Metastatic Uterine Adenocarcinoma in a Sable Antelope (Hippotragus niger)
Abstract
Simple Summary
Abstract
1. Introduction
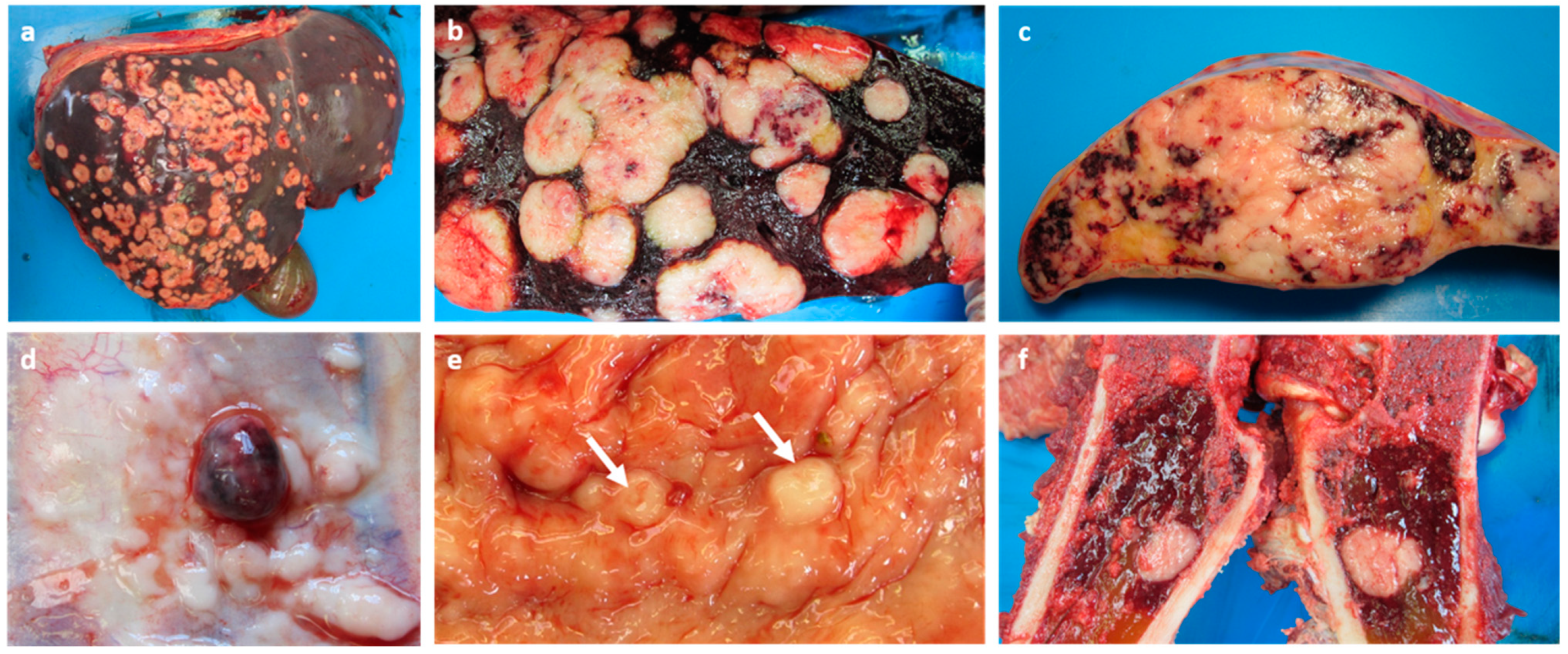
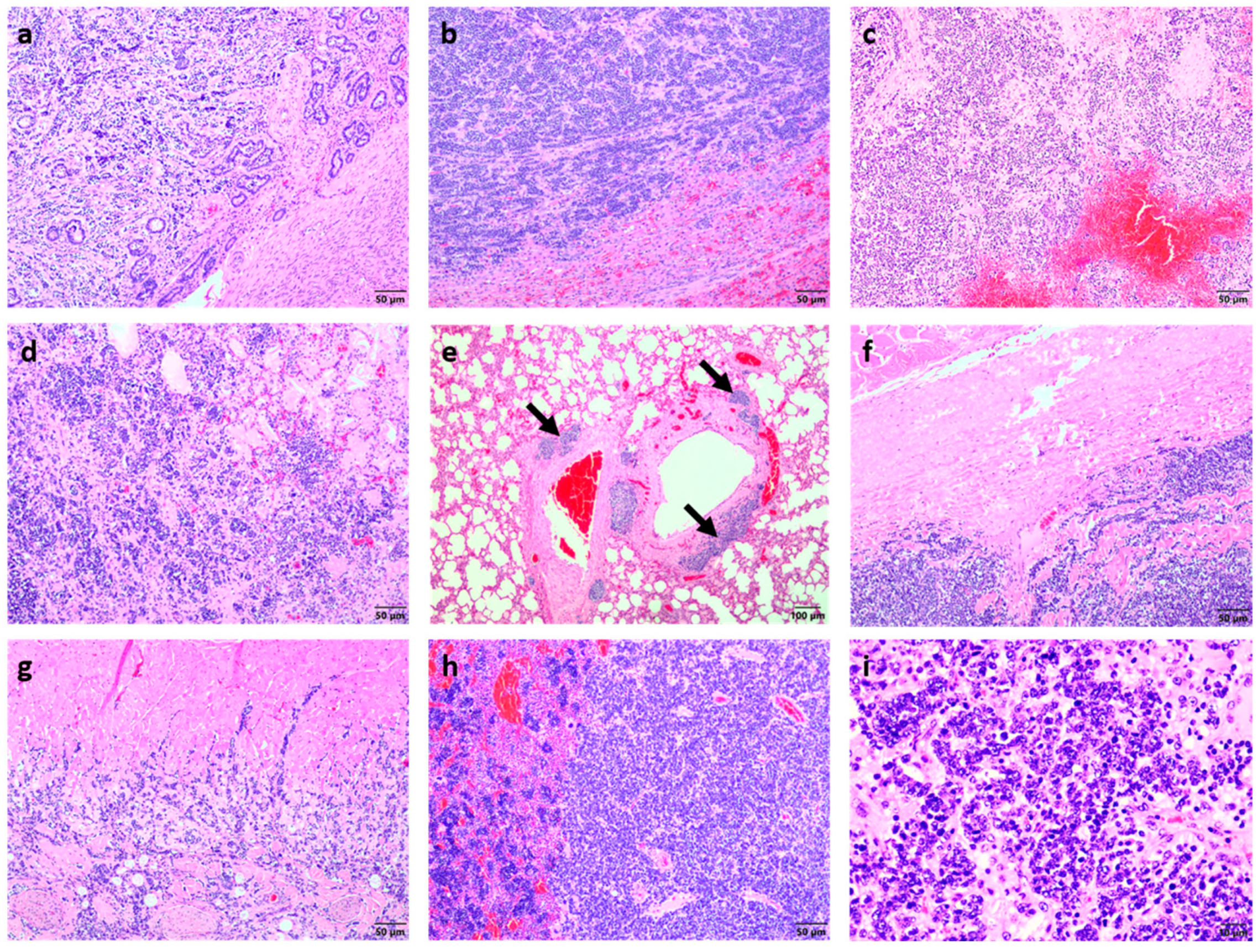
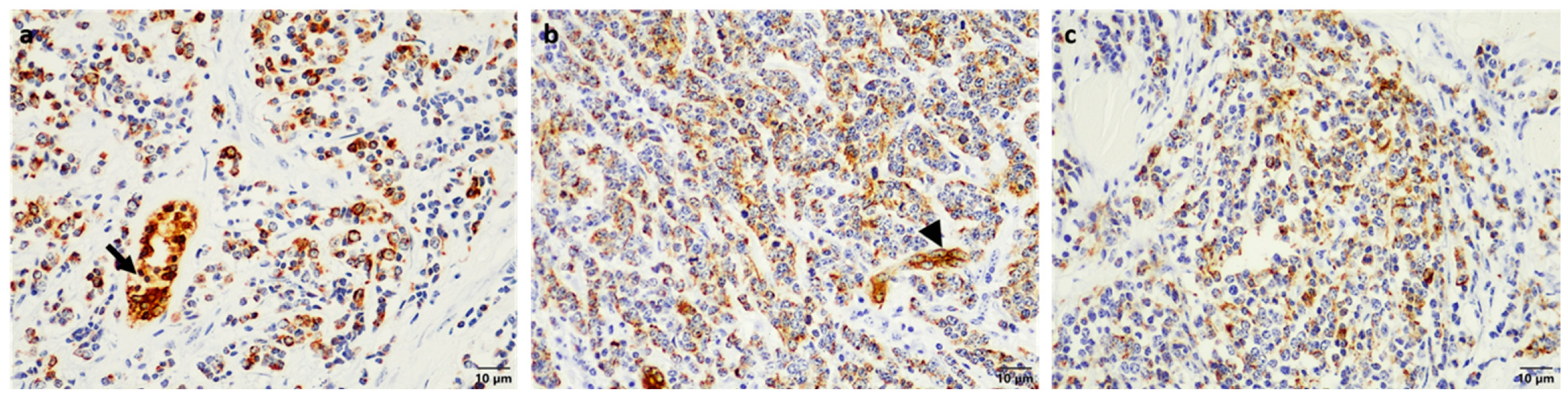
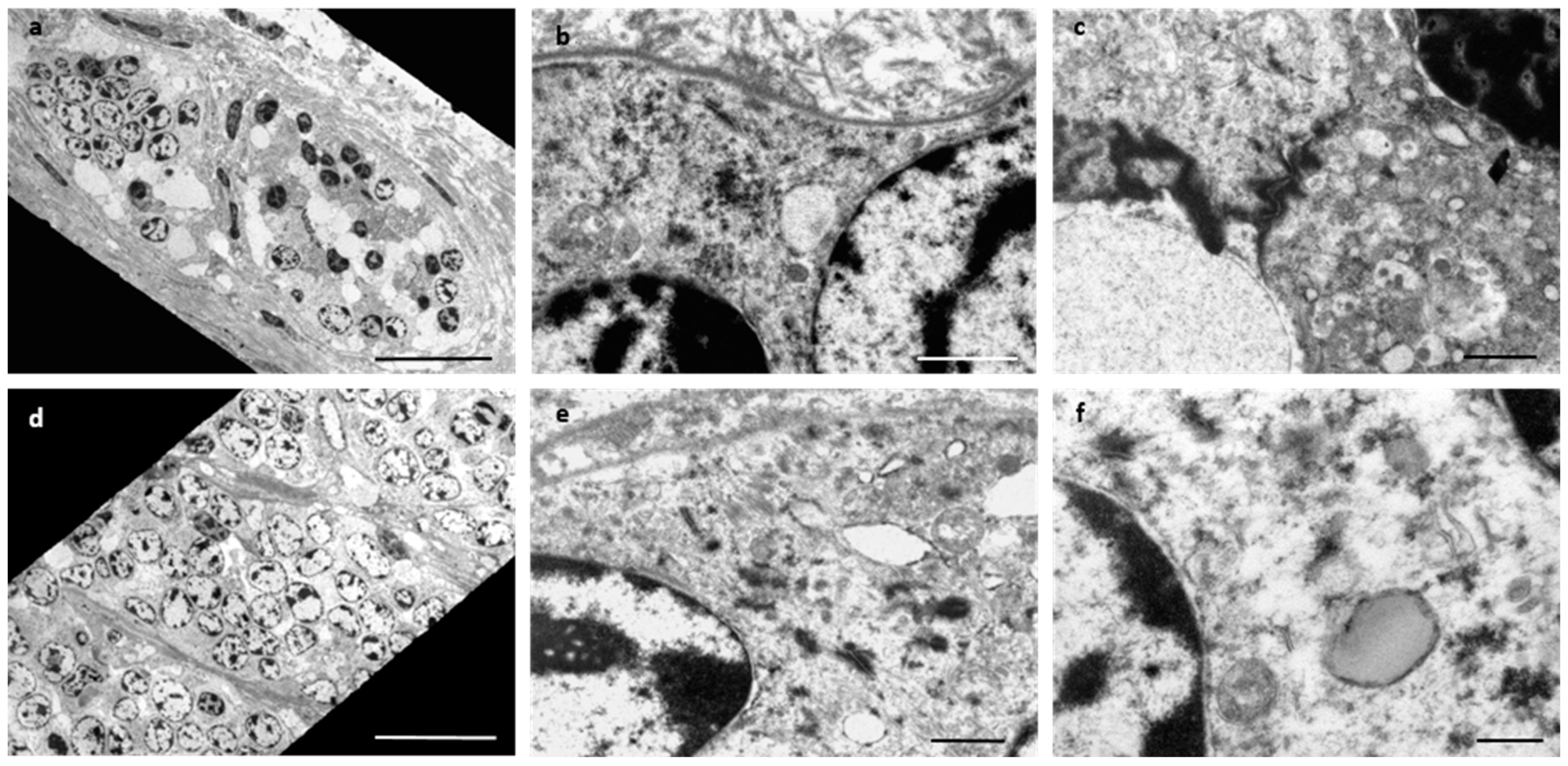
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cotchin, E. Spontaneous uterine cancer in animals. Br. J. Cancer 1964, 18, 209–277. [Google Scholar] [CrossRef]
- Agnew, D.D.; MacLachlan, N.J. Chapter 16: Tumors of the Genital Systems. In Tumors of Domestic Animals, 5th ed.; Meuten, D.J., Ed.; John Wiley & Sons, Inc.: Ames, IO, USA, 2017; pp. 689–722. [Google Scholar] [CrossRef]
- SEER. 2021. Available online: https://seer.cancer.gov/statfacts/html/corp.html (accessed on 8 June 2022).
- Anderson, C.; Pratschke, K. Uterine adenocarcinoma with abdominal metastases in an ovariohysterectomised cat. J. Feline Med. Surg. 2011, 13, 44–47. [Google Scholar] [CrossRef]
- Pires, M.D.A.; Catarino, J.C.; Vilhena, H.; Faim, S.; Neves, T.; Freire, A.; Seixas, F.; Orge, L.; Payan-Carreira, R. Co-existing monophasic teratoma and uterine adenocarcinoma in a female dog. Reprod. Domest. Anim. 2019, 54, 1044–1049. [Google Scholar] [CrossRef]
- Quéré, E.; Bourzac, C.; Farfan, M.; Losada, A.; Volmer, C.; Mespoulhès-Rivière, C. Standing Hand-Assisted Laparoscopic Diagnosis and Treatment of a Rare Case of Uterine Adenocarcinoma in an 18-Year-Old Mare. J. Equine Vet. Sci. 2019, 79, 39–44. [Google Scholar] [CrossRef]
