The Effect of Needle Reuse on Piglet Skin Puncture Force
Abstract
:1. Introduction
2. Materials and Methods
2.1. Survey
2.2. Laboratory Based Studies
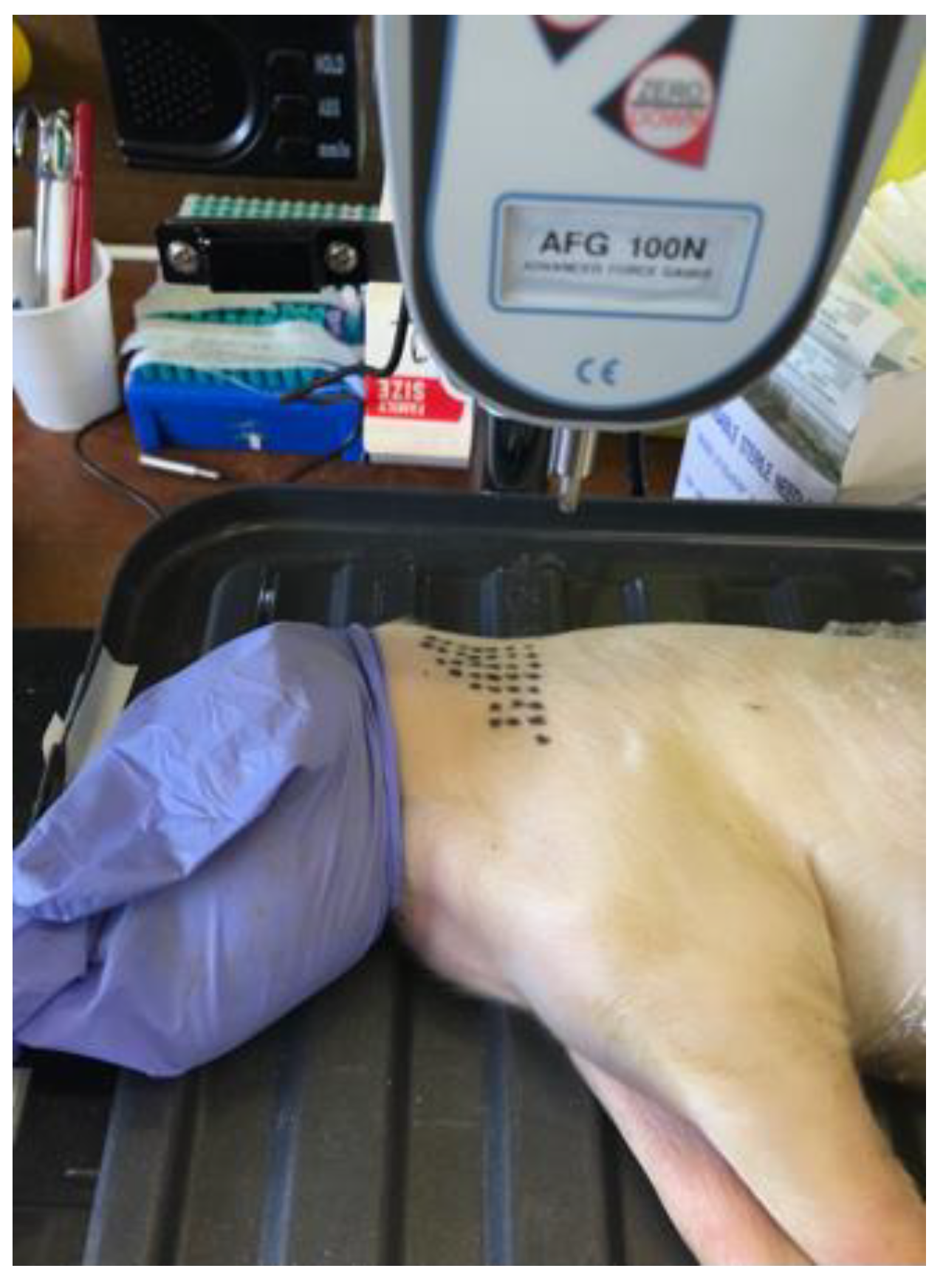
2.2.1. Piglet Cadaver Studies
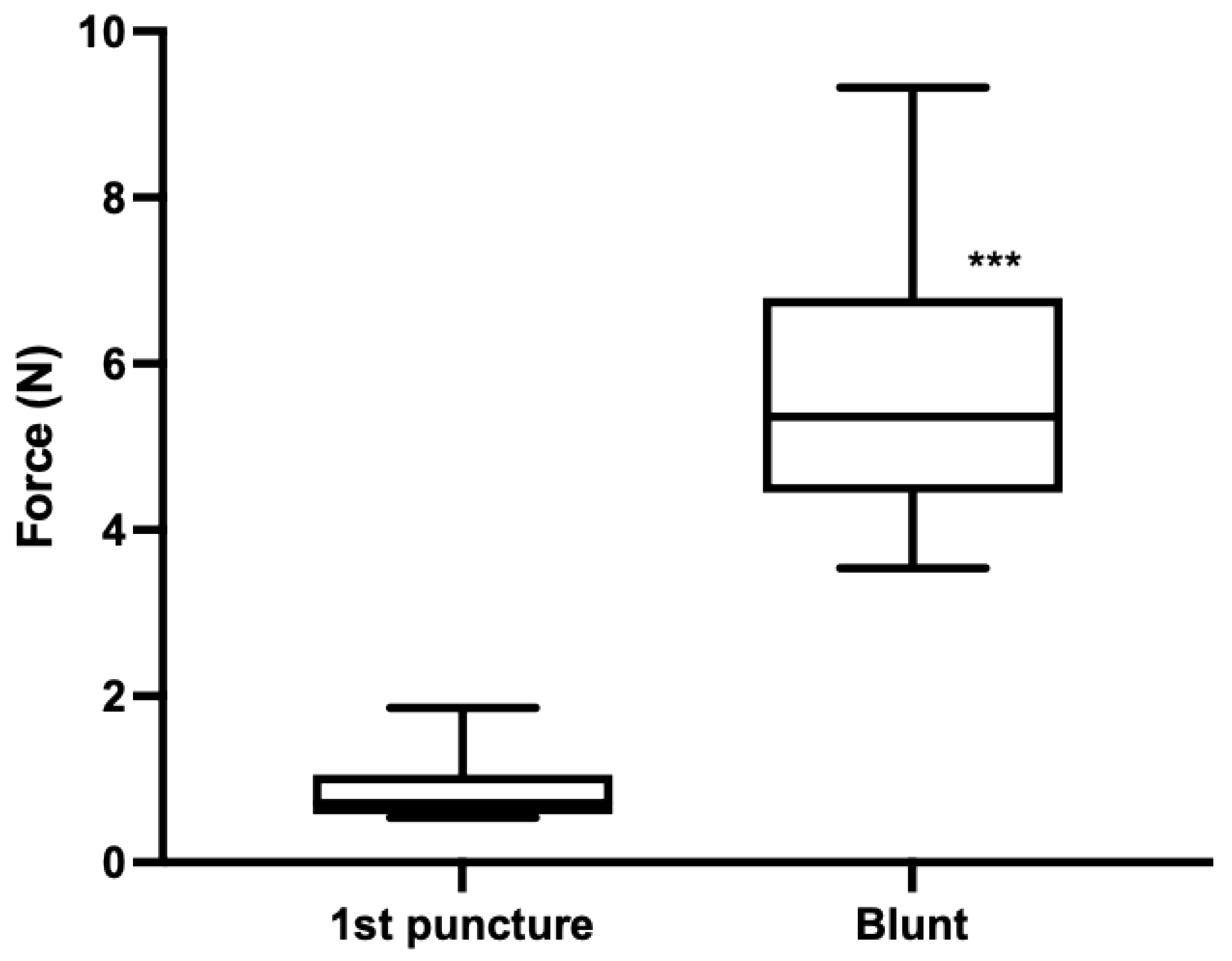
- Experiment 1: First-time use (×1) needles (n = 18) versus blunt needles (n = 17), prepared by scraping 10 times on concrete. Single punctures into the neck alternated between ×1 and blunt needles. One piglet cadaver was used.
