Influence of Butorphanol, Buprenorphine and Levomethadone on Sedation Quality and Postoperative Analgesia in Horses Undergoing Cheek Tooth Extraction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Animals and Group Selection
2.3. Preparation, Premedication and Treatment
2.4. Sedation Protocol
2.5. Assessment of Sedation Quality, Surgical Conditions and Severity of Extraction
2.6. Pre- and Postoperative Measurements
2.6.1. Pain Scores
2.6.2. Blood Samples
2.6.3. Locomotor Activity
2.7. Data Analysis
3. Results
3.1. Animals
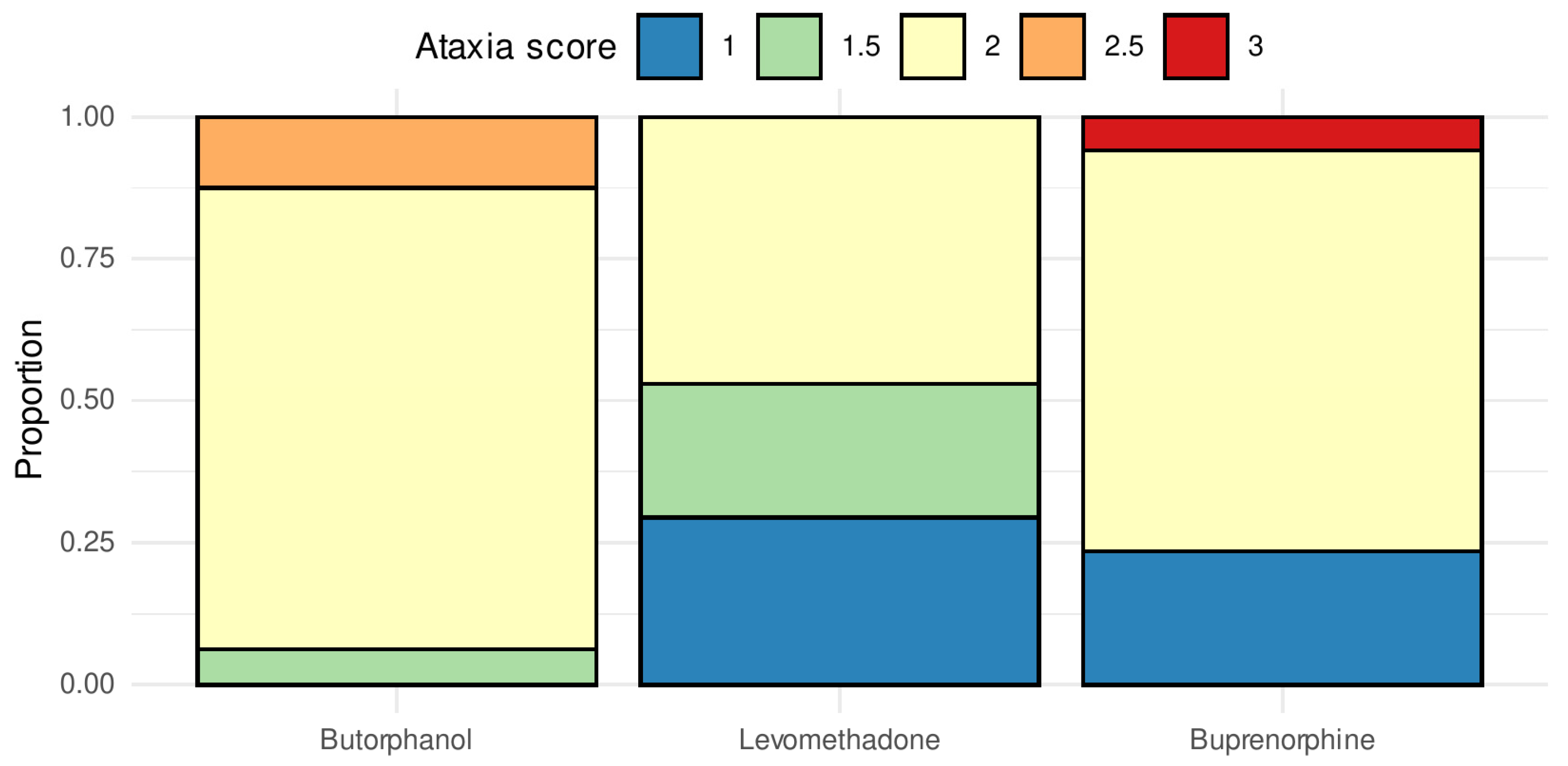
3.2. Sedation and Ataxia
3.3. Assessment of Sedation Quality, Surgical Conditions and Severity of Extraction
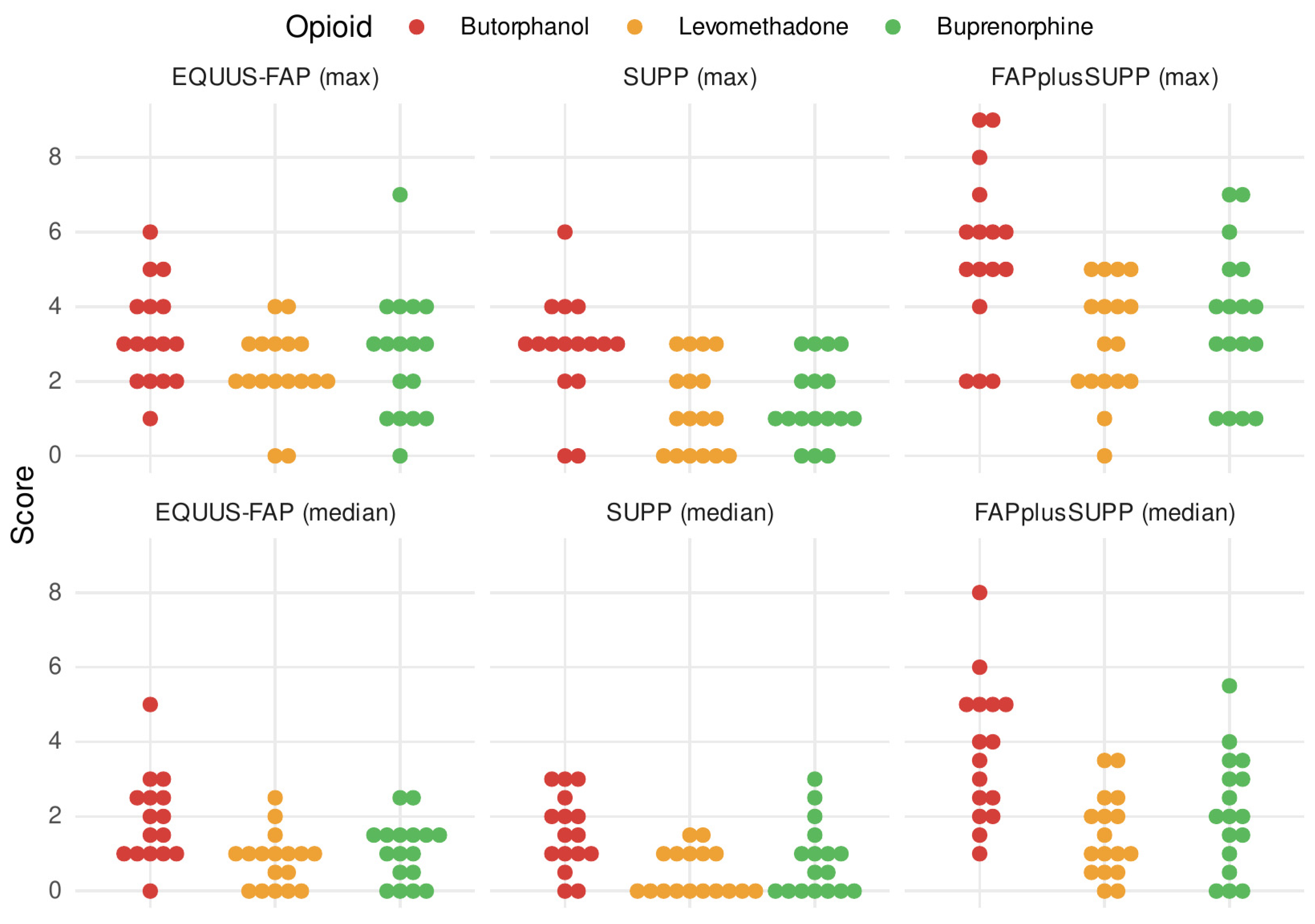
3.4. Pain Scores
3.5. Development of Pain Level over Time
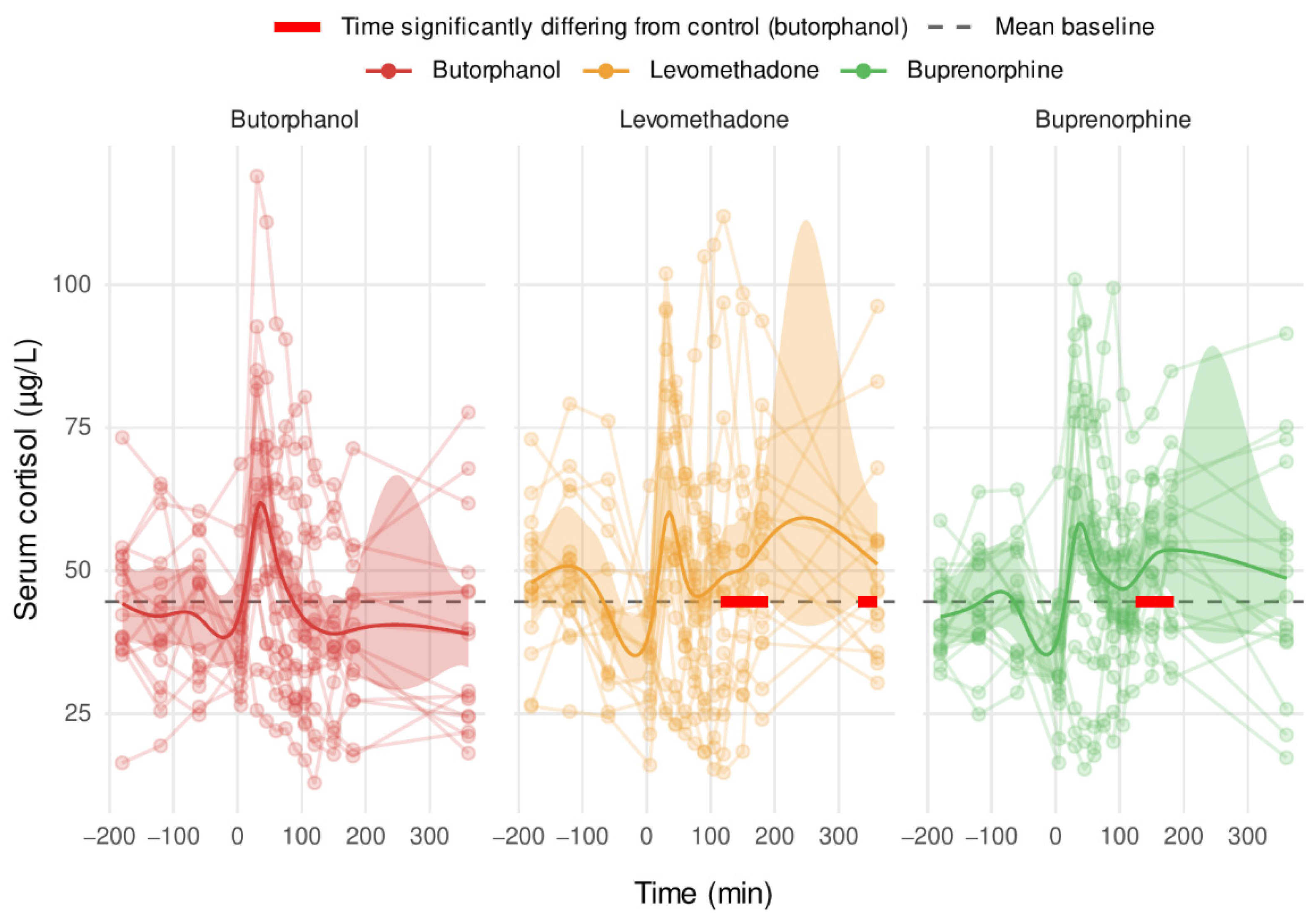
3.6. Serum Cortisol
3.7. Locomotor Activity
4. Discussion
4.1. Sedation and Ataxia
4.2. Pain Scores
4.3. Cortisol
4.4. Locomotor Activity
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- KuKanich, B.; Wiese, A. Opioids. In Veterinary Anaesthesia and Analgesia; Grimm, K.A., Lamont, L.A., Tranquilli, W.J., Greene, S.A., Robertson, S.A., Eds.; Wiley Online Library: Oxford, UK, 2015; pp. 207–222. [Google Scholar]
- Bettschart-Wolfensberger, R.; Clarke, K.W.; Vainio, O.; Aliabadi, F.S.; Demuth, D. Pharmacokinetics of medetomidine in ponies and elaboration of a medetomidine infusion regime which provides a constant level of sedation. Res. Vet. Sci. 1999, 67, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Ringer, S.K.; Portier, K.G.; Fourel, I.; Bettschart-Wolfensberger, R. Development of a xylazine constant rate infusion with or without butorphanol for standing sedation of horses. Vet. Anaesth. Analg. 2012, 39, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Gozalo-Marcilla, M.; de Oliveira, A.R.; Fonseca, M.W.; Possebon, F.S.; Pelligand, L.; Taylor, P.M.; Luna, S.P.L. Sedative and antinociceptive effects of different detomidine constant rate infusions, with or without methadone in standing horses. Equine Vet. J. 2019, 51, 530–536. [Google Scholar] [CrossRef] [PubMed]
