Immunohistochemical Expression of Platelet-Derived Growth Factor Receptor β (PDGFR-β) in Canine Cutaneous Peripheral Nerve Sheath Tumors: A Preliminary Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples and Histopathology
2.2. Immunohistochemistry (IHC)
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bostock, D.E.; Dye, M.T. Prognosis after surgical excision of canine fibrous connective tissue sarcomas. Vet. Pathol. 1980, 17, 581–588. [Google Scholar] [CrossRef]
- Dernell, W.S.; Withrow, S.J.; Kuntz, C.A.; Powers, B.E. Principles of treatment for soft tissue sarcomas. Clin. Tech. Small Anim. Pract. 1998, 13, 59–64. [Google Scholar] [CrossRef]
- Ehrhart, N. Soft-tissue sarcomas in dogs: A review. J. Am. Anim. Hosp. Assoc. 2005, 41, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Mayer, M.N.; Larue, S.M. Soft tissue sarcomas in dogs. Can. Vet. J. 2005, 46, 1048–1052. [Google Scholar] [PubMed]
- Liptak, J.M.; Christensen, N.I. Soft Tissue Sarcomas. In Withrow & MacEwen’s Small Animal Clinical Oncology, 6th ed.; Vail, D.M., Thamm, D.H., Liptak, J.M., Eds.; Elsevier Saunders: St. Louis, MO, USA, 2019; Chapter 22; pp. 404–431. [Google Scholar]
- Suzuki, S.; Uchida, K.; Nakayama, H. The effects of tumor location on diagnostic criteria for canine malignant nerve sheath tumors (MPNSTs) and the markers for distinction between canine MPNSTs and canine perivascular wall tumors. Vet. Pathol. 2014, 51, 722–736. [Google Scholar] [CrossRef]
- LeCouteur, R.A.; Withrow, S.J. Tumors of the nervous system. In Small Animal Clinical Oncology, 4th ed.; Withrow, S.J., MacEwen, E.G., Eds.; Elsevier Saunders: St. Louis, MO, USA, 2007; pp. 659–685. [Google Scholar]
- Koestner, A.; Armed Forces Institute of Pathology; WHO Collaborating Center for Worlwide Reference on Comparative Oncology. Histological Classification of Tumors of the Nervous System of Domestic Animals; Armed Forces Institute of Pathology in cooperation with the American Registry of Pathology and the World Health Organization Collaborating Center for Worldwide Reference on Comparative Oncology: Washington, DC, USA, 1999; pp. 37–38. [Google Scholar]