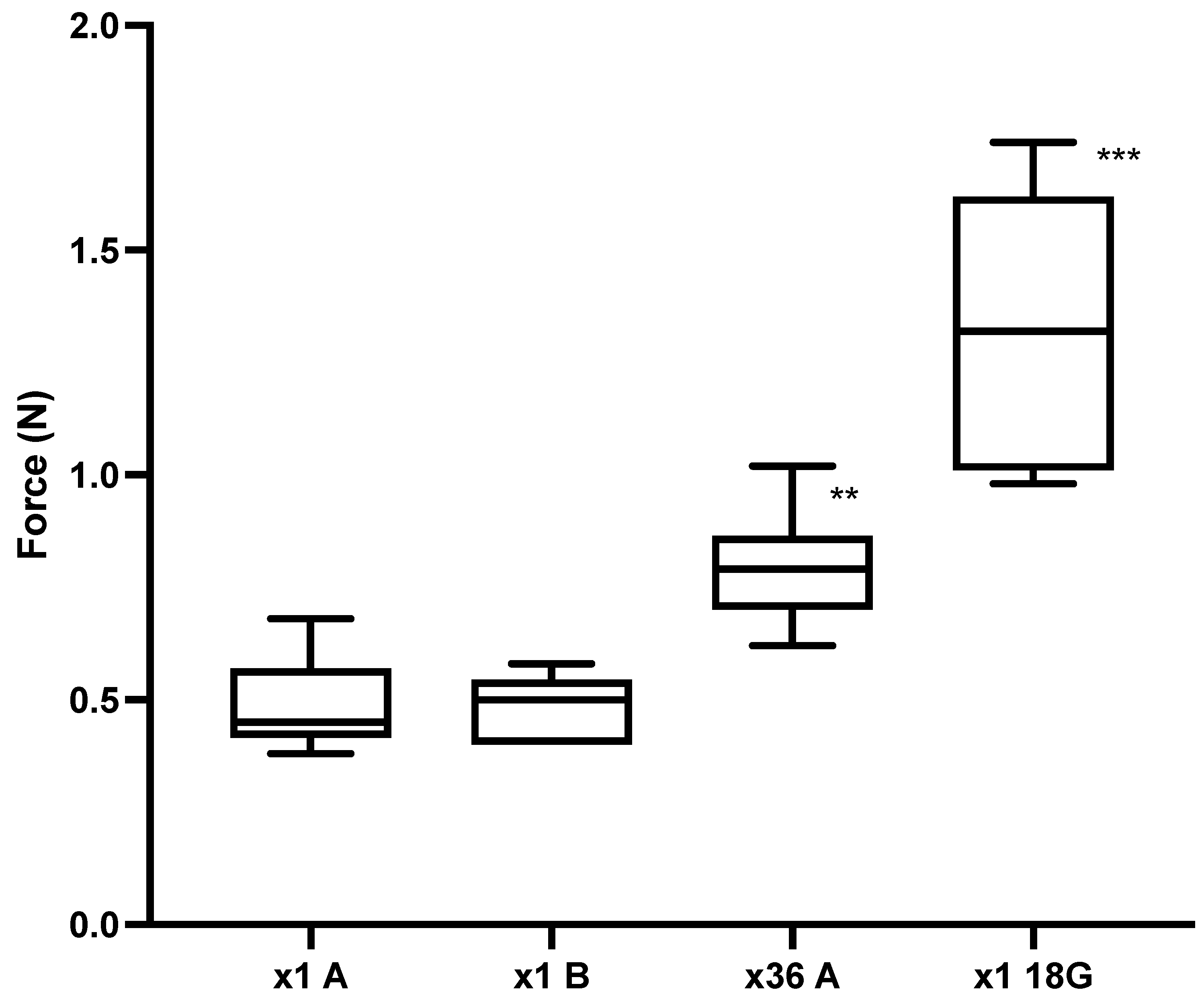
- Experiment 2: First-time use (×1) versus ×36 needle use. Puncture 1 into the neck of a piglet cadaver (n = 10) (×1A). The needles were then injected randomly 34 times across the remaining cadaver with the 36th puncture into the neck (n = 10) (×36A). To assess whether the piglet cadaver dried whilst the ×36A needles were being prepared, the puncture of the ×36A needles were alternated with a new set of needles (×1B) (n = 10). The 18 G ½” needles (n = 5) were used as a positive control (18 G) as puncture force has previously been shown to increase with needle diameter [24]. One piglet cadaver was used.
- Experiment 3: The design was the same as for Experiment 2 except that for each test the group n = 9, and the needles previously blunted by puncture into chamois leather 17 times (n = 6), were used as a positive control. One piglet cadaver was used.