- Robert, N.; Posthaus, H. Uterine adenocarcinoma in a captive sika deer. J. Wildl. Dis. 1999, 35, 141–144. [Google Scholar] [CrossRef]
- Duncan, C.; Powers, J.; Davis, T.; Spraker, T. Abomasal and uterine adenocarcinomas with ovarian metastasis in a captive elk (Cervus elaphus nelsoni). J. Vet. Diagn. Investig. 2007, 19, 560–563. [Google Scholar] [CrossRef]
- Baum, B. Not Just Uterine Adenocarcinoma-Neoplastic and Non-Neoplastic Masses in Domestic Pet Rabbits (Oryctolagus cuniculus): A Review. Vet. Pathol. 2021, 58, 890–900. [Google Scholar] [CrossRef]
- Settai, K.; Kondo, H.; Shibuya, H. Assessment of reported uterine lesions diagnosed histologically after ovariohysterectomy in 1,928 pet rabbits (Oryctolagus cuniculus). J. Am. Vet. Med. Assoc. 2020, 257, 1045–1050. [Google Scholar] [CrossRef]
- Dukes, T.W.; Bundza, A.; Corner, A.H. Bovine Neoplasms Encountered in Canadian Slaughterhouses: A Summary. Can. Vet. J. 1982, 23, 28–30. [Google Scholar]
- Harmon, B.G.; Munday, J.S.; Crane, M.M. Diffuse cystic endometrial hyperplasia and metastatic endometrial adenocarcinoma in a vietnamese pot-bellied pig (Sus scrofa). J. Vet. Diagn. Investig. 2004, 16, 587–589. [Google Scholar] [CrossRef]
- Cannon, C.Z.; Godfrey, V.L.; King-Herbert, A.; Nielsen, J.N. Metastatic uterine adenocarcinoma in an 8-year-old gilt. J. Am. Assoc. Lab. Anim. Sci. 2009, 48, 795–800. [Google Scholar]
- Golbar, H.; Izawa, T.; Kuwamura, M.; Ito, S.; Yamate, J. Uterine adenocarcinoma with prominent desmoplasia in a geriatric miniature pig. J. Vet. Med. Sci. 2010, 72, 253–256. [Google Scholar] [CrossRef][Green Version]
