Inflammatory, Mechanical and Infectious Complications Associated with Peripheral Intravenous Catheters in Dogs and Cats: A Risk Factor Analysis
Abstract
:1. Introduction
2. Materials and Methods
Statistical Analysis
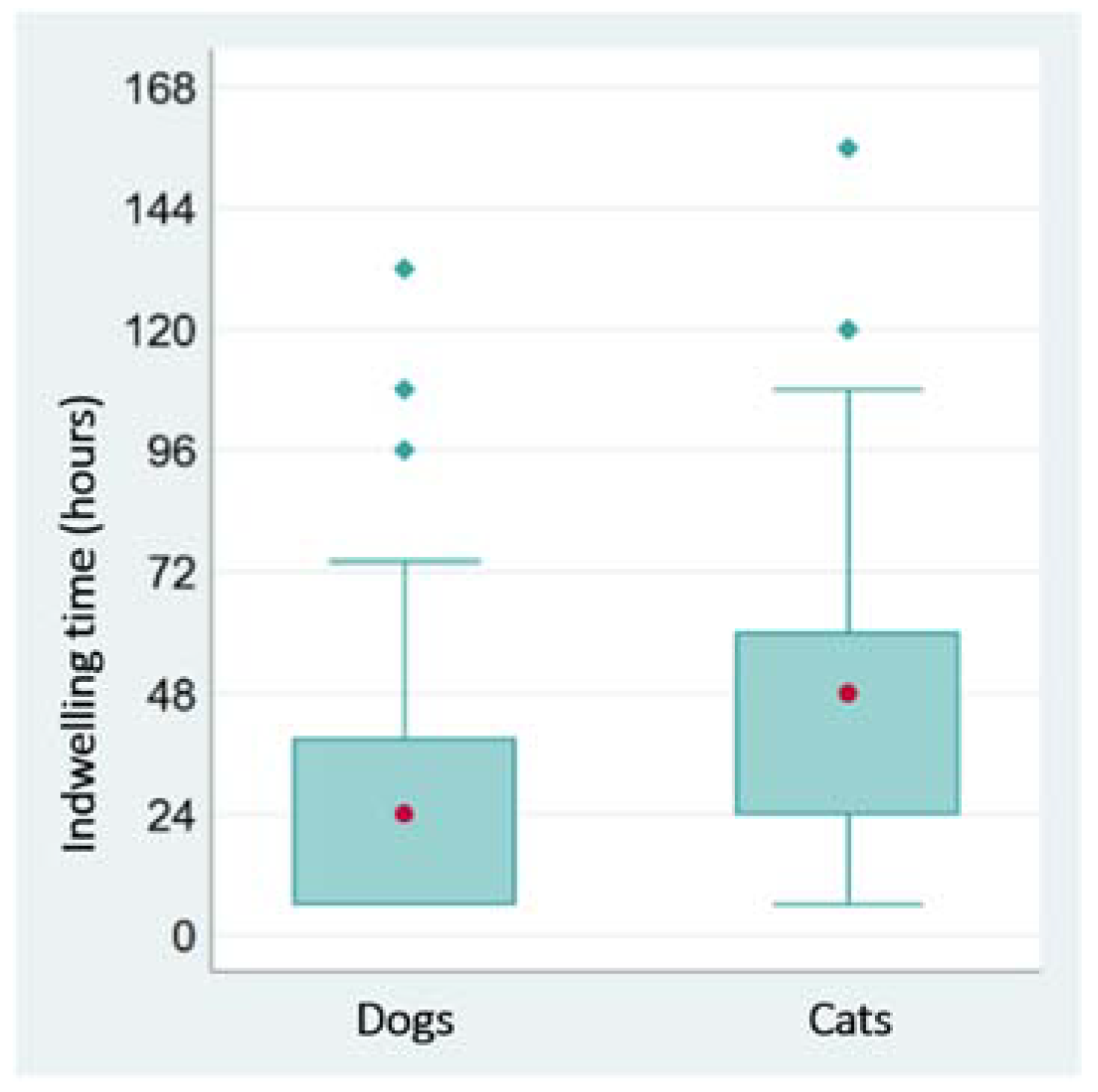
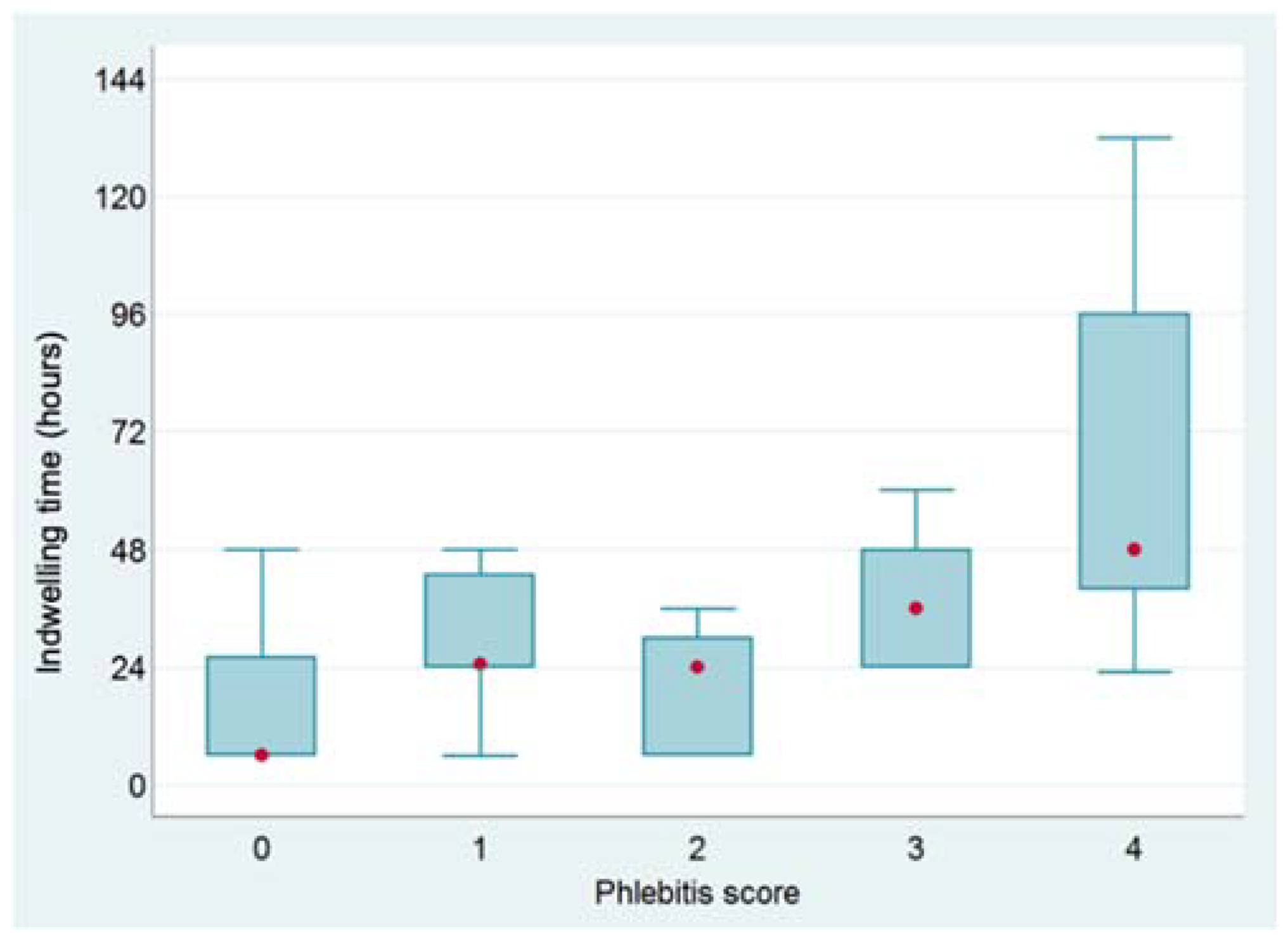
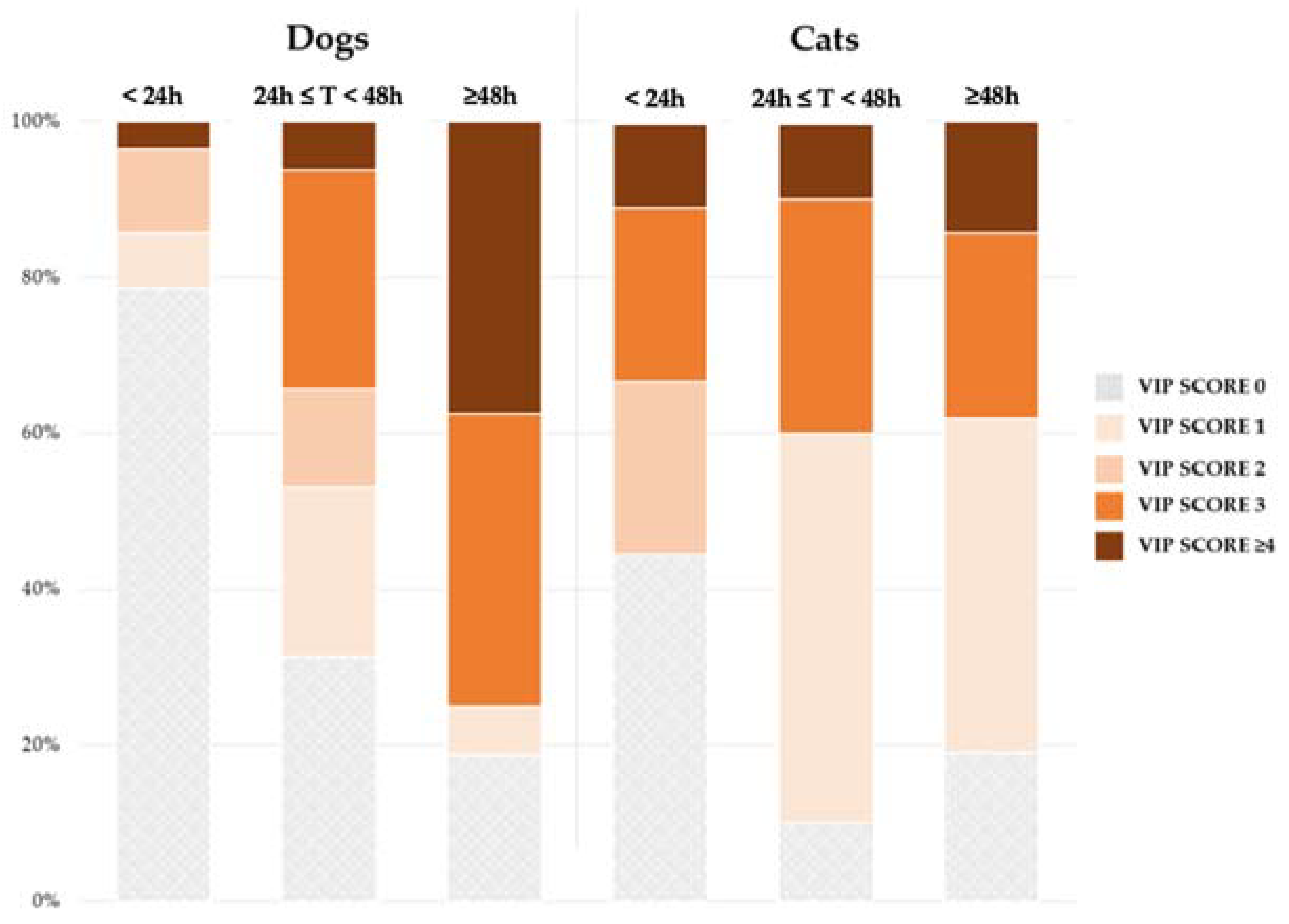
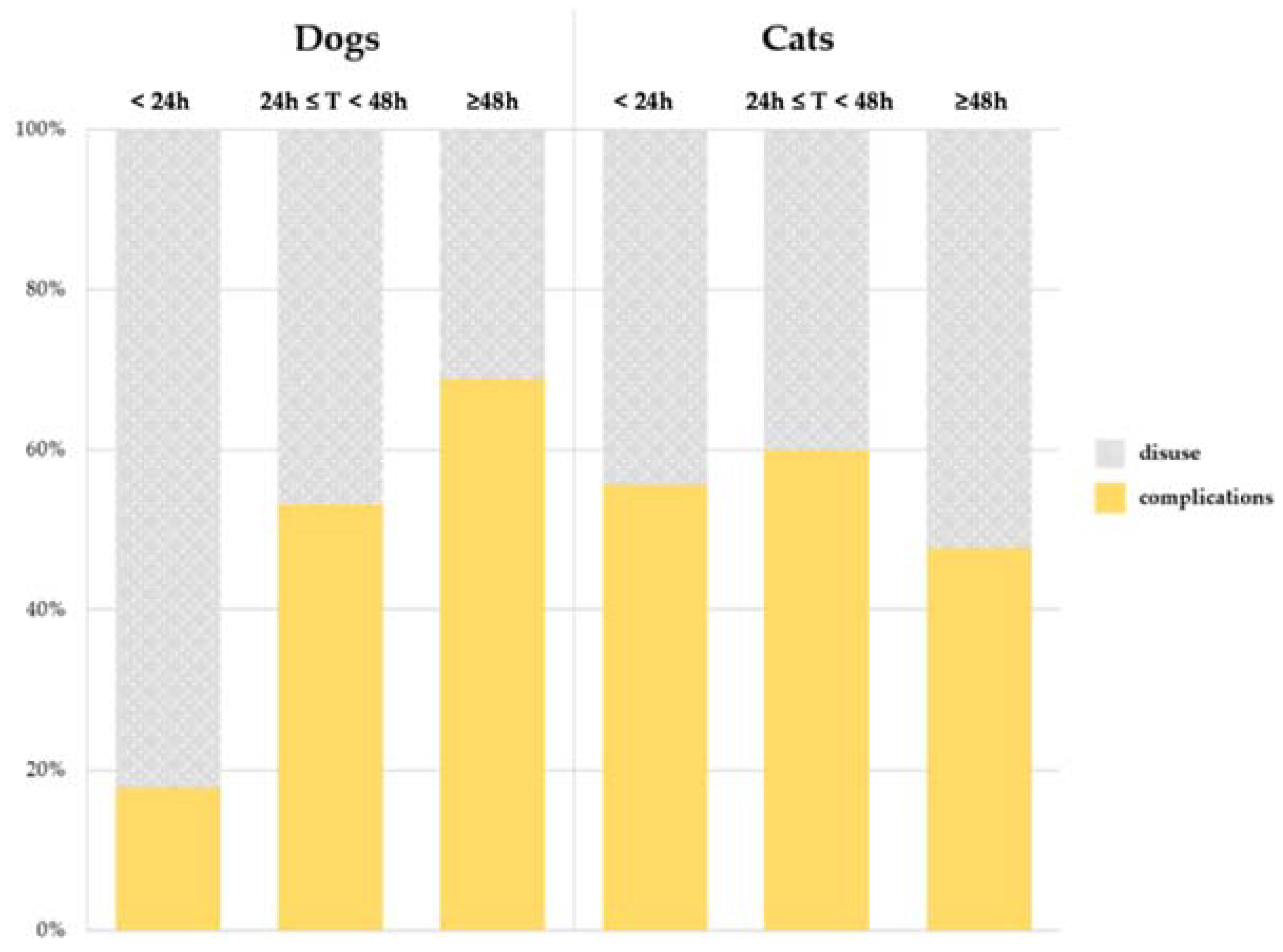
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chebroux, A.; Leece, E.A.; Brearley, J.C. Ease of intravenous catheterisation in dogs and cats: A comparative study of two peripheral catheters. J. Small Anim. Pract. 2015, 56, 242–246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adamantos, S.; Brodbelt, D.; Moores, A.L. Prospective evaluation of complications associated with jugular venous catheter use in a veterinary hospital. J. Small Anim. Pract. 2010, 51, 254–257. [Google Scholar] [CrossRef] [PubMed]
- Polderman, K.H.; Girbes, A.R. Central venous catheter use: Part 1: Mechanical complications. Intensive Care Med. 2002, 28, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Polderman, K.; Girbes, A. Central venous catheter use: Part 2: Infectious complications. Intensive Care Med. 2002, 28, 18–28. [Google Scholar] [CrossRef]
- Eggimann, P.; Pittet, D. Overview of catheter-related infections with special emphasis on prevention based on educational programs. Clin. Microbiol. Infect. 2002, 8, 295–309. [Google Scholar] [CrossRef] [Green Version]
- Alexandrou, E.; Ray-Barruel, G.; Carr, P.J.; Frost, S.A.; Inwood, S.; Higgins, N.; Lin, F.; Alberto, L.; Mermel, L.; Rickard, C.M.; et al. Use of Short Peripheral Intravenous Catheters: Characteristics, Management, and Outcomes Worldwide. J. Hosp. Med. 2018, 13. [Google Scholar] [CrossRef] [Green Version]