- Gozalo-Marcilla, M.; Luna, S.P.; Gasthuys, F.; Pollaris, E.; Vlaminck, L.; Martens, A.; Haspeslagh, M.; Schauvliege, S. Clinical applicability of detomidine and methadone constant rate infusions for surgery in standing horses. Vet. Anaesth. Analg. 2019, 46, 325–334. [Google Scholar] [CrossRef]
- Haunhorst, F.R.; Hopster, K.; Schmicke, M.; Bienert-Zeit, A.; Kästner, S. Clinical effect of buprenorphine or butorphanol, in combination with detomidine and diazepam, on sedation and postoperative pain after cheek tooth extraction in horses. Can. Vet. J. La Rev. Vet. Can. 2022, 63, 39–46. [Google Scholar]
- Corletto, F.; Raisis, A.A.; Brearley, J.C. Comparison of morphine and butorphanol as pre-anaesthetic agents in combination with romifidine for field castration in ponies. Vet. Anaesth. Analg. 2005, 32, 16–22. [Google Scholar] [CrossRef]
- Clarke, K.W.; England, G.C.; Goossens, L. Sedative and cardiovascular effects of romifidine, alone and in combination with butorphanol, in the horse. J. Vet. Anaesth. 1991, 18, 25–29. [Google Scholar] [CrossRef]
- Kohn, C.W.; Muir, W.W., III. Selected Aspects of the Clinical Pharmacology of Visceral Analgesics and Gut Motility Modifying Drugs in the Horse. J. Vet. Intern. Med. 1988, 2, 85–91. [Google Scholar] [CrossRef]
- Skarda, R.T.; Muir, W.W. Comparison of electroacupuncture and butorphanol on respiratory and cardiovascular effects and rectal pain threshold after controlled rectal distention in mares. Am. J. Vet. Res. 2003, 64, 137–144. [Google Scholar] [CrossRef]
- Sanchez, L.C.; Elfenbein, J.R.; Robertson, S.A. Effect of acepromazine, butorphanol, or N-butylscopolammonium bromide on visceral and somatic nociception and duodenal Motility in conscious Horses. Am. J. Vet. Res. 2008, 69, 579–585. [Google Scholar] [CrossRef]
