Surveillance of Coxiella burnetii Shedding in Three Naturally Infected Dairy Goat Herds after Vaccination, Focusing on Bulk Tank Milk and Dust Swabs
Abstract
:1. Introduction
2. Materials and Methods
2.1. Herd History
2.1.1. Dairy Goat Herd A
2.1.2. Dairy Goat Herd B
2.1.3. Dairy Goat Herd C
2.2. Sample Collection
2.2.1. Blood Samples and Vaginal Swabs
2.2.2. Bulk Tank Milk and Dust Swabs
2.3. Vaccination Schedules and Breeding Management
2.4. Laboratory Analysis
2.5. Statistical Analyses
3. Results
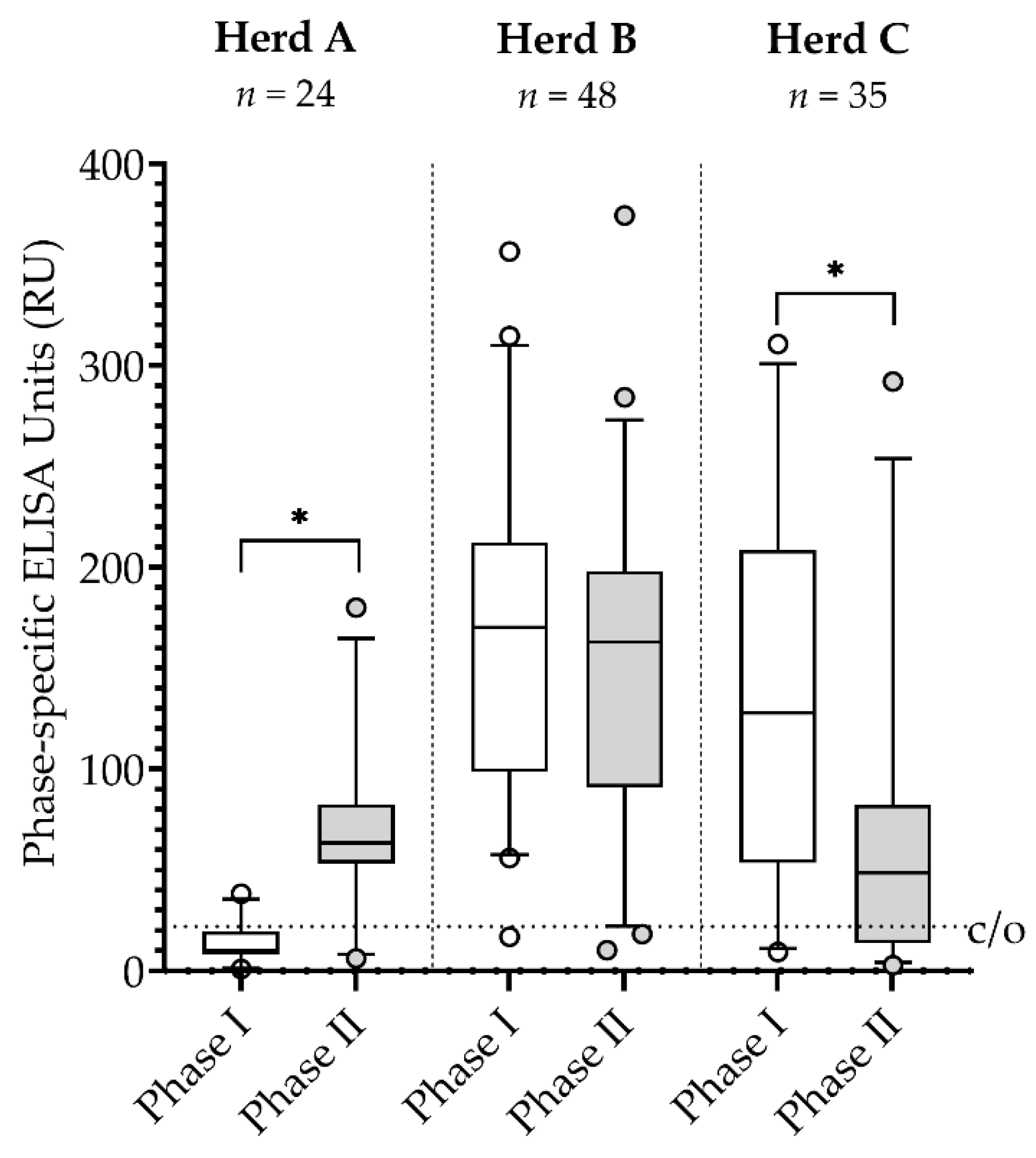
3.1. Serology
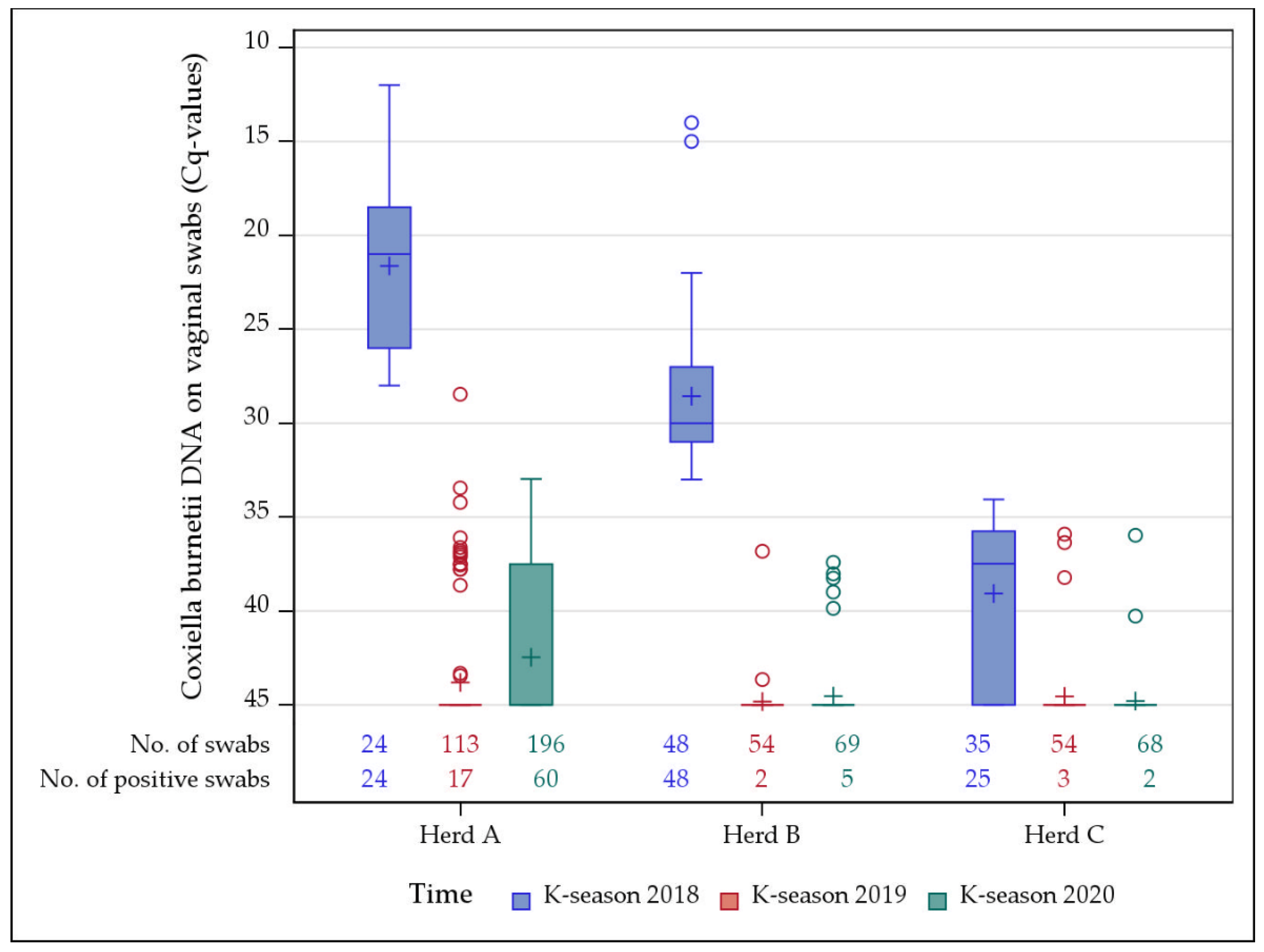
3.2. Vaginal Swabs
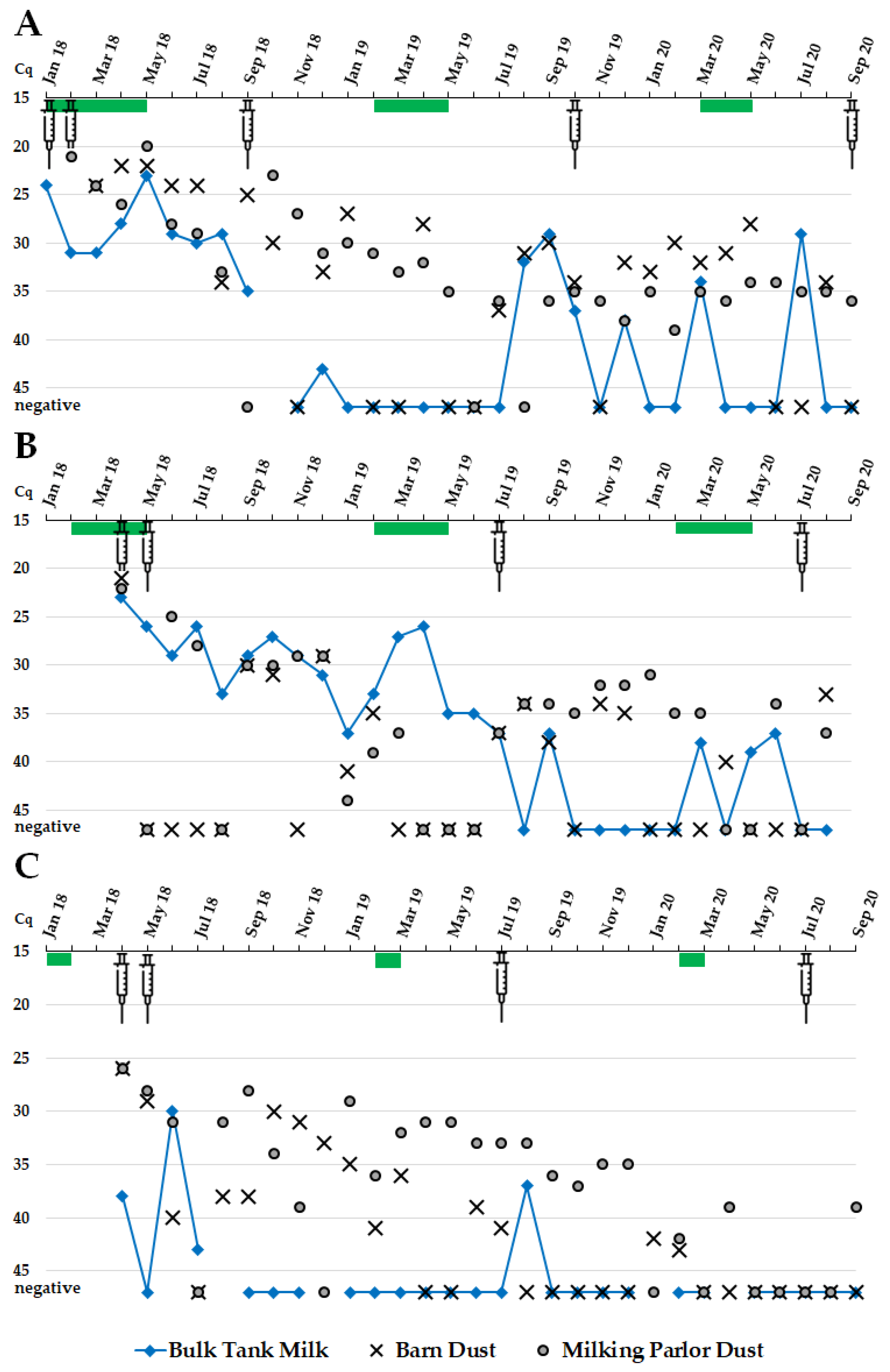
3.3. Bulk Tank Milk
3.4. Dust from Barns and Milking Parlors
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mertens, K.; Gerlach, C.; Neubauer, H.; Henning, K. Q fever—An Update. Curr. Clin. Microbiol. Rep. 2017, 4, 61–70. [Google Scholar] [CrossRef]
- Bauer, B.U.; Runge, M.; Campe, A.; Henning, K.; Mertens-Scholz, K.; Boden, K.; Sobotta, K.; Frangoulidis, D.; Knittler, M.R.; Matthiesen, S.; et al. Coxiella burnetii: A review focusing on infections in German sheep and goat flocks. Berl. Munch. Tierarztl. Wochenschr. 2020, 133, 184–200. [Google Scholar] [CrossRef]
- Bauer, B.; Prüfer, L.; Walter, M.; Ganter, I.; Frangoulidis, D.; Runge, M.; Ganter, M. Comparison of Coxiella burnetii excretion between sheep and goats naturally infected with one cattle-associated genotype. Pathogens 2020, 9, 652. [Google Scholar] [CrossRef]
- Rodolakis, A.; Berri, M.; Hechard, C.; Caudron, C.; Souriau, A.; Bodier, C.; Blanchard, B.; Camuset, P.; Devillechaise, P.; Natorp, J. Comparison of Coxiella burnetii shedding in milk of dairy bovine, caprine, and ovine herds. J. Dairy Sci. 2007, 90, 5352–5360. [Google Scholar] [CrossRef]
- Palmer, N.; Kierstead, M.; Key, D.; Williams, J.; Peacock, M.; Vellend, H. Placentitis and abortion in goats and sheep in Ontario caused by Coxiella burnetii. Can. Vet. J. 1983, 24, 60–61. [Google Scholar]
- Álvarez-Alonso, R.; Basterretxea, M.; Barandika, J.F.; Hurtado, A.; Idiazabal, J.; Jado, I.; Beraza, X.; Montes, M.; Liendo, P.; García-Pérez, A.L. A Q fever outbreak with a high rate of abortions in a dairy goat farm: Coxiella burnetii shedding, environmental contamination and viability. Appl. Environ. Microbiol. 2018, 84, e01650-18. [Google Scholar] [CrossRef] [Green Version]
