Incidence of Patellar Desmopathy in the Modified Maquet Technique with and without PRGF
Abstract
:1. Introduction
2. Materials and Methods
2.1. Clinical Trial
2.2. Data Collection
2.3. Radiographic Evaluation
2.4. Desmopathy Diagnosis
2.5. Statistical Method
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Moore, K.; READ, R. Cranial cruciate ligament rupture in the dog—A retrospective study comparing surgical techniques. Aust. Vet. J. 1995, 72, 281–285. [Google Scholar] [CrossRef] [PubMed]
- Lampman, T.J.; Lund, E.M.; Lipowitz, A.J. Cranial cruciate disease: Current status of diagnosis, surgery, and risk for disease. Vet. Comp. Orthop. Traumatol. 2003, 16, 122–126. [Google Scholar] [CrossRef]
- Fossum, T.W. Small Animal Surgery; Elsevier Health Sciences, Ed.; Elsevier Health Sciences: Philadelphia, PA, USA, 2013. [Google Scholar]
- De Rooster, H.; De Bruin, T.; Van Bree, H. Morphologic and functional features of the canine cruciate ligaments. Vet. Surg. 2006, 35, 769–780. [Google Scholar] [CrossRef] [PubMed]
- Witsberger, T.H.; Armando Villamil, J.; Schultz, L.G.; Hahn, A.W.; Cook, J.L. Prevalence of and risk factors for hip dysplasia and cranial cruciate ligament deficiency in dogs. J. Am. Vet. Med. Assoc. 2008, 232, 1818–1824. [Google Scholar] [CrossRef] [PubMed]
- Lafaver, S.; Miller, N.A.; Stubbs, W.P.; Taylor, R.A.; Boudrieau, R.J. Tibial tuberosity advancement for stabilization of the canine cranial cruciate ligament-deficient stifle joint: Surgical technique, early results, and complications in 101 dogs. Vet. Surg. 2007, 36, 573–586. [Google Scholar] [CrossRef]
- Montavon, P.M. Tibial tuberosity advancement (TTA) for the treatment of cranial cruciate disease in dogs: Evidences, technique and initial clinical results. In Proceedings of the 12th ESVOT Congress, Munich, Germany, 10–12 September 2004; pp. 254–255. [Google Scholar]
- Guerrero, T.G.; Makara, M.A.; Katiofsky, K.; Fluckiger, M.A.; Morgan, J.P.; Haessig, M.; Montavon, P.M. Comparison of Healing of the Osteotomy Gap after Tibial Tuberosity Advancement with and without Use of an Autogenous Cancellous Bone Graft. Vet. Surg. 2011, 40, 27–33. [Google Scholar] [CrossRef]
- Boudrieau, R.J. Tibial plateau leveling osteotomy or tibial tuberosity advancement? Vet. Surg. 2009, 38, 1–22. [Google Scholar] [CrossRef]
- Pacchiana, P.D.; Morris, E.; Gillings, S.L.; Jessen, C.R.; Lipowitz, A.J. Surgical and postoperative complications associated with tibial plateau leveling osteotomy in dogs with cranial cruciate ligament rupture: 397 Cases (1998–2001). J. Am. Vet. Med. Assoc. 2003, 222, 184–193. [Google Scholar] [CrossRef]