- Kästner, S.B.R. Zur Anwendung von Butorphanol in der Veterinärmedizin. Tierarztl. Prax. Ausg. K Kleintiere Heimtiere 2008, 36, 393–398. [Google Scholar] [CrossRef]
- Giamberardino, M.A.; Vecchiet, L. Pathophysiology of Visceral Pain. Curr. Pain Headache Rep. 1997, 1, 23–33. [Google Scholar] [CrossRef]
- Türp, J.C.; Hugger, A.; Schindler, H. Praxisnahe diagnostische Klassifikation orofazialer Schmerzen. Schweiz. Mon. Zahnmed. 2004, 115, 459–466. [Google Scholar]
- Andaluz, A.; Moll, X.; Abellán, R.; Ventura, R.; Carbó, M.; Fresno, L.; García, F. Pharmacokinetics of buprenorphine after intravenous administration of clinical doses to dogs. Vet. J. 2009, 181, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Poller, C.; Hopster, K.; Rohn, K.; Kästner, S.B.R. Nociceptive thermal threshold testing in horses—Effect of neuroleptic sedation and neuroleptanalgesia at different stimulation sites. BMC Vet. Res. 2013, 9, 135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rigotti, C.; de Vries, A.; Taylor, P.M. Paper Buprenorphine provides better anaesthetic conditions than butorphanol for field castration in ponies: Results of a randomised clinical trial. Vet. Rec. 2014, 20, 623. [Google Scholar] [CrossRef] [PubMed]
- Carregaro, A.B.; Luna, S.P.L.; Mataqueiro, M.I.; Queiroz-neto, A. De Effects of buprenorphine on nociception and spontaneous locomotor activity in horses. Am. J. Vet. Res. 2007, 68, 246–250. [Google Scholar] [CrossRef]
- MSD Animal Health GmbH. L-Polamivet® ad Us. Vet.[N], Injektionslösung. Available online: https://www.vetpharm.uzh.ch/tak/02000000/00028503.01 (accessed on 1 April 2022).
- Kristensen, K.; Christensen, C.B.; Christrup, L.L. The mu1, mu2, delta, kappa opioid receptor binding profiles of methadone stereoisomers and morphine. Life Sci. 1994, 56, 45–50. [Google Scholar] [CrossRef]
- Gorman, A.L.; Elliott, K.J.; Inturrisi, C.E. The d- and l-isomers of methadone bind to the non-competitive site on the N-methyl-D-aspartate (NMDA) receptor in rat forebrain and spinal cord. Neurosci. Lett. 1997, 223, 5–8. [Google Scholar] [CrossRef]