- Gross, T.L.; Ihrke, P.J.; Walder, E.J.; Affolter, V.K. Neural and Perineural Tumors. In Skin Diseases of the Dog and Cat, 2nd ed.; Blackwell Science Ltd.: Oxford, UK, 2005; pp. 786–796. [Google Scholar]
- Silva, E.O.; Goiozo, P.F.I.; Pereira, L.G.; Headley, S.A.; Bracarense, A.F.R.L. Concomitant malignant pulmonary peripheral nerve sheath tumour and benign cutaneous peripheral nerve sheath tumour in a dog. J. Comp. Path. 2017, 157, 46–50. [Google Scholar] [CrossRef]
- Chijiwa, K.; Uchida, K.; Tateyama, S. Immunohistochemical evaluation of canine peripheral nerve sheath tumors and other soft tissue sarcomas. Vet. Pathol. 2004, 41, 307–318. [Google Scholar] [CrossRef]
- Vucicevic, I.; Marinkovic, D.; Kukolj, V.; Nesic, S.; Anicic, M.; Durdevic, B.; Aleksic-Kovacecic, S. Immunohistochemical distinguish between canine peripheral nerve sheath tumors and perivascular wall tumors. Acta Vet. Beograd. 2019, 69, 290–299. [Google Scholar] [CrossRef]
- Alvarez, R.H.; Kantarjian, H.M.; Cortes, J.E. Biology of platelet-derived growth factor and its involvement in disease. Mayo Clin. Proc. 2006, 81, 1241–1257. [Google Scholar] [CrossRef]
- Demoulin, J.B.; Essaghir, A. PDGF receptor signaling networks in normal and cancer cells. Cytokine Growth Factor Rev. 2014, 25, 273–283. [Google Scholar] [CrossRef]
- Liu, K.W.; Hu, B.; Cheng, S.Y. Platelet-derived growth factor receptor alpha in glioma: A bad seed. Chin. J. Cancer 2011, 30, 590–602. [Google Scholar] [CrossRef] [PubMed]
- Seger, R.; Krebs, E.G. The MAPK signaling cascade. FASEB J. 1995, 9, 726–735. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, K.M.; Anderson, N.G. The protein kinase B/Akt signalling pathway in human malignancy. Cell Signal. 2002, 14, 381–395. [Google Scholar] [CrossRef]
- Tokunaga, E.; Oki, E.; Egashira, A.; Sadanaga, N.; Morita, M.; Kakeji, Y.; Maehara, Y. Deregulation of the Akt pathway in human cancer. Curr. Cancer Drug Targets 2008, 8, 27–36. [Google Scholar] [CrossRef]
- Ostman, A.; Heldin, C.H. PDGF receptors as targets in tumor treatment. Adv. Cancer Res. 2007, 97, 247–274. [Google Scholar] [CrossRef]