- O’Grady, N.P.; Alexander, M.; Burns, L.A.; Dellinger, E.P.; Garland, J.; Heard, S.O.; Lipsett, P.A.; Masur, H.; Mermel, L.A.; Pearson, M.L.; et al. Guidelines for the Prevention of Intravascular Catheter-related Infections. Clin. Infect. Dis. 2011, 52, e162–e193. [Google Scholar] [CrossRef] [Green Version]
- Rickard, C.M.; Webster, J.; Wallis, M.C.; Marsh, N.; McGrail, M.R.; French, V.; Foster, L.; Gallagher, P.; Gowardman, J.R.; Zhang, L.; et al. Routine versus clinically indicated replacement of peripheral intravenous catheters: A randomised controlled equivalence trial. Lancet 2012, 380, 1066–1074. [Google Scholar] [CrossRef] [Green Version]
- Webster, J.; Osborne, S.; Rickard, C.M.; Marsh, N. Clinically-indicated replacement versus routine replacement of peripheral venous catheters. Cochrane Database Syst. Rev. 2019, 1, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Mathews, K.A.; Brooks, M.J.; Valliant, A.E. A Prospective Study Of Intravenous Catheter Contamination. J. Vet. Emerg. Crit. Care 1996, 6, 33–43. [Google Scholar] [CrossRef]
- Lobetti, R.G.; Joubert, K.E.; Picard, J.; Carstens, J.; Pretorius, E. Bacterial colonization of intravenous catheters in young dogs suspected to have parvoviral enteritis. J. Am. Vet. Med. Assoc. 2002, 220, 1321–1324. [Google Scholar] [CrossRef] [PubMed]
- Marsh-Ng, M.L.; Burney, D.P.; Garcia, J. Surveillance of Infections Associated With Intravenous Catheters in Dogs and Cats in an Intensive Care Unit. J. Am. Anim. Hosp. Assoc. 2007, 43, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Jones, I.D.; Case, A.M.; Stevens, K.B.; Boag, A.; Rycroft, A.N. Factors contributing to the contamination of peripheral intravenous catheters in dogs and cats. Vet. Rec. 2009, 164, 616–618. [Google Scholar] [CrossRef] [PubMed]