- Agerholm, J.S. Coxiella burnetii associated reproductive disorders in domestic animals—A critical review. Acta Vet. Scand. 2013, 55, 13. [Google Scholar] [CrossRef] [Green Version]
- van den Brom, R.; Vellema, P. Q fever outbreaks in small ruminants and people in the Netherlands. Small Rumin. Res. 2009, 86, 74–79. [Google Scholar] [CrossRef]
- Brooke, R.J.; Kretzschmar, M.E.; Mutters, N.T.; Teunis, P.F. Human dose response relation for airborne exposure to Coxiella burnetii. BMC Infect. Dis. 2013, 13, 488. [Google Scholar] [CrossRef] [Green Version]
- Reedijk, M.; Van Leuken, J.P.G.; Van Der Hoek, W. Particulate matter strongly associated with human Q fever in The Netherlands: An ecological study. Epidemiol. Infect. 2013, 141, 2623–2633. [Google Scholar] [CrossRef] [Green Version]
- van Roeden, S.E.; Holsboer, E.W.; Oosterheert, J.J.; van Kats, J.P.; van Beckhoven, J.; Hogema, B.M.; van Wijk, M.J. Seroprevalence of Coxiella burnetii antibodies and chronic Q fever among post-mortal and living donors of tissues and cells from 2010 to 2015 in The Netherlands. Eurosurveillance 2018, 23, 17–00384. [Google Scholar] [CrossRef] [Green Version]
- Porten, K.; Rissland, J.; Tigges, A.; Broll, S.; Hopp, W.; Lunemann, M.; van Treeck, U.; Kimmig, P.; Brockmann, S.O.; Wagner-Wiening, C.; et al. A super-spreading ewe infects hundreds with Q fever at a farmers’ market in Germany. BMC Infect. Dis. 2006, 6, 147. [Google Scholar] [CrossRef] [Green Version]
- Signs, K.A.; Stobierski, M.G.; Gandhi, T.N. Q fever cluster among raw milk drinkers in Michigan, 2011. Clin. Infect. Dis. 2012, 55, 1387–1389. [Google Scholar] [CrossRef] [Green Version]
- Raoult, D.; Marrie, T.J.; Mege, J.L. Natural history and pathophysiology of Q fever. Lancet Infect. Dis. 2005, 5, 219–226. [Google Scholar] [CrossRef]
- Morroy, G.; Keijmel, S.P.; Delsing, C.E.; Bleijenberg, G.; Langendam, M.; Timen, A.; Bleeker-Rovers, C.P. Fatigue following acute Q-fever: A systematic literature review. PLoS ONE 2016, 11, e0155884. [Google Scholar] [CrossRef] [Green Version]
- Angelakis, E.; Raoult, D. Q fever. Vet. Micorbiol. 2010, 140, 297–309. [Google Scholar] [CrossRef] [Green Version]
- Georgiev, M.; Alfonso, A.; Neubauer, H.; Needham, H.; Thiéry, R.; Rodolakis, A.; Roest, H.I.J.; Stärk, K.D.; Stegeman, J.A.; Vellema, P.; et al. Q fever in humans and farm animals in four European countries, 1982 to 2010. Eurosurveillance 2013, 18, 20407. [Google Scholar] [CrossRef]