- Toffalini, F.; Demoulin, J.B. New insights into the mechanisms of hematopoietic cell transformation by activated receptor tyrosine kinases. Blood 2010, 116, 2429–2437. [Google Scholar] [CrossRef]
- Paulsson, J.; Ehnman, M.; Ostman, A. PDGF receptors in tumor biology: Prognostic and predictive potential. Future Oncol. 2014, 10, 1695–1708. [Google Scholar] [CrossRef]
- Peng, Y.; Guo, J.J.; Liu, Y.M.; Wu, X.L. MicroRNA-34A inhibits the growth, invasion and metastasis of gastric cancer by targeting PDGFR and MET expression. Biosci. Rep. 2000, 34, 247–256. [Google Scholar] [CrossRef]
- Aoki, M.; Nabeshima, K.; Koga, K.; Hamasaki, M.; Suzumiya, J.; Tamura, K.; Iwasaki, H. Imatinib mesylate inhibits cell invasion of malignant peripheral nerve sheath tumor induced by platelet-derived growth factor-BB. Lab. Investig. 2007, 87, 767–779. [Google Scholar] [CrossRef]
- Ohishi, J.; Aoki, M.; Nabeshima, K.; Suzumiya, J.; Takeuchi, T.; Ogose, A.; Hazokazi, M.; Yamashita, Y.; Owasaki, H. Imatinib mesylate inhibits cell growth of malignant peripheral nerve sheath tumors in vitro through supression of PDGFR-β. BMC Cancer 2013, 13, 224. [Google Scholar] [CrossRef]
- Maniscalco, L.; Iussich, S.; Morello, E.; Martano, M.; Biolatti, B.; Riondato, F.; Salda, L.D.; Romanucci, M.; Malatesta, D.; Bongiovanni, L.; et al. PDGFs and PDGFRs in canine osteossarcoma: New targets for innovative therapeutic strategies in comparative oncology. Vet. J. 2013, 195, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Gramer, I.; Killick, D.; Scase, T.; Chandry, D.; Marrington, M.; Blackwood, I. Expression of VEGFR and PDGFR-α/-β in 187 canine nasal carcinomas. Vet. Comp. Oncol. 2016, 15, 1041–1050. [Google Scholar] [CrossRef] [PubMed]
- Walters, L.; Martin, O.; Price, J.; Sulla, M.M. Expression of receptor tyrosine kinase targets PDGFR-β, VEGFR-2 and KIT in canine transitional cell carcinoma. Vet. Comp. Oncol. 2018, 16, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.J.; Newman, S.J.; Durtschi, D.C.; LeBlanc, A.K. Expression of PDGFR-β and Kit in canine anal sac apocrine gland adenocarcinoma using tissue immunohistochemistry. Vet. Comp. Oncol. 2011, 10, 74–79. [Google Scholar] [CrossRef]