- Guzmán Ramos, P.J.; Fernández Pérez, C.; Ayllón Santiago, T.; Baquero Artigao, M.R.; Ortiz-Díez, G. Incidence of and associated factors for bacterial colonization of intravenous catheters removed from dogs in response to clinical complications. J. Vet. Intern. Med. 2018, 32, 1084–1091. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lippert, A.C.; Fulton, R.B., Jr.; Parr, A.M. The Journal of the American Animal Hospital Association (USA). Nosocom. Infect. Surveill. Small Anim. Intensive Care Unit 1988, 24, 627–636. [Google Scholar]
- Scott Weese, J. Antimicrobial resistance in companion animals. Anim. Health Res. Rev. 2008, 9, 169–176. [Google Scholar] [CrossRef]
- Trissel, L.A. Handbook on Injectable Drugs, 11th ed.; American Society of Health-System Pharmacists: Bethesda, MD, USA, 2001; Volume 1, ISBN 1-58528-016-X. [Google Scholar]
- Gorski, L.A. The 2016 Infusion Therapy Standards of Practice. Home Healthc. Now 2017, 35, 10–18. [Google Scholar] [CrossRef]
- Gorski, L.A.; Stranz, M.; Cook, L.S.; Joseph, J.M.; Kokotis, K.; Sabatino-Holmes, P.; Van Gosen, L. Development of an Evidence-Based List of Noncytotoxic Vesicant Medications and Solutions. J. Infus. Nurs. 2017, 40, 26–40. [Google Scholar] [CrossRef]
- Keller, S.C.; Dzintars, K.; Gorski, L.A.; Williams, D.; Cosgrove, S.E. Antimicrobial Agents and Catheter Complications in Outpatient Parenteral Antimicrobial Therapy. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2018, 38, 476–481. [Google Scholar] [CrossRef]
- Jackson, A. Infection control--a battle in vein: Infusion phlebitis. Nurs. Times 1998, 94, 68–71. [Google Scholar]
- Vigier, J.P.; Merckx, J.; Coquin, J.Y.; Flaud, P.; Guiffant, G. The use of a hydrodynamic bench for experimental simulation of flushing venous catheters: Impact on the technique. Itbm-Rbm 2005, 26, 147–149. [Google Scholar] [CrossRef]
- Magiorakos, A.-P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milton, A.A.P. Nosocomial Infections and their Surveillance in Veterinary Hospitals. Adv. Anim. Vet. Sci. 2015, 3, 1–24. [Google Scholar] [CrossRef] [Green Version]