- Schatzmann, U.; Armbruster, S.; Stucki, F.; Busato, A.; Kohler, I. Analgesic Effect of Butorphanol and Levomethadone in Detomidine Sedated Horses. J. Vet. Med. Ser. A Physiol. Pathol. Clin. Med. 2001, 48, 337–342. [Google Scholar] [CrossRef]
- Marroum, P.J.; Webb, A.I.; Aeschbacher, G.; Curry, S.H. Pharmacokinetics and pharmacodynamics of acepromazine in horses. Am. J. Vet. Res. 1994, 55, 1428–1433. [Google Scholar] [PubMed]
- Knych, H.K.; Seminoff, K.; McKemie, D.S.; Kass, P.H. Pharmacokinetics, pharmacodynamics, and metabolism of acepromazine following intravenous, oral, and sublingual administration to exercised Thoroughbred horses. J. Vet. Pharmacol. Ther. 2018, 41, 522–535. [Google Scholar] [CrossRef] [PubMed]
- Pascoe, P.J.; Taylor, P.M. Effects of dopamine antagonists on alfentanil-induced locomotor activity in horses. Vet. Anaesth. Analg. 2003, 30, 165–171. [Google Scholar] [CrossRef] [PubMed]
- van Loon, J.P.A.M.; Van Dierendonck, M.C. Monitoring equine head-related pain with the Equine Utrecht University scale for facial assessment of pain (EQUUS-FAP). Vet. J. 2017, 220, 88–90. [Google Scholar] [CrossRef] [PubMed]
- Pehkonen, J.; Karma, L.; Raekallio, M. Behavioral Signs Associated With Equine Periapical Infection in Cheek Teeth. J. Equine Vet. Sci. 2019, 77, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Ayala, I.; Martos, N.F.; Silvan, G.; Gutierrez-Panizo, C.; Clavel, J.G.; Illera, J.C. Cortisol, adrenocorticotropic hormone, serotonin, adrenaline and noradrenaline serum concentrations in relation to disease and stress in the horse. Res. Vet. Sci. 2012, 93, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Müller, T.M.; Hopster, K.; Bienert-Zeit, A.; Rohn, K.; Kästner, S.B.R. Effect of butorphanol, midazolam or ketamine on romifidine based sedation in horses during standing cheek tooth removal. BMC Vet. Res. 2017, 13, 381. [Google Scholar] [CrossRef] [Green Version]