- Iussich, S.; Maniscalco, L.; Di Sciuva, A.; Iotti, B.; Morello, E.; Martano, M.; Gattino, F.; Buracco, P.; De Maria, R. PDGFRs expression in dogs affected by malignant oral melanomas: Correlation with prognosis. Vet. Comp. Oncol. 2016, 15, 462–469. [Google Scholar] [CrossRef]
- Altamura, G.; Uberti, B.; Galiero, G.; Martano, M.; Pirro, A.; Russo, M.; Borzacchiello, G. Expression and activation of platelet-derived growth factor β receptor, mitogen-activated protein/extracelular signal-regulated kinase kinase (MEK) and extracelular signal-regulated kinase (ERK) in canine mammary tumours. Res. Vet. Sci. 2017, 110, 29–33. [Google Scholar] [CrossRef]
- Avallone, G.; Pellegrino, V.; Roccabianca, P.; Lepri, E.; Crippa, L.; Beha, G.; De Tolla, L.; Sarli, G. Tyrosine Kinase Receptor Expression in Canine Liposarcoma. Vet. Pathol. 2017, 54, 212–217. [Google Scholar] [CrossRef]
- Avallone, G.; Stefanello, D.; Boracchi, P.; Gelain, M.E.; Tresoldi, E.; Roccabianca, P.; Ferrari, R.; Turin, L. Growth Factors and COX2 Expression in Canine Perivascular Wall Tumors. Vet. Pathol. 2015, 52, 1034–1040. [Google Scholar] [CrossRef]
- Asa, S.A.; Murai, A.; Murakami, M.; Hoshino, H.; Mori, T.; Maruo, K.; Khater, A.; El-sawak, A.; el-Aziz, E.A.; Yanai, T.; et al. Expression of platelet-derived growth factor and its receptors in spontaneous canine hemangiosarcoma and cutaneous hemangioma. Histol. Histopathol. 2012, 27, 601–607. [Google Scholar] [CrossRef]
- Koestner, A.; Higgins, R.J.; Meuten, D.J. Primary Tumors of the peripheral nervous system. In Tumors in Domestic Animals, 4th ed.; Iowa State University Press: Ames, IA, USA, 2002; pp. 731–735. [Google Scholar]
- Schulman, F.Y.; Johnson, T.O.; Facemire, P.R.; Fanburg-Smith, J.C. Feline peripheral nerve sheath tumors: Histologic, immunohistochemical and clinicopathological correlation (59 Tumors in 53 cats). Vet. Pathol. 2009, 46, 1166–1180. [Google Scholar] [CrossRef]