- Carey, K.; Aiken, S.W.; DiResta, G.R.; Herr, L.G.; Monette, S. Radiographic and clinical changes of the patellar tendon after tibial plateau leveling osteotomy: 94 Cases (2001–2003). Vet. Comp. Orthop. Traumatol. 2005, 18, 235–242. [Google Scholar] [CrossRef]
- Mattern, K.L.; Berry, C.R.; Peck, J.N.; De Haan, J.J. Radiographic and ultrasonographic evaluation of the patellar ligament following tibial plateau leveling osteotomy. Vet. Radiol. Ultrasound 2006, 47, 185–191. [Google Scholar] [CrossRef]
- Kühn, K.; Ohlerth, S.; Makara, M.; Hässig, M.; Guerrero, T.M. Radiographic and ultrasonographic evaluation of the patellar ligament following tibial tuberosity advancement. Vet. Radiol. Ultrasound 2011, 52, 466–471. [Google Scholar] [CrossRef] [PubMed]
- Pettitt, R.; Cripps, P.; Baker, M.; Hattersley, R.; Lorenz, N.; Mcconnell, F. Radiographic and ultrasonographic changes of the patellar ligament following tibial tuberosity advancement in 25 dogs. Vet. Comp. Orthop. Traumatol. 2014, 27, 216–221. [Google Scholar] [PubMed]
- DeSandre-Robinson, D.M.; Tano, C.A.; Fiore, K.L.; Prytherch, B. Radiographic evaluation and comparison osteotomy and tibial tuberosity advancement. J. Am. Vet. Med. Assoc. 2017, 250, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Etchepareborde, S.; Brunel, L.; Bollen, G.; Balligand, M. Preliminary experience of a modified maquet technique for repair of cranial cruciate ligament rupture in dogs. Vet. Comp. Orthop. Traumatol. 2011, 24, 223–227. [Google Scholar] [CrossRef]
- Ness, M.G. The Modified Maquet Procedure (MMP) in Dogs: Technical Development and Initial Clinical Experience. J. Am. Anim. Hosp. Assoc. 2016, 52, 242–250. [Google Scholar] [CrossRef]
- Retallack, L.M.; Daye, R.M. A modified Maquet-tibial tuberosity advancement technique for treatment of canine cranial cruciate ligament disease: Short term outcome and complications. Vet. Surg. 2018, 47, 44–51. [Google Scholar] [CrossRef]
- Anitua, E.; Sánchez, M.; Zalduendo, M.M.; De La Fuente, M.; Prado, R.; Orive, G.; Andía, I. Fibroblastic response to treatment with different preparations rich in growth factors. Cell Prolif. 2009, 42, 162–170. [Google Scholar] [CrossRef]
- Intini, G. The use of platelet-rich plasma in bone reconstruction therapy. Biomaterials 2009, 30, 4956–4966. [Google Scholar] [CrossRef]