- Suthar, N.; Roy, S.; Call, D.R.; Besser, T.E.; Davis, M.A. An Individual-Based Model of Transmission of Resistant Bacteria in a Veterinary Teaching Hospital. PLoS ONE 2014, 9, e98589. [Google Scholar] [CrossRef]
- Weese, J.S. Infection control in veterinary practice; the time is now. J. Small Anim. Pract. 2011, 52, 507–508. [Google Scholar] [CrossRef]
- Stull, J.W.; Weese, J.S. Hospital-Associated Infections in Small Animal Practice. Vet. Clin. N. Am. Small Anim. Pract. 2015, 45, 217–233. [Google Scholar] [CrossRef]
- Bush, K.; Odunayo, A.; Hedges, K.; Guieu, L.-V.; Smith, R.; Okafor, C. Peripheral Intravenous Catheter Complications in Hospitalized Cats: An Observational Pilot Study. Top. Companion Anim. Med. 2020, 41, 100456. [Google Scholar] [CrossRef]
- Seguela, J.; Pages, J.-P. Bacterial and fungal colonisation of peripheral intravenous catheters in dogs and cats. J. Small Anim. Pract. 2011, 52, 531–535. [Google Scholar] [CrossRef]
- Pasalioglu, K.B.; Kaya, H. Catheter indwell time and phlebitis development during peripheral intravenous catheter administration. Pak. J. Med. Sci. 2014, 30, 725–730. [Google Scholar] [CrossRef]
- Affolter, V.K.; Moore, P.F. Histologie features of normal canine and feline skin. Clin. Dermatol. 1994, 12, 491–497. [Google Scholar] [CrossRef]
- Lloyd, J. Minimising Stress for Patients in the Veterinary Hospital: Why It Is Important and What Can Be Done about It. Vet. Sci. 2017, 4, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carney, H.C.; Little, S.; Brownlee-Tomasso, D.; Harvey, A.M.; Mattox, E.; Robertson, S.; Rucinsky, R.; Manley, D.S. AAFP and ISFM Feline-Friendly Nursing Care Guidelines. J. Feline Med. Surg. 2012, 14, 337–349. [Google Scholar] [CrossRef] [PubMed]