- Joshi, R. Learning from eponyms: Jose Verocay and Verocay bodies, Antoni A and B areas, Nils Antoni and Schwannomas. Indian Derm. Online J. 2012, 3, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, M.; Naruko, T.; Ikura, Y.; Komatsu, R.; Iwasa, Y.; Kitabayashi, C.; Inoue, T.; Itoh, A.; Yoshiyama, M.; Ueda, M. A decline in platelet activation and inflammatory cell infiltration is associated with the phenotypic redifferentiation of neointimal smooth muscle cells after bare-metal stent implantation in acute coronary syndrome. J. Atheroscler. Thromb. 2010, 17, 675–687. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Asa, S.A.; Mori, T.; Maruo, K.; Khater, A.; El-sawak, A.; el-Aziz, E.A.; Yanai, T.; Sakai, H. Analysis of genomic mutation and immunohistochemistry of platelet-derived growth factor receptors in canine vascular tumours. Vet. Comp. Oncol. 2013, 13, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, S.; Amorim, I.; Rema, A.; Faria, F.; Gartner, F. Molecular heterogeneity of canine cutaneous peripheral nerve sheath tumors: A drawback in the diagnosis refinement. In Vivo 2016, 30, 819–827. [Google Scholar] [CrossRef][Green Version]
- Watanabe, T.; Oda, Y.; Tamiya, S.; Kinukawa, N.; Masuda, K.; Tsuneyoshi, M. Malignant peripheral nerve sheath tumours: High Ki67 labelling index is the significant prognostic indicator. Histopathology 2001, 39, 187–197. [Google Scholar] [CrossRef]
- Heaton, C.M.; Fernandes, A.F.A.; Jark, P.C.; Pan, X. Evaluation of toceranib for treatment of apocrine gland sac anal adenocarcinoma in dogs. J. Vet. Intern. Med. 2020, 34, 873–881. [Google Scholar] [CrossRef]
- Dennis, M.M.; McSporran, K.D.; Bacon, N.J.; Schulman, F.Y.; Foster, R.A.; Powers, B.E. Prognostic factors for cutaneous and subcutaneous soft tissues sarcomas in dogs. Vet. Pathol. 2011, 48, 73–84. [Google Scholar] [CrossRef]
- Pender, A.; Jones, R.L. Olaratumab: A platelet-derived growth factor receptor-α-blocking antibody for the treatment of soft tissue sarcoma. Clin. Pharmacol. 2017, 9, 159–164. [Google Scholar] [CrossRef]
- Chang, K.K.; Yoon, C.; Yi, B.C.; Tap, W.D.; Simon, M.C.; Yoon, S.S. Platelet-derived growth factor receptor-α and -β promote cancer stem cell phenotypes in sarcomas. Oncogenesis 2018, 7, 47. [Google Scholar] [CrossRef]