- Ilha, M.R.; Newman, S.J.; van Amstel, S.; Fecteau, K.A.; Rohrbach, B.W. Uterine lesions in 32 female miniature pet pigs. Vet. Pathol. 2010, 47, 1071–1075. [Google Scholar] [CrossRef]
- Napier, J.E.; Murray, S.; Garner, M.M.; Viner, T.; Murphy, H. Uterine leiomyomas in three captive eastern Bongo (Tragelaphus eurycerus isaaci). J. Zoo Wildl. Med. 2005, 36, 709–711. [Google Scholar] [CrossRef]
- Harwood, D.G.; Griffiths, W.H.; Bradshaw, J.M.; Gilbert, T. Uterine endometrial carcinoma associated with dystocia in a captive scimitar-horned oryx (Oryx dammah). Vet. Rec. 2009, 164, 661–662. [Google Scholar] [CrossRef]
- Estes, R.D. Hippotragus niger SABLE ANTELOPE in The Mammals of Africa. VI. Pigs, Hippopotamuses, Chevrotain, Giraffes, Deer, and Bovids; Kingdon, J.S., Hoffmann, M., Eds.; Bloomsbury Publishing: London, UK, 2013; pp. 556–565. [Google Scholar] [CrossRef]
- IUCN SSC Antelope Specialist Group. Hippotragus niger. The IUCN Red List of Threatened Species 2017: E.T10170A50188654. 2017. Available online: https://www.iucnredlist.org/species/10170/50188654 (accessed on 8 June 2022). [CrossRef]
- Haefele, H.J.; Guthrie, A.; Trupkiewicz, J.G.; Garner, M.M. Oropharyngeal teratoma in a neonatal sable antelope (Hippotragus niger). J. Zoo Wildl. Med. 2008, 39, 266–269. [Google Scholar] [CrossRef]