- Crisi, P.E.; De Santis, F.; Giordano, M.V.; Cerasoli, I.; Colucci, F.; Di Tommaso, M.; Luciani, A. Evaluation of eutectic lidocaine/prilocaine cream for jugular blood sampling in cats. J. Feline Med. Surg. 2021, 23, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Loveday, H.P.; Wilson, J.A.; Pratt, R.J.; Golsorkhi, M.; Tingle, A.; Bak, A.; Browne, J.; Prieto, J.; Wilcox, M. epic3: National Evidence-Based Guidelines for Preventing Healthcare-Associated Infections in NHS Hospitals in England. J. Hosp. Infect. 2014, 86, S1–S70. [Google Scholar] [CrossRef]
- Zhang, L.; Cao, S.; Marsh, N.; Ray-Barruel, G.; Flynn, J.; Larsen, E.; Rickard, C.M. Infection risks associated with peripheral vascular catheters. J. Infect. Prev. 2016, 17, 207–213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leonard, F.C.; Abbott, Y.; Rossney, A.; Quinn, P.J.; O’Mahony, R.; Markey, B.K. Methicillin-resistant Staphylococcus aureus isolated from a veterinary surgeon and five dogs in one practice. Vet. Rec. 2006, 158, 155–159. [Google Scholar] [CrossRef] [PubMed]
| Species/Sex | Number of Subjects | Age (months) | Bodyweight (kg) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Total | Un-Neutered | Neutered | Median | Min | Max | Median | Min | Max | |
| Dogs | 76 | 63 | 13 | 60 | 2 | 144 | 14 | 1.5 | 55 |
| Males | 53 | 46 | 7 | 58 | 2 | 144 | 14.3 | 2 | 55 |
| Females | 23 | 17 | 6 | 66 | 3 | 144 | 13.6 | 1.5 | 25 |
| Cats | 40 | 29 | 11 | 54 | 4 | 144 | 3 | 1.5 | 5.5 |
| Males | 28 | 22 | 6 | 56 | 6 | 144 | 3.7 | 1.5 | 5.5 |
| Females | 12 | 7 | 5 | 49 | 4 | 132 | 2.9 | 1.9 | 4 |
| Variables | Dogs | Cats | |||
|---|---|---|---|---|---|
| N | % | N | % | ||
| Site of insertion | Cephalic vein | 71 | 93% | 36 | 90% |
| Saphenous vein | 5 | 7% | 4 | 10% | |
| Sedation | No | 55 | 72% | 28 | 70% |
| Yes | 21 | 28% | 12 | 30% | |
| Previous placement of PIVC at the same site | No | 57 | 75% | 28 | 70% |
| Yes | 19 | 25% | 12 | 30% | |