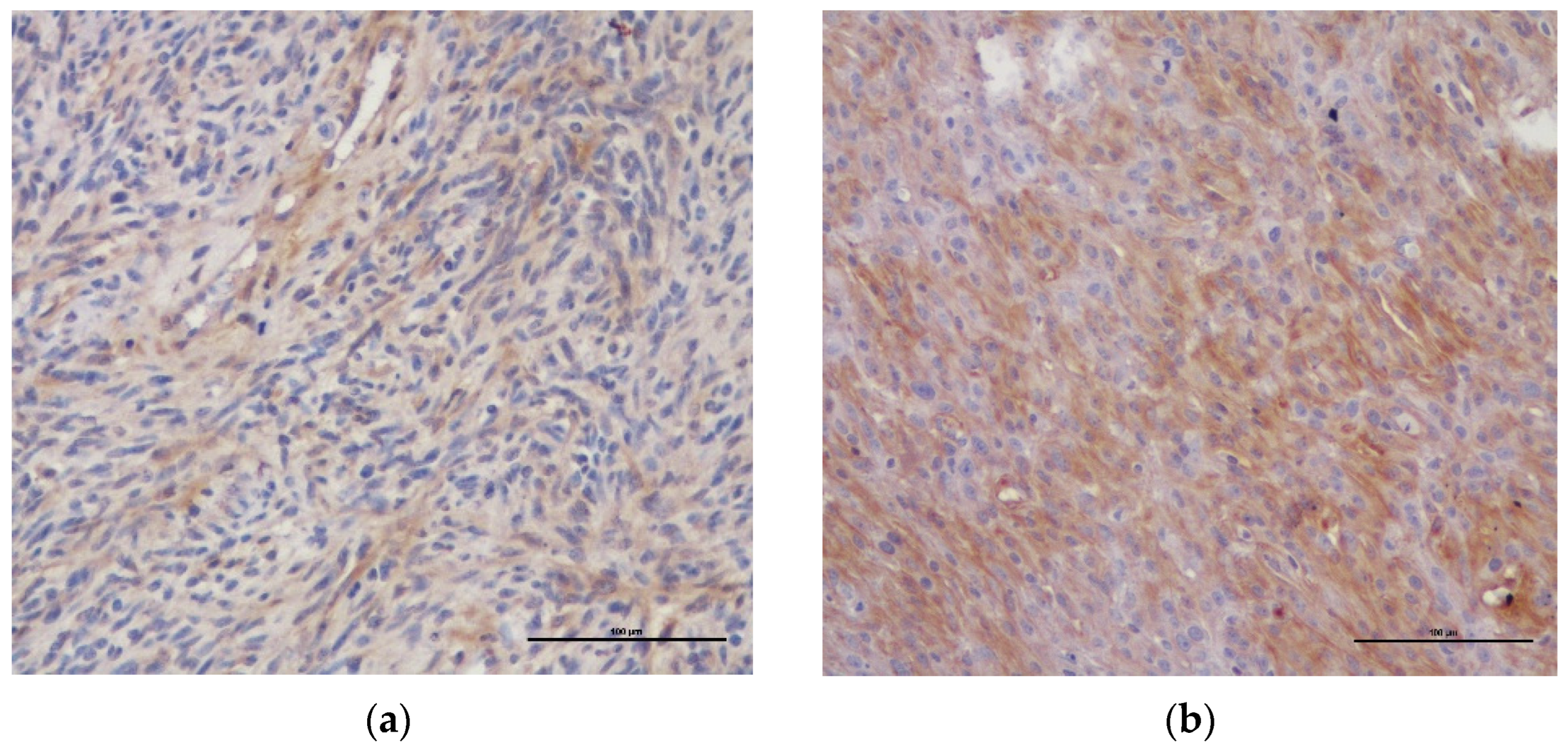
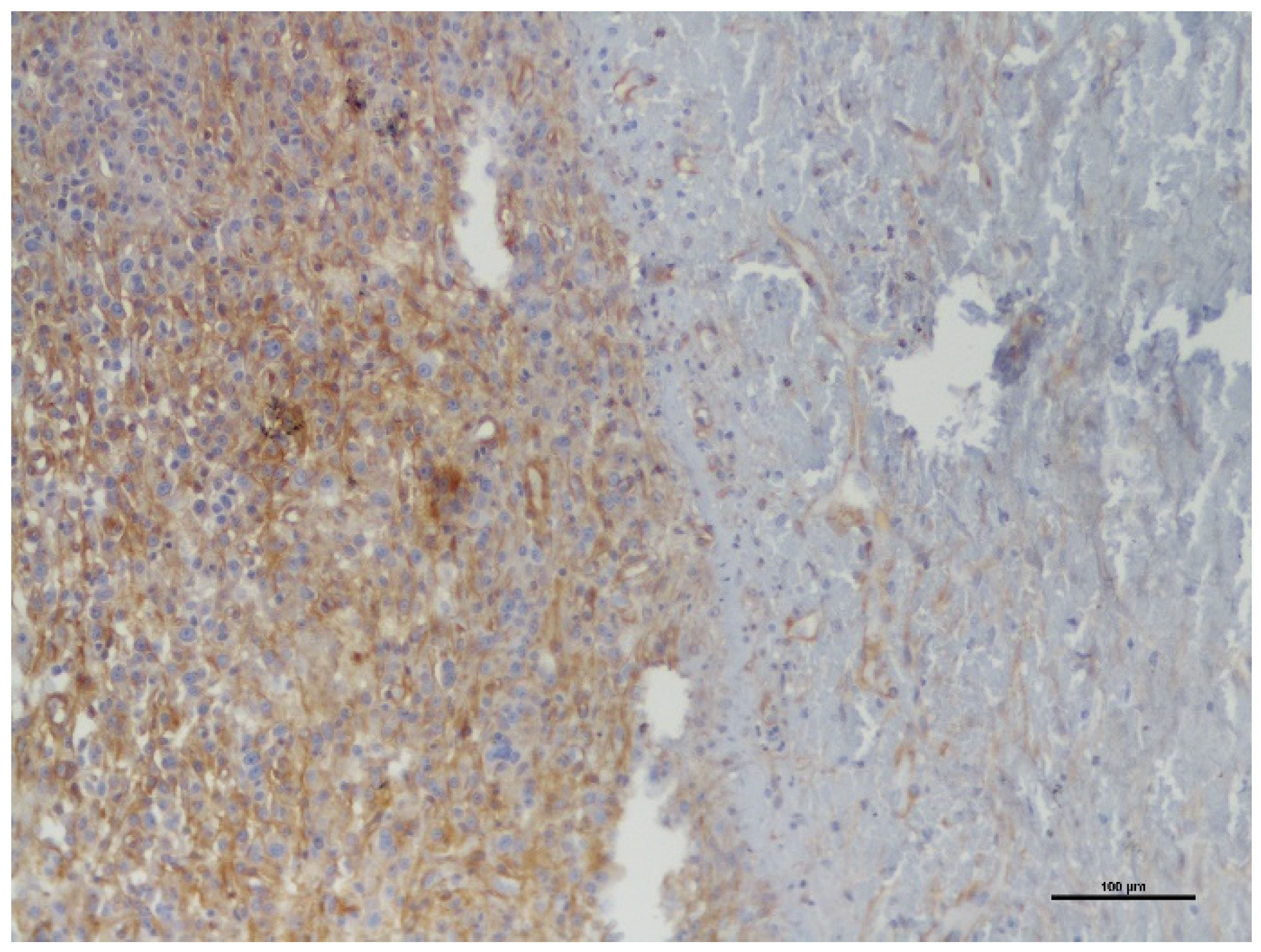
| Case Nr. | Breed | Age (Years) | Gender | Type of Tumor | Location of Tumor | Dimensions of Tumor (cm) | Ki-67 PI (%) | % PDGFR-β Expression |
|---|---|---|---|---|---|---|---|---|
| 1 | M. Schnauzer | 13 | M | B | T | 2.5 × 2.5 | 60.8 | ≤25% |
| 2 | Mixed breed | 12 | F | B | L | 4 × 3.5 | 15.8 | >25% |
| 3 | Poodle | 9 | M | B | NR | 3 × 2.5 | 36.3 | >25% |
| 4 | X Rottweiler | 8 | M | B | NR | NR | 4.3 | ≤25% |
| 5 | Mixed breed | 8 | M | B | L | 4 × 2.8 | 1.8 | ≤25% |
| 6 | Mixed breed | 10 | F | B | L | 2 × 1 | 3.8 | ≤25% |
| 7 | G. Shepherd | 14 | F | B | T | 4 × 2 | 14.1 | ≤25% |
| 8 | Poodle | 13 | M | B | L | 1.2 × 1.2 | 1.2 | ≤25% |
| 9 | Boxer | 9 | M | B | T | 2 × 1 | 2.8 | >25% |
| 10 | Port. Pointer | 9 | M | M | T | 8 × 8 | 38.9 | >25% |
| 11 | St. Bernard | 10 | F | M | L | 10 × 10 | 62.6 | ≤25% |
| 12 | Siberian husky | 13 | F | M | L | 4.5 × 4 | 14.1 | >25% |
| 13 | Labr. Retriever | 2 | M | M | T | 3 × 3 | 23.5 | >25% |
| 14 | Boxer | 7 | M | M | L | 4.5 × 3.5 | 26.2 | >25% |
| 15 | Boxer | 7 | F | M | T | 6.5 × 6 | 21.4 | >25% |
| 16 | Mixed breed | 12 | M | M | L | 3 × 2 | 54.0 | ≤25% |
| 17 | Mixed breed | 12 | F | M | T | 10.5 × 8 | 6.4 | >25% |
| 18 | Dalmatian | 10 | F | M | L | 4 × 2.5 | 10.2 | >25% |
| 19 | Boxer | 8 | F | M | L | 4 × 2 | 2.8 | >25% |
| Variable | Nr. of Tumors | BPNST (Number; %) | MPNST (Number; %) | p Value |
|---|---|---|---|---|
| Gender | ||||
| Female | 9 | 3 (33.3) | 6 (66.7) | 0.24 |
| Male | 10 | 6 (60.0) | 4 (40.0) | |
| Breed * | ||||
| Small | 3 | 3 (100.0) | 0 (0) | 0.05 |
| Medium, Large, Giant | 11 | 3 (27.3) | 8 (72.7) | |
| Tumor Location * | ||||
| Trunk | 7 | 3 (42.9) | 4 (57.1) | 0.64 |
| Limbs | 10 | 4 (40.0) | 6 (60.0) | |
| Tumor size * | ||||
| <3 cm | 4 | 4 (100.0) | 0 (0) | 0.02 |
| ≥3 cm | 14 | 4 (28.6) | 10 (71.4) |
| Variable | Nr. of Tumors | Mean Ki-67 | Standard Deviation (SD) | p Value (ANOVA) |
|---|---|---|---|---|