- van Dyk, E.; Bosman, A.M.; van Wilpe, E.; Williams, J.H.; Bengis, R.G.; van Heerden, J.; Venter, E.H. Detection and characterisation of papillomavirus in skin lesions of giraffe and sable antelope in South Africa. S. Afr. Vet. Assoc. 2011, 82, 80–85. [Google Scholar] [CrossRef]
- Kurotaki, T.; Kokoshima, H.; Kitamori, F.; Kitamori, T.; Tsuchitani, M. A case of adenocarcinoma of the endometrium extending into the leiomyoma of the uterus in a rabbit. J. Vet. Med. Sci. 2007, 69, 981–984. [Google Scholar] [CrossRef]
- Haist, V.; Hirschfeld, S.G.; Mallig, C.; Fehr, M.; Baumgärtner, W. Pathologic fracture of the femur due to endometrial adenocarcinoma metastasis in a female pet rabbit (Oryctolagus cuniculi). Berl. Munch. Tierarztl. Wochenschr. 2010, 123, 346–351. [Google Scholar]
- Miller, M.A.; Ramos-Vara, J.A.; Dickerson, M.F.; Johnson, G.C.; Pace, L.W.; Kreeger, J.M.; Turnquist, S.E.; Turk, J.R. Uterine neoplasia in 13 cats. J. Vet. Diagn. Investig. 2003, 15, 515–522. [Google Scholar] [CrossRef]
- Pérez-Martínez, C.; García-Fernández, R.A.; Escudero, A.; Ferreras, M.C.; García-Iglesias, M.J. Expression of cytokeratins and vimentin in normal and neoplastic tissue from the bovine female reproductive tract. J. Comp. Pathol. 2001, 124, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Moll, R.; Franke, W.W.; Schiller, D.L.; Geiger, B.; Krepler, R. The catalog of human cytokeratins: Patterns of expression in normal epithelia, tumors and cultured cells. Cell 1982, 31, 11–24. [Google Scholar] [CrossRef]