- Sommeling, C.E.; Heyneman, A.; Hoeksema, H.; Verbelen, J.; Stillaert, F.B.; Monstrey, S. The use of platelet-rich plasma in plastic surgery: A systematic review. J. Plast. Reconstr. Aesthetic Surg. 2013, 66, 301–311. [Google Scholar] [CrossRef]
- Fabbro, M.D.; Bortolin, M.; Taschieri, S.; Ceci, C.; Weinstein, R.L. Antimicrobial properties of platelet-rich preparations. A systematic review of the current pre-clinical evidence. Platelets 2016, 27, 276–285. [Google Scholar] [CrossRef]
- Valiño-Cultelli, V.; Varela-López, Ó.; González-Cantalapiedra, A. Preliminary clinical and radiographic evaluation of a novel resorbable implant of polylactic acid (Pla) for tibial tuberosity advancement (tta) by modified maquet technique (mmt). Animals 2021, 11, 1271. [Google Scholar] [CrossRef] [PubMed]
- Valiño-Cultelli, V.; Varela-López, Ó.; González-Cantalapiedra, A. Does prgf work? A prospective clinical study in dogs with a novel polylactic acid scaffold injected with prgf using the modified maquet technique. Animals 2021, 11, 2404. [Google Scholar] [CrossRef] [PubMed]
- Cook, J.L.; Evans, R.; Conzemius, M.G.; Lascelles, B.D.X.; McIlwraith, C.W.; Pozzi, A.; Clegg, P.; Innes, J.; Schulz, K.; Houlton, J.; et al. Proposed Definitions and Criteria for Reporting Time Frame, Outcome, and Complications For Clinical Orthopedic Studies in Veterinary Medicine. Vet. Surg. 2010, 39, 905–908. [Google Scholar] [CrossRef] [PubMed]
- Simpson, A.H.; Mills, L.; Noble, B. The role of growth factors and related agents in accelerating fracture healing. J. Bone Jt. Surg.-Ser. B 2006, 88, 701–705. [Google Scholar] [CrossRef] [PubMed]
- Veillette, C.J.H.; McKee, M.D. Growth factors—BMPs, DBMs, and buffy coat products: Are there any proven differences amongst them? Injury 2007, 38, S38–S48. [Google Scholar] [CrossRef]
- Tsiridis, E.; Upadhyay, N.; Giannoudis, P. Molecular aspects of fracture healing:Which are the important molecules? Injury 2007, 38, S11–S25. [Google Scholar] [CrossRef]
- Marx, R.E. Platelet-rich plasma (PRP): What is PRP and what is not PRP? Implant Dent. 2001, 10, 225–228. [Google Scholar] [CrossRef]
- Sánchez, M.; Anitua, E.; Azofra, J.; Prado, R.; Muruzabal, F.; Andia, I. Ligamentization of Tendon Grafts Treated with an Endogenous Preparation Rich in Growth Factors: Gross Morphology and Histology. Arthrosc.-J. Arthrosc. Relat. Surg. 2010, 26, 470–480. [Google Scholar] [CrossRef]
