMed]
- Rage, E.; Marusic, C.; Lico, C.; Baschieri, S.; Donini, M. Current state-of-the-art in the use of plants for the production of recombinant vaccines against infectious bursal disease virus. Appl. Microbiol. Biotechnol. 2020, 104, 2287–2296. [Google Scholar] [CrossRef]
- Firdaus, F.Z.; Skwarczynski, M.; Toth, I. Developments in Vaccine Adjuvants. Methods Mol. Biol. 2022, 2412, 145–178. [Google Scholar]
- Shende, P.; Waghchaure, M. Combined vaccines for prophylaxis of infectious conditions. Artif. Cells Nanomed. Biotechnol. 2019, 47, 696–705. [Google Scholar] [CrossRef] [Green Version]
- Alving, C.R.; Matyas, G.R.; Torres, O.; Jalah, R.; Beck, Z. Adjuvants for vaccines to drugs of abuse and addiction. Vaccine 2014, 32, 5382–5389. [Google Scholar] [CrossRef] [Green Version]
- Pei, M.; Liang, J.; Zhang, C.; Wang, X.; Zhang, C.; Ma, G.; Sun, H. Chitosan/calcium phosphates nanosheet as a vaccine carrier for effective cross-presentation of exogenous antigens. Carbohydr. Polym. 2019, 224, 115172. [Google Scholar] [CrossRef]
- Mousavi, S.; Ghotaslou, R.; Kordi, S.; Khoramdel, A.; Aeenfar, A.; Kahjough, S.; Akbarzadeh, A. Antibacterial and antifungal effects of chitosan nanoparticles on tissue conditioners of complete dentures. Int. J. Biol. Macromol. 2018, 18, 881–885. [Google Scholar] [CrossRef]
- Hamedi, H.; Moradi, S.; Hudson, S.; Tonelli, A. Chitosan based hydrogels and their applications for drug delivery in wound dressings: A review. Carbohydr. Polym. 2018, 199, 445–460. [Google Scholar] [CrossRef] [PubMed]
- Katsarov, P.; Pilicheva, B.; Uzunova, Y.; Gergov, G.; Kassarova, M. Chemical cross-linking: A feasible approach to prolong doxylamine/pyridoxine release from spray-dried chitosan microspheres. Eur. J. Pharm. Sci. 2018, 123, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; He, B.; Mi, P. Calcium phosphate nanocarriers for drug delivery to tumors: Imaging, therapy and theranostics. Biomater. Sci. 2019, 7, 3942–3960. [Google Scholar] [CrossRef] [PubMed]
- Mulens-Arias, V.; Rojas, J.; Pérez-Yagüe, S.; Morales, M.; Barber, D.J.B. Polyethylenimine-coated SPIONs trigger macrophage activation through TLR-4 signaling and ROS production and modulate podosome dynamics. Biomaterials 2015, 52, 494–506. [Google Scholar] [CrossRef] [PubMed]
- Choi, R.J.; Mohamad Zobir, S.Z.; Alexander-Dann, B.; Sharma, N.; Ma, M.K.L.; Lam, B.Y.H.; Yeo, G.S.H.; Zhang, W.; Fan, T. Combination of Ginsenosides Rb2 and Rg3 Promotes Angiogenic Phenotype of Human Endothelial Cells via PI3K/Akt and MAPK/ERK Pathways. Front. Pharmacol. 2021, 12, 618773. [Google Scholar] [CrossRef] [PubMed]
- Lee, S. Anti-Metastatic and Anti-Inflammatory Effects of Matrix Metalloproteinase Inhibition by Ginsenosides. Biomedicines 2021, 9, 198. [Google Scholar] [CrossRef]
- Yu, J.; Shi, F.S.; Hu, S. Improved immune responses to a bivalent vaccine of Newcastle disease and avian influenza in chickens by ginseng stem-leaf saponins. Vet. Immunol. Immunopathol. 2015, 167, 147–155. [Google Scholar] [CrossRef]
- Rivera, E.; Ekholm Pettersson, F.; Inganäs, M.; Paulie, S.; Grönvik, K.O. The Rb1 fraction of ginseng elicits a balanced Th1 and Th2 immune response. Vaccine 2005, 23, 5411–5419. [Google Scholar] [CrossRef]
- Su, X.; Pei, Z.; Hu, S. Ginsenoside Re as an adjuvant to enhance the immune response to the inactivated rabies virus vaccine in mice. Int. Immunopharmacol. 2014, 20, 283–289. [Google Scholar] [CrossRef]
- Song, X.; Chen, J.; Sakwiwatkul, K.; Li, R.; Hu, S. Enhancement of immune responses to influenza vaccine (H3N2) by ginsenoside Re. Int. Immunopharmacol. 2010, 10, 351–356. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Zhang, X.; Xu, L.; Ouyang, D.; Liu, K.; Pan, H.; He, J.; He, X. Ginsenoside Rg1 regulates innate immune responses in macrophages through differentially modulating the NF-κB and PI3K/Akt/mTOR pathways. Int. Immunopharmacol. 2014, 23, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Ho, I.C.; Miaw, S.C. Regulation of IL-4 Expression in Immunity and Diseases. Adv. Exp. Med. Biol. 2016, 941, 31–77. [Google Scholar] [PubMed]