- Anderson, A.D.; Kruszon-Moran, D.; Loftis, A.D.; McQuillan, G.; Nicholson, W.L.; Priestley, R.A.; Candee, A.J.; Patterson, N.E.; Massung, R.F. Seroprevalence of Q fever in the United States, 2003–2004. Am. J. Trop. Med. Hyg. 2009, 81, 691–694. [Google Scholar] [CrossRef]
- Tozer, S.; Lambert, S.; Sloots, T.; Nissen, M. Q fever seroprevalence in metropolitan samples is similar to rural/remote samples in Queensland, Australia. Eur. J. Clin. Microbiol. Infect. Dis. 2011, 30, 1287. [Google Scholar] [CrossRef]
- Stoker, M.G.P.; Fiset, P. Phase variation of the Nine Mile and other strains of Rickettsia Burneti. Can. J. Microbiol. 1956, 2, 310–321. [Google Scholar] [CrossRef]
- Williams, J.; Johnston, M.; Peacock, M.; Thomas, L.; Stewart, S.; Portis, J. Monoclonal antibodies distinguish phase variants of Coxiella burnetii. Infect. Immun. 1984, 43, 421–428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roest, H.; Post, J.; van Gelderen, B.; van Zijderveld, F.G.; Rebel, J.M. Q fever in pregnant goats: Humoral and cellular immune responses. Vet. Res. 2013, 44, 67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sting, R.; Molz, K.; Philipp, W.; Bothe, F.; Runge, M.; Ganter, M. Quantitative real-time PCR and phase specific serology are mutually supportive in Q fever diagnostics in goats. Vet. Microbiol. 2013, 167, 600–608. [Google Scholar] [CrossRef] [PubMed]
- Hatchette, T.; Campbell, N.; Hudson, R.; Raoult, D.; Marrie, T.J. Natural history of Q fever in goats. Vector Borne Zoonotic Dis. 2003, 3, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Muleme, M.; Stenos, J.; Vincent, G.; Wilks, C.R.; Devlin, J.M.; Campbell, A.; Cameron, A.; Stevenson, M.A.; Graves, S.; Firestone, S.M. Peripartum dynamics of Coxiella burnetii infections in intensively managed dairy goats associated with a Q fever outbreak in Australia. Prev. Vet. Med. 2017, 139, 58–66. [Google Scholar] [CrossRef]
- Bontje, D.; Backer, J.; Hogerwerf, L.; Roest, H.; van Roermund, H. Analysis of Q fever in Dutch dairy goat herds and assessment of control measures by means of a transmission model. Prev. Vet. Med. 2016, 123, 71–89. [Google Scholar] [CrossRef] [Green Version]