- Fernández-Sarmiento, J.A.; Domínguez, J.M.; Granados, M.M.; Morgaz, J.; Navarrete, R.; Carrillo, J.M.; Gómez-Villamandos, R.J.; Muñoz-Rascón, P.; Martín de las Mulas, J.; Millán, Y.; et al. Histological Study of the Influence of Plasma Rich in Growth Factors (PRGF) on the Healing of Divided Achilles Tendons in Sheep. J Bone Jt. Surg Am. 2013, 95, 246–255. [Google Scholar] [CrossRef]
- Yoshioka, T.; Kanamori, A.; Washio, T.; Aoto, K.; Uemura, K.; Sakane, M.; Ochiai, N. The effects of plasma rich in growth factors (PRGF-Endoret) on healing of medial collateral ligament of the knee. Knee Surg. Sport. Traumatol. Arthrosc. 2013, 21, 1763–1769. [Google Scholar] [CrossRef]
- López-Nájera, D.; Rubio-Zaragoza, M.; Sopena-Juncosa, J.J.; Alentorn-Geli, E.; Cugat-Bertomeu, R.; Fernández-Sarmiento, J.A.; Domínguez-Pérez, J.M.; García-Balletbó, M.; Primo-Capella, V.J.; Carrillo-Poveda, J.M. Effects of plasma rich in growth factors (PRGF) on biomechanical properties of Achilles tendon repair. Knee Surg. Sport. Traumatol. Arthrosc. 2016, 24, 3997–4004. [Google Scholar] [CrossRef] [PubMed]
- Barastegui, D.; Alentorn-Geli, E.; Gotecha, D.; Rius, M.; Navarro, J.; Cuscó, X.; Seijas, R.; Cugat, R. Treatment of Partial Posterior Cruciate Ligament Injuries with Platelet-Rich Plasma in Growth Factors (PRGF) Intraligamentous Infiltration and a Specific Knee Brace. Surg. J. 2021, 07, e30–e34. [Google Scholar] [CrossRef] [PubMed]
- Griffin, X.L.; Smith, C.M.; Costa, M.L. The clinical use of platelet-rich plasma in the promotion of bone healing: A systematic review. Injury 2009, 40, 158–162. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, I.A.; Growney Kalaf, E.A.; Bowlin, G.L.; Sell, S.A. Platelet-rich plasma in bone regeneration: Engineering the delivery for improved clinical efficacy. Biomed Res. Int. 2014, 2014, 392398. [Google Scholar] [CrossRef] [PubMed]
- Jovani-Sancho, M.D.M.; Sheth, C.C.; Marqués-Mateo, M.; Puche-Torres, M. Platelet-Rich Plasma: A Study of the Variables that May Influence Its Effect on Bone Regeneration. Clin. Implant Dent. Relat. Res. 2016, 18, 1051–1064. [Google Scholar] [CrossRef] [PubMed]
- Mazzucco, L.; Medici, D.; Serra, M.; Panizza, R.; Rivara, G.; Orecchia, S.; Libener, R.; Cattana, E.; Levis, A.; Betta, P.G.; et al. The use of autologous platelet gel to treat difficult-to-heal wounds: A pilot study. Transfusion 2004, 44, 1013–1018. [Google Scholar] [CrossRef]
- Lee, H.-W.; Reddy, M.S.; Geurs, N.; Palcanis, K.G.; Lemons, J.E.; Rahemtulla, F.G.; Ho, K.-J.; Chen, D.-T.; Davis, C.R.; Feldman, D.S. Efficacy of Platelet-Rich Plasma on Wound Healing in Rabbits. J. Periodontol. 2008, 79, 691–696. [Google Scholar] [CrossRef]