| Size of the device | 26 Gauge | 6 | 8% | 2 | 5% |
| 24 Gauge | 4 | 5% | 12 | 30% | |
| 22 Gauge | 38 | 50% | 23 | 58% | |
| 20 Gauge | 28 | 37% | 3 | 8% | |
| Number of attempts | 1 | 67 | 88% | 31 | 78% |
| 2 | 6 | 8% | 4 | 10% | |
| 3 | 3 | 4% | 5 | 13% | |
| Difficulty | Easy | 69 | 91% | 31 | 78% |
| Difficult | 7 | 9% | 9 | 23% | |
| Reaction | No | 61 | 80% | 31 | 78% |
| Mild | 9 | 12% | 7 | 18% | |
| Moderate | 6 | 8% | 1 | 3% | |
| Strong | - | 0% | 1 | 3% | |
| Site quality | Good | 69 | 91% | 35 | 88% |
| Poor | 7 | 9% | 5 | 13% | |
| Vesicants/irritants | No | 37 | 49% | 13 | 33% |
| Yes | 39 | 51% | 27 | 68% | |
| Antibiotics | No | 53 | 70% | 19 | 48% |
| Yes | 23 | 30% | 21 | 53% | |
| Classes | Molecules | Dogs (n = 23) | Cats (n = 21) |
|---|---|---|---|
| Penicillins | Ampicillin | 3 | 1 |
| Potentiated Penicillins | Ampicillin-sulbactam | 3 | 10 |
| Potentiated Penicillins + Fluoroquinolones | Ampicillin-sulbactam + Enrofloxacin | 1 | 1 |
| Potentiated Penicillins + Nitromidazoles | Ampicillin-sulbactam + Metronidazole | 3 | - |
| 1st Generation Cephalosporins | Cefazolin | 5 | 5 |
| 3rd Generation Cephalosporins | Ceftazidime | 5 | 1 |
| Fluoroquinolones | Enrofloxacin | 3 | 2 |
| Nitromidazoles | Metronidazole | - | 1 |
| Variables | Modalities | Dogs | Cats | ||
|---|---|---|---|---|---|
| N | % | N | % | ||
| Visual Infusion Phlebitis scale | Score 0 | 35 | 46% | 9 | 23% |
| Score 1 | 10 | 13% | 14 | 35% | |
| Score 2 | 7 | 9% | 2 | 5% | |
| Score 3 | 15 | 20% | 10 | 25% | |
| Score 4 | 9 | 12% | 5 | 13% | |
| Score 5 | - | - | - | - | |
| Onset of complications | No | 43 | 57% | 19 | 48% |
| Yes | 33 | 43% | 21 | 52% | |
| Complication/reason for removal | Disuse * | 43 | 57% | 19 | 48% |
| Mechanic | 9 | 12% | 6 | 15% | |
| Inflammatory | 7 | 9% | 5 | 12% | |
| Mixed | 17 | 22% | 10 | 25% | |