- Wang, X.; Shi, J.; Li, Z.; Zhang, S.; Wu, H.; Jiang, Z.; Yang, C.; Tian, C. Facile one-pot preparation of chitosan/calcium pyrophosphate hybrid microflowers. ACS Appl. Mater. Interfaces 2014, 6, 14522–14532. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Bai, L.; Tan, Y.; Sun, J.; Qu, Q.; Shi, D.; Guo, S.; Liu, C. The immunoregulatory effect of sulfated Echinacea purpurea polysaccharide on chicken bone marrow-derived dendritic cells. Int. J. Biol. Macromol. 2019, 139, 1123–1132. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Chen, J.; Li, E.; Fan, Q.; Wang, D.; Zhang, C.; Li, P.; Li, X.; Chen, X.; Qiu, S.; et al. Solomonseal polysaccharide and sulfated Codonopsis pilosula polysaccharide synergistically resist Newcastle disease virus. PLoS ONE 2015, 10, e0117916. [Google Scholar] [CrossRef] [PubMed]
- Morales-Lange, B.; Ramírez-Cepeda, F.; Schmitt, P.; Guzmán, F.; Lagos, L.; Øverland, M.; Wong-Benito, V.; Imarai, M.; Fuentes, D.; Boltaña, S.; et al. Interferon Gamma Induces the Increase of Cell-Surface Markers (CD80/86, CD83 and MHC-II) in Splenocytes From Atlantic Salmon. Front. Immunol. 2021, 12, 666356. [Google Scholar] [CrossRef]
- Jinkawa, A.; Shimizu, M.; Nishida, K.; Kaneko, S.; Usami, M.; Sakumura, N.; Irabu, H.; Takakuwa, M.; Inoue, N.; Mizuta, M.; et al. Cytokine profile of macrophage activation syndrome associated with Kawasaki disease. Cytokine 2019, 119, 52–56. [Google Scholar] [CrossRef]
- Cabral-Hipólito, N.; Molina-Ramírez, B.S.; Castillo-Maldonado, I.; Meza-Velázquez, R.; García-Garza, R.; Gauna, S.V.; Delgadillo-Guzmán, D.; Hernández-Herrera, A.; Ramírez-Moreno, A.; Cruz, J.H.; et al. Tannic Acid Exhibits Adjuvant Activity by Enhancing Humoral and Cell-Mediated Immunity Against BSA as a Protein Antigen. Protein Pept. Lett. 2022, 29, 166–175. [Google Scholar] [CrossRef]
- Wang, H.; Zheng, Y.; Sun, Q.; Zhang, Z.; Zhao, M.; Peng, C.; Shi, S. Ginsenosides emerging as both bifunctional drugs and nanocarriers for enhanced antitumor therapies. J. Nanobiotechnology 2021, 19, 322. [Google Scholar] [CrossRef]
- Hanagata, N.; Li, X.; Chen, M.H.; Li, J.; Hattori, S. Double-stranded phosphodiester cytosine-guanine oligodeoxy-nucleotide complexed with calcium phosphate as a potent vaccine adjuvant for activating cellular and Th1-type humoral immunities. Int. J. Nanomed. 2017, 13, 43–62. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Zhou, B.; Wang, L.; Zhou, F.; Smith, N.; Saunders, D.; Towner, R.A.; Song, J.; Qu, J.; Chen, W.R. Biodegradable pH-responsive amorphous calcium carbonate nanoparticles as immunoadjuvants for multimodal imaging and enhanced photoimmunotherapy. J. Mater. Chem. B 2020, 8, 8261–8270. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Liu, Y.; Wang, L.; Liu, Y.; Zhang, W.; Fan, B.; Ma, X.; Yuan, Q.; Ma, G.; Su, Z. Enhanced humoral and cell-mediated immune responses generated by cationic polymer-coated PLA microspheres with adsorbed HBsAg. Mol. Pharm. 2014, 11, 1772–1784. [Google Scholar] [CrossRef] [PubMed]
- Mu, L.; Yin, X.; Wu, H.; Lei, Y.; Han, K.; Mo, J.; Guo, Z.; Li, J.; Ye, J. Mannose-Binding Lectin Possesses Agglutination Activity and Promotes Opsonophagocytosis of Macrophages with Calreticulin Interaction in an Early Vertebrate. J. Immunol. 2020, 205, 3443–3455. [Google Scholar] [CrossRef] [PubMed]
- Cortés, A.; Muñoz-Antoli, C.; Esteban, J.G.; Toledo, R. Th2 and Th1 Responses: Clear and Hidden Sides of Immunity against Intestinal Helminths. Trends Parasitol. 2017, 33, 678–693. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, X.; Li, H.; Zhang, L.; Zhang, X.; Zhao, L.; Zhang, G.; Cao, S.; Liu, Y. Chitosan/Calcium-Coated Ginsenoside Rb1 Phosphate Flower-like Microparticles as an Adjuvant to Enhance Immune Responses. Vet. Sci. 2022, 9, 355. https://doi.org/10.3390/vetsci9070355
Song X, Li H, Zhang L, Zhang X, Zhao L, Zhang G, Cao S, Liu Y. Chitosan/Calcium-Coated Ginsenoside Rb1 Phosphate Flower-like Microparticles as an Adjuvant to Enhance Immune Responses. Veterinary Sciences. 2022; 9(7):355. https://doi.org/10.3390/vetsci9070355
Chicago/Turabian StyleSong, Xinghui, Huijuan Li, Liheng Zhang, Xiaozhan Zhang, Li Zhao, Gaiping Zhang, Shengbo Cao, and Yunchao Liu. 2022. "Chitosan/Calcium-Coated Ginsenoside Rb1 Phosphate Flower-like Microparticles as an Adjuvant to Enhance Immune Responses" Veterinary Sciences 9, no. 7: 355. https://doi.org/10.3390/vetsci9070355
APA StyleSong, X., Li, H., Zhang, L., Zhang, X., Zhao, L., Zhang, G., Cao, S., & Liu, Y. (2022). Chitosan/Calcium-Coated Ginsenoside Rb1 Phosphate Flower-like Microparticles as an Adjuvant to Enhance Immune Responses. Veterinary Sciences, 9(7), 355. https://doi.org/10.3390/vetsci9070355