- Gil da Costa, R.M.; Santos, M.; Amorim, I.; Lopes, C.; Dias Pereira, P.; Faustino, A.M. An immunohistochemical study of feline endometrial adenocarcinoma. J. Comp. Pathol. 2009, 140, 254–259. [Google Scholar] [CrossRef] [PubMed]
- Saraiva, A.L.; Payan-Carreira, R.; Gärtner, F.; Fortuna da Cunha, M.R.; Rêma, A.; Faria, F.; Lourenço, L.M.; Pires Mdos, A. An immunohistochemical study on the expression of sex steroid receptors, Ki-67 and cytokeratins 7 and 20 in feline endometrial adenocarcinomas. BMC Vet. Res. 2015, 11, 204. [Google Scholar] [CrossRef]
- Lingard, D.R.; Dickinson, E.O. Uterine adenocarcinoma with metastasis in the cow. J. Am. Vet. Med. Assoc. 1966, 148, 913–915. [Google Scholar]
- Garcia-Iglesias, M.J.; Bravo-Moral, A.M.; Perez-Martinez, C.; Ferreras-Estrada, M.C.; Martinez-Rodriguez, J.M.; Escudero-Diez, A. Incidence and pathomorphology of uterine tumours in the cow. Zentralbl. Veterinarmed. A 1995, 42, 421–429. [Google Scholar] [CrossRef]
- Stilwell, G.; Peleteiro, M.C. Uterine adenocarcinoma with pulmonary, liver and mesentery metastasis in a holstein cow. Vet. Med. Int. 2010, 2010, 727856. [Google Scholar] [CrossRef]
- Payan-Carreira, R.; Saraiva, A.L.; Santos, T.; Vilhena, H.; Sousa, A.; Santos, C.; Pires, M.A. Feline endometrial adenocarcinoma in females <1 year old: A description of four cases. Reprod. Domest. Anim. 2013, 48, e70–e77. [Google Scholar] [CrossRef]
- Payne-Johnson, C.E.; Kelly, D.F.; Davies, P.T. Endometrial carcinoma in a young dog. J. Comp. Pathol. 1986, 96, 463–467. [Google Scholar] [CrossRef]