- Canevari, J.T.; Firestone, S.M.; Vincent, G.; Campbell, A.; Tan, T.; Muleme, M.; Cameron, A.W.N.; Stevenson, M.A. The prevalence of Coxiella burnetii shedding in dairy goats at the time of parturition in an endemically infected enterprise and associated milk yield losses. BMC Vet. Res. 2018, 14, 353. [Google Scholar] [CrossRef] [Green Version]
- Achard, D.; Rodolakis, A. Q fever vaccination in ruminants: A critical review. In The Principles and Practice of Q Fever; Caetano Simoes, J.C., Ferreira, A.S., de Silva, G.J., Eds.; Nova Science Publishers Inc.: New York, NY, USA, 2017; pp. 367–389. [Google Scholar]
- Arricau-Bouvery, N.; Souriau, A.; Bodier, C.; Dufour, P.; Rousset, E.; Rodolakis, A. Effect of vaccination with phase I and phase II Coxiella burnetii vaccines in pregnant goats. Vaccine 2005, 23, 4392–4402. [Google Scholar] [CrossRef]
- De Cremoux, R.; Rousset, E.; Touratier, A.; Audusseau, G.; Nicollet, P.; Ribaud, D.; David, V.; Le Pape, M. Assessment of vaccination by a phase I Coxiella burnetii-inactivated vaccine in goat herds in clinical Q fever situation. FEMS Immunol. Med. Microbiol. 2012, 64, 104–106. [Google Scholar] [CrossRef] [Green Version]
- Rousset, E.; Durand, B.; Champion, J.L.; Prigent, M.; Dufour, P.; Forfait, C.; Marois, M.; Gasnier, T.; Duquesne, V.; Thiery, R.; et al. Efficiency of a phase 1 vaccine for the reduction of vaginal Coxiella burnetii shedding in a clinically affected goat herd. Clin. Microbiol. Infect. 2009, 15 (Suppl. S2), 188–189. [Google Scholar] [CrossRef]
- Hogerwerf, L.; van den Brom, R.; Roest, H.I.J.; Bouma, A.; Vellema, P.; Pieterse, M.; Dercksen, D.P.; Nielen, M. Reduction of Coxiella burnetii prevalence by vaccination of goats and sheep, The Netherlands. Emerg. Infect. Dis. 2011, 17, 379–386. [Google Scholar] [CrossRef] [PubMed]
- van den Brom, R.; Santman-Berends, I.; Luttikholt, S.; Moll, L.; Van Engelen, E.; Vellema, P. Bulk tank milk surveillance as a measure to detect Coxiella burnetii shedding dairy goat herds in the Netherlands between 2009 and 2014. J. Dairy Sci. 2015, 98, 3814–3825. [Google Scholar] [CrossRef] [PubMed]
- van den Brom, R.; van Engelen, E.; Luttikholt, S.; Moll, L.; van Maanen, K.; Vellema, P. Coxiella burnetii in bulk tank milk samples from dairy goat and dairy sheep farms in The Netherlands in 2008. Vet. Rec. 2012, 170, 310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jansen, W.; Cargnel, M.; Boarbi, S.; Mertens, I.; Van Esbroeck, M.; Fretin, D.; Mori, M. Belgian bulk tank milk surveillance program reveals the impact of a continuous vaccination protocol for small ruminants against Coxiella burnetii. Transbound. Emerg. Dis. 2021, 1–12. [Google Scholar] [CrossRef]