- Tremaine, H. Oral extraction of equine cheek teeth. Equine Vet. Educ. 2004, 16, 151–158. [Google Scholar] [CrossRef]
- van Loon, J.P.A.M.; Van Dierendonck, M.C. Monitoring acute equine visceral pain with the Equine Utrecht University Scale for Composite Pain Assessment (EQUUS-COMPASS) and the Equine Utrecht University Scale for Facial Assessment of Pain (EQUUS-FAP): A scale-construction study. Vet. J. 2015, 206, 356–364. [Google Scholar] [CrossRef]
- Team, R.C. R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Wood, S.N. Fast stable restricted maximum likelihood and marginal likelihood estimation of semiparametric generalized linear models. J. R. Stat. Soc. 2011, 73, 3–36. [Google Scholar] [CrossRef] [Green Version]
- Wood, S.N.; Pia, N.; Saefken, B. Smoothing parameter and model selection for general smooth models (with discussion). ournal Am. Stat. Assoc. 2016, 111, 1548–1575. [Google Scholar] [CrossRef]
- de Grauw, J.; van Loon, T. Clinical effects of two doses of butorphanol with detomidine for intravenous premedication of healthy warmblood horses. Vet. Anaesth. Analg. 2020, 47, 681–685. [Google Scholar] [CrossRef] [PubMed]
- Kruljc, P.; Nemec, A. Electroencephalographic and electromyographic changes during the use of detomidine and detomidine-butorphanol combination in standing horses. Acta Vet. Hung. 2006, 54, 35–42. [Google Scholar] [CrossRef]
- Taylor, P.; Coumbe, K.; Henson, F.; Scott, D.; Taylor, A. Evaluation of sedation for standing clinical procedures in horses using detomidine combined with buprenorphine. Vet. Anaesth. Analg. 2014, 41, 14–24. [Google Scholar] [CrossRef] [Green Version]
- Studer, N.; Diez Bernai, S.; Thormann, W.; Levionnois, O.; Spadavecchia, C. The anti-nociceptive effects of levomethadone in standing horses sedated with romifidine. Vet. Anaesth. 2020, 48, 104743. [Google Scholar] [CrossRef]