- Sontas, B.H.; Erdogan, Ö.; Apaydin Enginler, S.Ö.; Yilmaz, Ö.T.; Şennazli, G.; Ekici, H. Endometrial adenocarcinoma in two young queens. J. Small Anim. Pract. 2013, 54, 156–159. [Google Scholar] [CrossRef]
- Pena, F.J.; Gines, J.A.; Duque, J.; Vieitez, V.; Martinez-Pérez, R.; Madejón, L.; Nuñez Martinez, I.; Moran, J.M.; Fernández-García, S. Endometrial adenocarcinoma and mucometra in a 6-year-old Alaska Malamute dog. Reprod. Domest. Anim. 2006, 41, 189–190. [Google Scholar] [CrossRef] [PubMed]
- Choi, U.S. A Case of Uterine Adenocarcinoma in A Spayed Female Dog. J. Vet. Clin. 2013, 30, 53–56. [Google Scholar]
- Preissel, A.K.; Brugger, N.; Stassen, T.; Nuss, K. Treatment of a uterine adenocarcinoma in a miniature pig by ovariohysterectomy. Schweiz. Arch. Tierheilkd. 2009, 151, 229–232. [Google Scholar] [CrossRef] [PubMed]
- McOnie, R.C.; Noel, A.M.; Fubini, S.L.; Reesink, H.L. Surgical treatment of uterine neoplasia in 13 production size pigs with a comparison to pot-bellied pigs. Vet. Surg. 2021, 50, 1434–1442. [Google Scholar] [CrossRef] [PubMed]
- Meier, H. Carcinoma of the uterus in the cat: Two cases. Cornell Vet. 1956, 46, 188–200. [Google Scholar]
- Špoljarić, B.; Vince, S.; Folnožić, I.; Medven Zagradišnik, L.; Huber, D.; Gudan Kurilj, A.; Grabarević, Ž. Endometrial Mucinous Adenocarcinoma in a Bitch with Pyometra-A Case Report. Top. Companion Anim. Med. 2019, 36, 16–21. [Google Scholar] [CrossRef]
- Cave, T.A.; Hine, R.; Howie, F.; Thompson, H.; Argyle, D.J. Uterine carcinoma in a 10-month-old golden retriever. J. Small Anim. Pract. 2002, 43, 133–135. [Google Scholar] [CrossRef]