| Tumor Type | ||||
| Benign | 9 | 15.66 | 20.32 | 0.28 |
| Malignant | 10 | 26.01 | 20.09 | |
| PDGFR-β expression | ||||
| ≤25% | 8 | 25.33 | 28.38 | 0.45 |
| >25% | 11 | 18.04 | 12.48 |
| Variable | Nr. of Tumors | Tumors with ≤25% PDGFR-β Expression (Number; %) | Tumors with >25% PDGFR-β Expression (Number; %) | p Value |
|---|---|---|---|---|
| Tumor type | ||||
| Benign | 9 | 6 (66.7) | 3 (33.3) | 0.05 |
| Malignant | 10 | 2 (20.0) | 8 (80.0) | |
| Tumor Location * | ||||
| Trunk | 7 | 2 (28.6) | 5 (71.4) | 0.35 |
| Limbs | 10 | 5 (50.0) | 5 (50.0) | |
| Tumor size * | ||||
| <3 cm | 4 | 3 (75.0) | 1 (25.0) | 0.13 |
| ≥3 cm | 14 | 4 (28.6) | 10 (71.4) | |
| Ki-67 Index | ||||
| <20% | 11 | 5 (45.5) | 6 (54.5) | 0.55 |
| ≥20% | 8 | 3 (37.5) | 5 (62.5) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aluai-Cunha, C.; Matos, A.; Amorim, I.; Carvalho, F.; Rêma, A.; Santos, A. Immunohistochemical Expression of Platelet-Derived Growth Factor Receptor β (PDGFR-β) in Canine Cutaneous Peripheral Nerve Sheath Tumors: A Preliminary Study. Vet. Sci. 2022, 9, 345. https://doi.org/10.3390/vetsci9070345
Aluai-Cunha C, Matos A, Amorim I, Carvalho F, Rêma A, Santos A. Immunohistochemical Expression of Platelet-Derived Growth Factor Receptor β (PDGFR-β) in Canine Cutaneous Peripheral Nerve Sheath Tumors: A Preliminary Study. Veterinary Sciences. 2022; 9(7):345. https://doi.org/10.3390/vetsci9070345
Chicago/Turabian StyleAluai-Cunha, Catarina, Augusto Matos, Irina Amorim, Fátima Carvalho, Alexandra Rêma, and Andreia Santos. 2022. "Immunohistochemical Expression of Platelet-Derived Growth Factor Receptor β (PDGFR-β) in Canine Cutaneous Peripheral Nerve Sheath Tumors: A Preliminary Study" Veterinary Sciences 9, no. 7: 345. https://doi.org/10.3390/vetsci9070345
APA StyleAluai-Cunha, C., Matos, A., Amorim, I., Carvalho, F., Rêma, A., & Santos, A. (2022). Immunohistochemical Expression of Platelet-Derived Growth Factor Receptor β (PDGFR-β) in Canine Cutaneous Peripheral Nerve Sheath Tumors: A Preliminary Study. Veterinary Sciences, 9(7), 345. https://doi.org/10.3390/vetsci9070345