- Jodełko, A.; Szymańska-Czerwińska, M.; Rola, J.G.; Niemczuk, K. Molecular detection of Coxiella burnetii in small ruminants and genotyping of specimens collected from goats in Poland. BMC Vet. Res. 2021, 17, 341. [Google Scholar] [CrossRef]
- Khalili, M.; Diali, H.G.; Mirza, H.N.; Mosavi, S.M. Detection of Coxiella burnetii by PCR in bulk tank milk samples from dairy caprine herds in southeast of Iran. Asian Pac. J. Trop. Dis. 2015, 5, 119–122. [Google Scholar] [CrossRef]
- Carrié, P.; Barry, S.; Rousset, E.; de Crémoux, R.; Sala, C.; Calavas, D.; Perrin, J.B.; Bronner, A.; Gasqui, P.; Gilot-Fromont, E.; et al. Swab cloths as a tool for revealing environmental contamination by Q fever in ruminant farms. Transbound. Emerg. Dis. 2019, 66, 1202–1209. [Google Scholar] [CrossRef]
- Zendoia, I.I.; Barandika, J.F.; Hurtado, A.; López, C.M.; Alonso, E.; Beraza, X.; Ocabo, B.; García-Pérez, A.L. Analysis of environmental dust in goat and sheep farms to assess Coxiella burnetii infection in a Q fever endemic area: Geographical distribution, relationship with human cases and genotypes. Zoonoses Public Health 2021, 68, 666–676. [Google Scholar] [CrossRef]
- Álvarez-Alonso, R.; Zendoia, I.I.; Barandika, J.F.; Jado, I.; Hurtado, A.; López, C.M.; García-Pérez, A.L. Monitoring Coxiella burnetii infection in naturally infected dairy sheep flocks throughout four lambing seasons and investigation of viable bacteria. Front. Vet. Sci. 2020, 7, 352. [Google Scholar] [CrossRef]
- Joulié, A.; Laroucau, K.; Bailly, X.; Prigent, M.; Gasqui, P.; Lepetitcolin, E.; Blanchard, B.; Rousset, E.; Sidi-Boumedine, K.; Jourdain, E. Circulation of Coxiella burnetii in a naturally infected flock of dairy sheep: Shedding dynamics, environmental contamination, and genotype diversity. Appl. Environ. Microbiol. 2015, 81, 7253–7260. [Google Scholar] [CrossRef] [Green Version]