- Dönselmann Im Sande, P.; Hopster, K.; Kästner, S. Einfluss von Morphin, Butorphanol und Levomethadon in unterschiedlicher Dosierung auf den thermischen nozizeptiven Schwellenwert bei Pferden. Tierärztliche Prax. Ausg. G Grosstiere Nutztiere 2017, 45, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Hopster, K. Analgesie bei viszeralen Schmerzen—Was hilft wann und wie lange? Pferde Spieg. 2017, 20, 107–112. [Google Scholar] [CrossRef]
- Löscher, W.; Ungemach, F.R.; Kroker, R. Pharmakotherapie bei Haus-Und Nutztieren; Löscher, W., Ungemach, F.R., Kroker, R., Eds.; Enke: Bayern, Germany, 2014; Volume 96, p. 179. [Google Scholar]
- Price, D.D.; Mayer, D.J.; Mao, J.; Caruso, F.S. NMDA-receptor antagonists and opioid receptor interactions as related to analgesia and tolerance. J. Pain Symptom Manag. 2000, 19, 7–11. [Google Scholar] [CrossRef]
- Woolf, C.J.; Thompson, S.W.N. The induction and maintenance of central sensitization is dependent on N-methyl-d-aspartic acid receptor activation; implications for the treatment of post-injury pain hypersensitivity states. Pain 1991, 44, 293–299. [Google Scholar] [CrossRef]
- Thomson, P.J.; Rood, J.P. Preemtive analgesia reduces postoperative pain experience following oral day case surgery. Ambul. Surg. 1995, 3, 144–147. [Google Scholar] [CrossRef]
- Gottschalk, A.; Wu, C.L. New concepts in acute pain therapy. Ann. Long-Term Care 2004, 12, 18–24. [Google Scholar]
- Love, E.J.; Taylor, P.M.; Murrell, J.; Whay, H.R. Effects of acepromazine, butorphanol and buprenorphine on thermal and mechanical nociceptive thresholds in horses. Equine Vet. J. 2012, 44, 221–225. [Google Scholar] [CrossRef] [PubMed]
- van Loon, J.P.A.M.; Macri, L. Objective assessment of chronic pain in horses using the horse chronic pain scale (Hcps): A scale-construction study. Animals 2021, 11, 1826. [Google Scholar] [CrossRef] [PubMed]
- Boman, S. Plasma Cortisol Concentrations after Treatment with Methadone Alone or Together with Acepromazine or Detomidine in Horses; Dept. of Biomedical Sciences and Veterinary Public Health: Uppsala, Sweden, 2013. [Google Scholar]


| Score | 0 | 1 | 2 |