- Yasin, H.K.; Taylor, A.H.; Ayakannu, T. A Narrative Review of the Role of Diet and Lifestyle Factors in the Development and Prevention of Endometrial Cancer. Cancers 2021, 13, 2149. [Google Scholar] [CrossRef]
- Raglan, O.; Kalliala, I.; Markozannes, G.; Cividini, S.; Gunter, M.J.; Nautiyal, J.; Gabra, H.; Paraskevaidis, E.; Martin-Hirsch, P.; Tsilidis, K.K.; et al. Risk factors for endometrial cancer: An umbrella review of the literature. Int. J. Cancer 2019, 145, 1719–1730. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
van der Weyden, L.; Bezuidenhout, A.; van Wilpe, E.; O’Dell, N. Metastatic Uterine Adenocarcinoma in a Sable Antelope (Hippotragus niger). Vet. Sci. 2022, 9, 339. https://doi.org/10.3390/vetsci9070339
van der Weyden L, Bezuidenhout A, van Wilpe E, O’Dell N. Metastatic Uterine Adenocarcinoma in a Sable Antelope (Hippotragus niger). Veterinary Sciences. 2022; 9(7):339. https://doi.org/10.3390/vetsci9070339
Chicago/Turabian Stylevan der Weyden, Louise, Anien Bezuidenhout, Erna van Wilpe, and Nicolize O’Dell. 2022. "Metastatic Uterine Adenocarcinoma in a Sable Antelope (Hippotragus niger)" Veterinary Sciences 9, no. 7: 339. https://doi.org/10.3390/vetsci9070339
APA Stylevan der Weyden, L., Bezuidenhout, A., van Wilpe, E., & O’Dell, N. (2022). Metastatic Uterine Adenocarcinoma in a Sable Antelope (Hippotragus niger). Veterinary Sciences, 9(7), 339. https://doi.org/10.3390/vetsci9070339