- Pallua, N.; Wolter, T.; Markowicz, M. Platelet-rich plasma in burns. Burns 2010, 36, 4–8. [Google Scholar] [CrossRef]
- Villela, D.L.; Santos, V.L. Evidence on the use of platelet-rich plasma for diabetic ulcer: A systematic review. Growth Factors 2010, 28, 111–116. [Google Scholar] [CrossRef]
- Ostvar, O.; Shadvar, S.; Yahaghi, E.; Azma, K.; Fayyaz, A.F.; Ahmadi, K.; Nowrouzian, I. Effect of platelet-rich plasma on the healing of cutaneous defects exposed to acute to chronic wounds: A clinico-histopathologic study in rabbits. Diagn. Pathol. 2015, 10, 85. [Google Scholar] [CrossRef]
- Wallace, J.L.; Dicay, M.; McKnight, W.; Dudar, G.K. Platelets accelerate gastric ulcer healing through presentation of vascular endothelial growth factor. Br. J. Pharmacol. 2006, 148, 274. [Google Scholar] [CrossRef] [PubMed]
- Alio, J.L.; Colecha, J.R.; Pastor, S.; Rodriguez, A.; Artola, A. Symptomatic dry eye treatment with autologous platelet-rich plasma. Ophthalmic Res. 2007, 39, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Martínez, C.E.; Smith, P.C.; Palma Alvarado, V.A. The influence of platelet-derived products on angiogenesis and tissue repair: A concise update. Front. Physiol. 2015, 6, 290. [Google Scholar] [CrossRef] [PubMed]
- López, S.; Vilar, J.M.; Sopena, J.J.; Damià, E.; Chicharro, D.; Carrillo, J.M.; Cuervo, B.; Rubio, M. Assessment of the efficacy of platelet-rich plasma in the treatment of traumatic canine fractures. Int. J. Mol. Sci. 2019, 20, 1075. [Google Scholar] [CrossRef] [PubMed]
- Ross, M.; Worrell, T.W. Thigh and calf girth following knee injury and surgery. J. Orthop. Sports Phys. Ther. 1998, 27, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Millis, D.; Levine, D.; Mynatt, T. Changes in muscle mass following transection of the cranial cruciate ligament and immediate stifle stabilization. In Proceedings of the First International Symposium on Rehabilitation and Physical Therapy in Veterinary Medicine; Oregon State University: Corvalis, OR, USA, 1999; pp. 7–11. [Google Scholar]
- Steinberg, E.J.; Prata, R.G.; Palazzini, K.; Brown, D.C. Tibial tuberosity advancement for treatment of CrCL injury: Complications and owner satisfaction. J. Am. Anim. Hosp. Assoc. 2011, 47, 250–257. [Google Scholar] [CrossRef]
- Wolf, R.E.; Scavelli, T.D.; Hoelzler, M.G.; Fulcher, R.P.; Bastian, R.P. CCL Surgical and postoperative complications associated with tibial tuberosity advancement for cranial cruciate ligament rupture in dogs: 458 cases (2007–2009). J. Am. Vet. Med. Assoc. 2012, 240, 1481–1487. [Google Scholar] [CrossRef]