| Bacterial culture | Negative | 60 | 79% | 32 | 80% |
| Positive | 16 | 21% | 8 | 20% | |
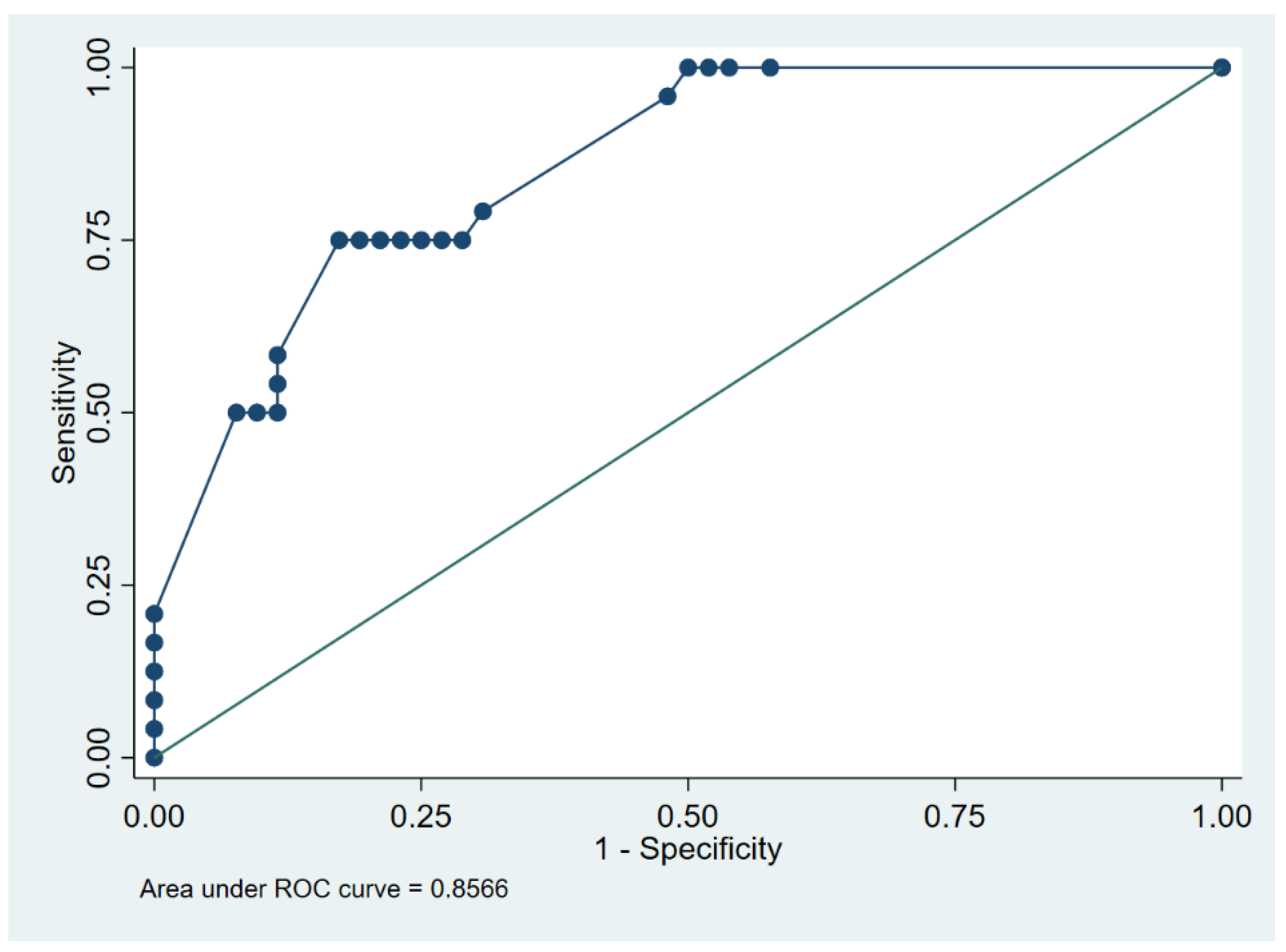
| Cut-Point (h) | Sensitivity | Specificity | Classified | Youden’s J |
|---|---|---|---|---|
| (≥6) | 100% | 0% | 31.6% | 0.00 |
| (≥12) | 100% | 42.3% | 60.5% | 0.42 |
| (≥13) | 100% | 46.2% | 63.2% | 0.46 |
| (≥20) | 100% | 48.1% | 64.5% | 0.48 |
| (≥23) | 100% | 50.0% | 65.8% | 0.50 |
| (≥24) | 95.8% | 51.9% | 65.8% | 0.48 |
| (≥25) | 79.2% | 69.2% | 72.4% | 0.48 |
| (≥26) | 75.0% | 71.2% | 72.4% | 0.46 |
| (≥27) | 75.0% | 73.1% | 73.7% | 0.48 |
| (≥29) | 75.0% | 75.0% | 75.0% | 0.50 |
| (≥30) | 75.0% | 76.9% | 76.3% | 0.52 |
| (≥32) | 75.0% | 78.9% | 77.6% | 0.54 |
| (≥35) | 75.0% | 80.8% | 79.0% | 0.56 |
| (≥36) | 75.0% | 82.7% | 80.3% | 0.58 |
| (≥38) | 58.3% | 88.5% | 79.0% | 0.47 |
| (≥40) | 54.2% | 88.5% | 77.6% | 0.43 |
| (≥43) | 50.0% | 88.5% | 76.3% | 0.38 |
| (≥47) | 50.0% | 90.4% | 77.6% | 0.40 |
| (≥48) | 50.0% | 92.3% | 79.0% | 0.42 |
| (≥60) | 20.8% | 100% | 75.0% | 0.21 |
| (≥74) | 16.7% | 100% | 73.7% | 0.17 |
| (≥96) | 12.5% | 100% | 72.4% | 0.13 |
| (≥108) | 8.3% | 100% | 71.1% | 0.08 |
| (≥132) | 4.2% | 100% | 69.7% | 0.04 |
| (>132) | 0% | 100% | 68.4% | 0.00 |
| Isolate | Species | PEN | AMI | MAC | CEF-1 | CEF-2 | CEF-3 | CEF-4 | FLUO | LINC | SXT | CARB | Tot. Classes R |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Escherichia coli | D | R | R | S | - | R | R | S | R | - | S | - | 5 |
| Escherichia coli | D | R | R | S | - | R | R | - | R | - | S | R | 6 |
| Enterobacter aerogenes | C | R | S | S | - | R | S | S | S | - | S | R | 3 |
| Enterobacter aerogenes | C | R | S | S | - | R | S | S | S | - | S | R | 3 |
| Enterobacter aerogenes | C | R | S | S | - | R | S | S | S | - | S | R | 3 |
| Plesiomonas shigelloides | D | R | S | R | R | R | S | R | S | - | S | - | 5 |