- de Bruin, A.; de Groot, A.; de Heer, L.; Bok, J.; Hamans, M.; van Rotterdam, B.; Wielinga, P.; Janse, I. Detection of Coxiella burnetii in complex matrices by using multiplex qPCR during a major Q fever outbreak in the Netherlands. Appl. Environ. Microbiol. 2011, 77, 6516–6523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schimmer, B.; Lenferink, A.; Schneeberger, P.; Aangenend, H.; Vellema, P.; Hautvast, J.; van Duynhoven, Y. Seroprevalence and risk factors for Coxiella burnetii (Q fever) seropositivity in dairy goat farmers’ households in The Netherlands, 2009–2010. PLoS ONE 2012, 7, e42364. [Google Scholar] [CrossRef]
- Bauer, B.U.; Knittler, M.R.; Herms, T.L.; Frangoulidis, D.; Matthiesen, S.; Tappe, D.; Runge, M.; Ganter, M. Multispecies Q fever outbreak in a mixed dairy goat and cattle farm based on a new bovine-associated genotype of Coxiella burnetii. Vet. Sci. 2021, 8, 252. [Google Scholar] [CrossRef] [PubMed]
- Hogerwerf, L.; Borlée, F.; Still, K.; Heederik, D.; van Rotterdam, B.; de Bruin, A.; Nielen, M.; Wouters, I.M. Detection of Coxiella burnetii DNA in inhalable airborne dust samples from goat farms after mandatory culling. Appl. Environ. Microbiol. 2012, 78, 5410–5412. [Google Scholar] [CrossRef] [Green Version]
- Bauer, B.U.; Knittler, M.R.; Prüfer, T.L.; Wolf, A.; Matthiesen, S.; Runge, M.; Ganter, M. Humoral immune response to Q fever vaccination of three sheep flocks naturally pre-infected with Coxiella burnetii. Vaccine 2021, 39, 1499–1507. [Google Scholar] [CrossRef] [PubMed]
- Frangoulidis, D.; Walter, M.C.; Antwerpben, M.; Zimmermann, P.; Janowetz, B.; Alex, M.; Böttcher, J.; Henning, K.; Hilbert, A.; Ganter, M.; et al. Molecular analysis of Coxiella burnetii in Germany reveals evolution of unique clonal clusters. Int. J. Med. Microbiol. 2014, 304, 868–876. [Google Scholar] [CrossRef] [PubMed]
- Berri, M.; Rousset, E.; Champion, J.; Russo, P.; Rodolakis, A. Goats may experience reproductive failures and shed Coxiella burnetii at two successive parturitions after a Q fever infection. Res. Vet. Sci. 2007, 83, 47–52. [Google Scholar] [CrossRef]
- Guatteo, R.; Seegers, H.; Joly, A.; Beaudeau, F. Prevention of Coxiella burnetii shedding in infected dairy herds using a phase I C. burnetii inactivated vaccine. Vaccine 2008, 26, 4320–4328. [Google Scholar] [CrossRef]
- de Cremoux, R.; Rousset, E.; Touratier, A.; Audusseau, G.; Nicollet, P.; Ribaud, D.; David, V.; Le Pape, M. Coxiella burnetii vaginal shedding and antibody responses in dairy goat herds in a context of clinical Q fever outbreaks. FEMS Immunol. Med. Microbiol. 2012, 64, 120–122. [Google Scholar] [CrossRef] [Green Version]