|---|---|---|---|
| Palpation of the diseased area | No reaction | Mild reaction | Refused palpation |
| Heart rate (bpm) | 24–44 | 45–52 | >52 |
| Food intake | Present | - | Not present |
| Difficulties with mastication/ quidding | no difficulties/ no quidding | - | difficulties/quidding observed |
| BUT | LEV | BUP | |
|---|---|---|---|
| Total number | 16 | 17 | 17 |
| Sex (M/F/MC *) | 0/7/9 | 0/9/8 | 1/7/9 |
| Age (y) Median [Min–Max], | 15 [10–21] | 13 [12–18] | 12 [9–18] |
| Breed (DH/P/TB/WB **) | 1/4/2/9 | 0/5/2/10 | 3/1/2/11 |
| Weight (kg) Median [Min–Max], | 555 [436–577] | 566 [453–539] | 537 [430–599] |
| Jaw [Mxl/Mxl + Mand/Mand ***] | 9/1/6 | 9/0/8 | 9/0/8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Emanuel, D.; Kästner, S.B.R.; Delarocque, J.; Grob, A.J.; Bienert-Zeit, A. Influence of Butorphanol, Buprenorphine and Levomethadone on Sedation Quality and Postoperative Analgesia in Horses Undergoing Cheek Tooth Extraction. Vet. Sci. 2022, 9, 174. https://doi.org/10.3390/vetsci9040174
Emanuel D, Kästner SBR, Delarocque J, Grob AJ, Bienert-Zeit A. Influence of Butorphanol, Buprenorphine and Levomethadone on Sedation Quality and Postoperative Analgesia in Horses Undergoing Cheek Tooth Extraction. Veterinary Sciences. 2022; 9(4):174. https://doi.org/10.3390/vetsci9040174
Chicago/Turabian StyleEmanuel, Daphna, Sabine B. R. Kästner, Julien Delarocque, Anne J. Grob, and Astrid Bienert-Zeit. 2022. "Influence of Butorphanol, Buprenorphine and Levomethadone on Sedation Quality and Postoperative Analgesia in Horses Undergoing Cheek Tooth Extraction" Veterinary Sciences 9, no. 4: 174. https://doi.org/10.3390/vetsci9040174
APA StyleEmanuel, D., Kästner, S. B. R., Delarocque, J., Grob, A. J., & Bienert-Zeit, A. (2022). Influence of Butorphanol, Buprenorphine and Levomethadone on Sedation Quality and Postoperative Analgesia in Horses Undergoing Cheek Tooth Extraction. Veterinary Sciences, 9(4), 174. https://doi.org/10.3390/vetsci9040174