| Pseudomonas aeruginosa | D | - | S | - | - | - | R | R | S | - | - | R | 3 |
| Pseudomonas aeruginosa | D | - | R | - | - | - | S | S | R | - | - | R | 3 |
| Serratia marcescens | D | S | R | S | R | S | S | S | S | - | R | - | 3 |
| Staphylococcus Intermedius Group | D | R | S | S | R | R | R | R | R | R | S | - | 7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Crisi, P.E.; De Santis, F.; Aste, G.; Tiscar, P.G.; Mosca, F.; Gasparini, A.; Felici, A.; Ferroni, L.; Miglio, A.; Di Tommaso, M.; et al. Inflammatory, Mechanical and Infectious Complications Associated with Peripheral Intravenous Catheters in Dogs and Cats: A Risk Factor Analysis. Vet. Sci. 2022, 9, 118. https://doi.org/10.3390/vetsci9030118
Crisi PE, De Santis F, Aste G, Tiscar PG, Mosca F, Gasparini A, Felici A, Ferroni L, Miglio A, Di Tommaso M, et al. Inflammatory, Mechanical and Infectious Complications Associated with Peripheral Intravenous Catheters in Dogs and Cats: A Risk Factor Analysis. Veterinary Sciences. 2022; 9(3):118. https://doi.org/10.3390/vetsci9030118
Chicago/Turabian StyleCrisi, Paolo Emidio, Francesca De Santis, Giovanni Aste, Pietro Giorgio Tiscar, Francesco Mosca, Agostina Gasparini, Andrea Felici, Laura Ferroni, Arianna Miglio, Morena Di Tommaso, and et al. 2022. "Inflammatory, Mechanical and Infectious Complications Associated with Peripheral Intravenous Catheters in Dogs and Cats: A Risk Factor Analysis" Veterinary Sciences 9, no. 3: 118. https://doi.org/10.3390/vetsci9030118
APA StyleCrisi, P. E., De Santis, F., Aste, G., Tiscar, P. G., Mosca, F., Gasparini, A., Felici, A., Ferroni, L., Miglio, A., Di Tommaso, M., & Luciani, A. (2022). Inflammatory, Mechanical and Infectious Complications Associated with Peripheral Intravenous Catheters in Dogs and Cats: A Risk Factor Analysis. Veterinary Sciences, 9(3), 118. https://doi.org/10.3390/vetsci9030118