- van den Brom, R.; van Engelen, E.; Vos, J.; Luttikholt, S.J.M.; Moll, L.; Roest, H.I.J.; van der Heijden, H.M.J.F.; Vellema, P. Detection of Coxiella burnetii in the bulk tank milk from a farm with vaccinated goats, by using a specific PCR technique. Small Rumin. Res. 2013, 110, 150–154. [Google Scholar] [CrossRef]
- Lucchese, L.; Capello, K.; Barberio, A.; Zuliani, F.; Stegeman, A.; Ceglie, L.; Guerrini, E.; Marangon, S.; Natale, A. IFAT and ELISA phase I/phase II as tools for the identification of Q fever chronic milk shedders in cattle. Vet. Microbiol. 2015, 179, 102–108. [Google Scholar] [CrossRef]
- Böttcher, J.; Frangoulidis, D.; Schumacher, M.; Janowetz, B.; Gangl, A.; Alex, M. The impact of Q fever-phase-specific milk serology for the diagnosis of puerperal and chronic milk shedding of C. burnetii in dairy cows. Berl. Munch. Tierarztl. Wochenschr. 2013, 126, 427–435. [Google Scholar] [CrossRef] [PubMed]
- Dubey, J.P.; Verma, S.K.; Ferreira, L.R.; Oliveira, S.; Cassinelli, A.B.; Ying, Y.; Kwok, O.C.H.; Tuo, W.; Chiesa, O.A.; Jones, J.L. Detection and survival of Toxoplasma gondii in milk and cheese from experimentally infected goats. J. Food Prot. 2014, 77, 1747–1753. [Google Scholar] [CrossRef] [PubMed]
- Addis, M.F.; Cubeddu, T.; Pilicchi, Y.; Rocca, S.; Piccinini, R. Chronic intramammary infection by Listeria monocytogenes in a clinically healthy goat—A case report. BMC Vet. Res. 2019, 15, 229. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.L.; Gonzalez-Juarrero, M.; Bowen, R.A. Evaluation of shedding, tissue burdens, and humoral immune response in goats after experimental challenge with the virulent Brucella melitensis strain 16M and the reduced virulence vaccine strain Rev. 1. PLoS ONE 2017, 12, e0185823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ilic, M.; Barbic, L.; Bogdanic, M.; Tabain, I.; Savic, V.; Kosanovic Licina, M.L.; Kaic, B.; Jungic, A.; Vucelja, M.; Angelov, V.; et al. Tick-borne encephalitis outbreak following raw goat milk consumption in a new micro-location, Croatia, June 2019. Ticks Tick Borne Dis. 2020, 11, 101513. [Google Scholar] [CrossRef]
- EFSA Panel on Biological Hazards. Scientific opinion on the public health risks related to the consumption of raw drinking milk. EFSA J. 2015, 13, 3940. [Google Scholar] [CrossRef] [Green Version]