- Bisgard, S.K.; Barnhart, M.D.; Shiroma, J.T.; Kennedy, S.C.; Schertel, E.R. The Effect of Cancellous Autograft and Novel Plate Design on Radiographic Healing and Postoperative Complications in Tibial Tuberosity Advancement for Cranial Cruciate-Deficient Canine Stifles. Vet. Surg. 2011, 40, 402–407. [Google Scholar] [CrossRef]
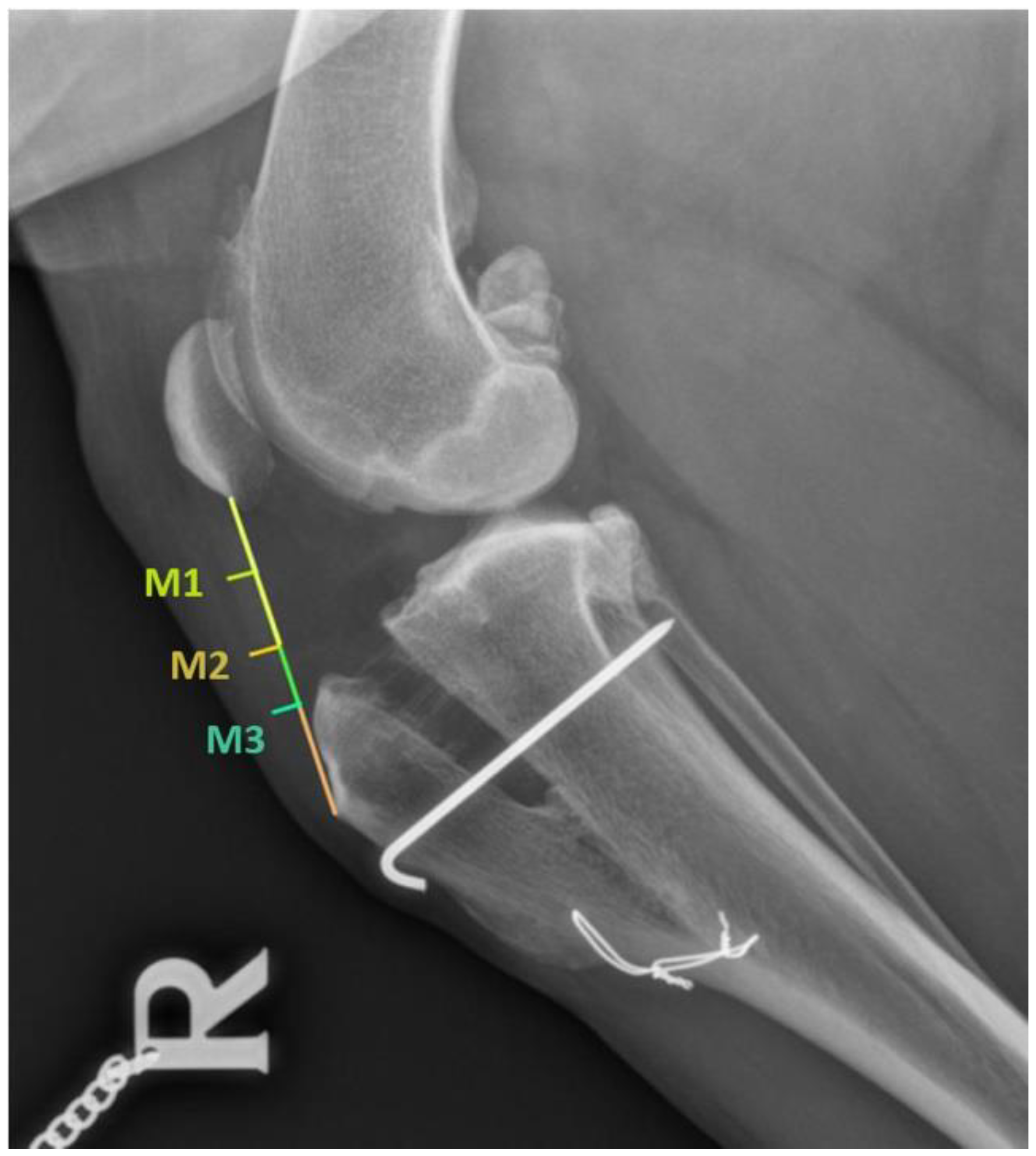
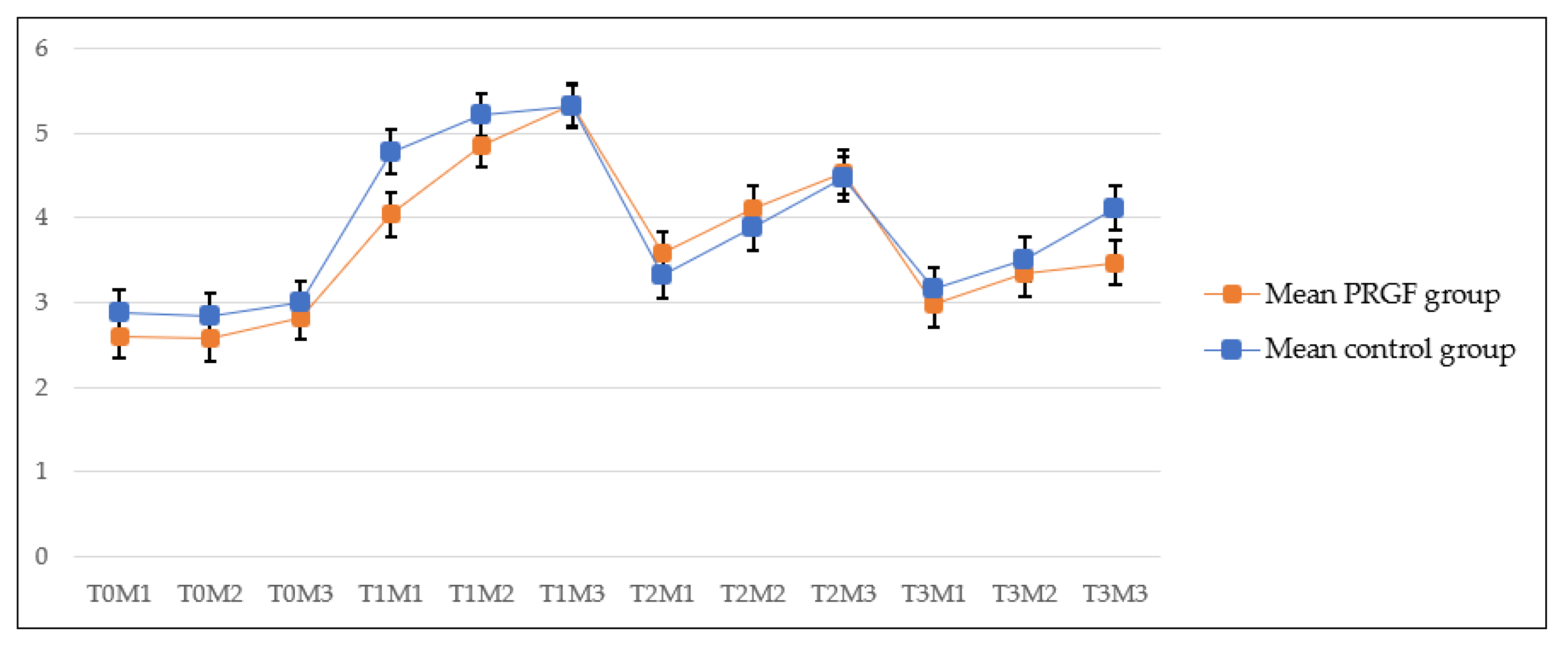
| CONTROL GROUP (n = 17) (mm) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre-surgery (T-0) | First follow-up (T-1) | Second follow-up (T-2) | Third follow-up (T-3) | |||||||||
| M-1 | M-2 | M-3 | M-1 | M-2 | M-3 | M-1 | M-2 | M-3 | M-1 | M-2 | M-3 | |
| 2.88 ± 0.73 | 2.84 ± 0.78 | 2.99 ± 0.81 | 4.77 ± 2.48 | 5.21 ± 2.50 | 5.31 ± 2.68 | 3.31 ± 1.48 | 3.88 ± 1.74 | 4.46 ± 1.83 | 3.15 ± 1.42 | 3.50 ± 1.58 | 4.11 ± 2.21 | |
| Mean | 2.90 | 5.09 | 3.88 | 3.58 | ||||||||
| PRGF GROUP (n = 18) (mm) | ||||||||||||
| Pre-surgery (T-0) | First follow-up (T-1) | Second follow-up (T-2) | Third follow-up (T-3) | |||||||||
| M-1 | M-2 | M-3 | M-1 | M-2 | M-3 | M-1 | M-2 | M-3 | M-1 | M-2 | M-3 | |
| 2.59 ± 0.73 | 2.57 ± 0.63 | 2.82 ± 0.56 | 4.03 ± 1.06 | 4.86 ± 2.27 | 5.33 ± 2.50 | 3.58 ± 1.05 | 4.10 ± 1.39 | 4.53 ± 1.68 | 2.96 ± 0.87 | 3.33 ± 1.31 | 3.46 ± 0.91 | |
| Mean | 2.66 | 4.74 | 4.07 | 3.25 | ||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valiño-Cultelli, V.; Varela-López, Ó.; González-Cantalapiedra, A. Incidence of Patellar Desmopathy in the Modified Maquet Technique with and without PRGF. Vet. Sci. 2022, 9, 180. https://doi.org/10.3390/vetsci9040180
Valiño-Cultelli V, Varela-López Ó, González-Cantalapiedra A. Incidence of Patellar Desmopathy in the Modified Maquet Technique with and without PRGF. Veterinary Sciences. 2022; 9(4):180. https://doi.org/10.3390/vetsci9040180
Chicago/Turabian StyleValiño-Cultelli, Victoria, Óscar Varela-López, and Antonio González-Cantalapiedra. 2022. "Incidence of Patellar Desmopathy in the Modified Maquet Technique with and without PRGF" Veterinary Sciences 9, no. 4: 180. https://doi.org/10.3390/vetsci9040180
APA StyleValiño-Cultelli, V., Varela-López, Ó., & González-Cantalapiedra, A. (2022). Incidence of Patellar Desmopathy in the Modified Maquet Technique with and without PRGF. Veterinary Sciences, 9(4), 180. https://doi.org/10.3390/vetsci9040180