- Hermans, M.H.; Huijsmans, C.R.; Schellekens, J.J.; Savelkoul, P.H.; Wever, P.C. Coxiella burnetii DNA in goat milk after vaccination with Coxevac®. Vaccine 2011, 29, 2653–2656. [Google Scholar] [CrossRef]
- Kersh, G.J.; Fitzpatrick, K.A.; Self, J.S.; Priestley, R.A.; Kelly, A.J.; Lash, R.R.; Marsden-Haug, N.; Nett, R.J.; Bjork, A.; Massung, R.F.; et al. Presence and persistence of Coxiella burnetii in the environment of goat farms associated with a Q fever outbreak. Appl. Environ. Microbiol. 2013, 79, 1697–1703. [Google Scholar] [CrossRef] [Green Version]
- Astobiza, I.; Barandika, J.F.; Ruiz-Fons, F.; Hurtado, A.; Povedano, I.; Juste, R.A.; García-Pérez, A.L. Coxiella burnetii shedding and environmental contamination at lambing in two highly naturally-infected dairy sheep flocks after vaccination. Res. Vet. Sci. 2011, 91, e58–e63. [Google Scholar] [CrossRef]
- Klee, S.R.; Tyczka, J.; Ellerbrok, H.; Franz, T.; Linke, S.; Baljer, G.; Appel, B. Highly sensitive real-time PCR for specific detection and quantification of Coxiella burnetii. BMC Microbiol. 2006, 6, 2. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bauer, B.U.; Schoneberg, C.; Herms, T.L.; Runge, M.; Ganter, M. Surveillance of Coxiella burnetii Shedding in Three Naturally Infected Dairy Goat Herds after Vaccination, Focusing on Bulk Tank Milk and Dust Swabs. Vet. Sci. 2022, 9, 102. https://doi.org/10.3390/vetsci9030102
Bauer BU, Schoneberg C, Herms TL, Runge M, Ganter M. Surveillance of Coxiella burnetii Shedding in Three Naturally Infected Dairy Goat Herds after Vaccination, Focusing on Bulk Tank Milk and Dust Swabs. Veterinary Sciences. 2022; 9(3):102. https://doi.org/10.3390/vetsci9030102
Chicago/Turabian StyleBauer, Benjamin U., Clara Schoneberg, T. Louise Herms, Martin Runge, and Martin Ganter. 2022. "Surveillance of Coxiella burnetii Shedding in Three Naturally Infected Dairy Goat Herds after Vaccination, Focusing on Bulk Tank Milk and Dust Swabs" Veterinary Sciences 9, no. 3: 102. https://doi.org/10.3390/vetsci9030102
APA StyleBauer, B. U., Schoneberg, C., Herms, T. L., Runge, M., & Ganter, M. (2022). Surveillance of Coxiella burnetii Shedding in Three Naturally Infected Dairy Goat Herds after Vaccination, Focusing on Bulk Tank Milk and Dust Swabs. Veterinary Sciences, 9(3), 102. https://doi.org/10.3390/vetsci9030102