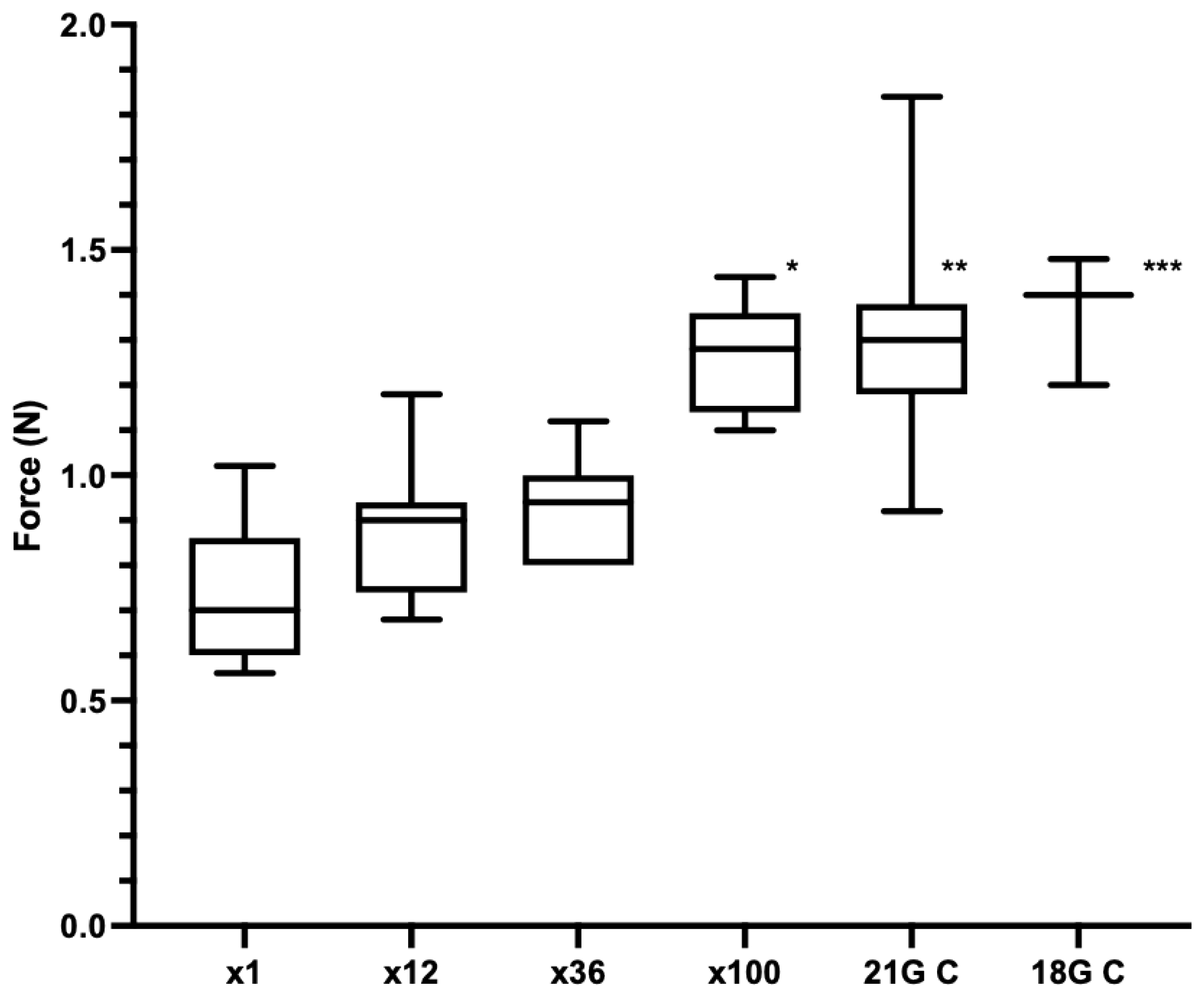
- Experiment 4: Comparison of ×1, ×12, ×36 and ×100 punctures. The repeat use needles were prepared by injecting randomly 11, 35 or 99 times (across four piglet cadavers), n = 7 per group. The 18 G ½” needles (18 G C) (n = 3) and the needles previously blunted by puncture into chamois leather 17 times (21 G C) (n = 6) were used as positive controls. The order that the needles were punctured into the neck of a fifth piglet cadaver were randomised using Research Randomizer. Five piglet cadavers were used in total.
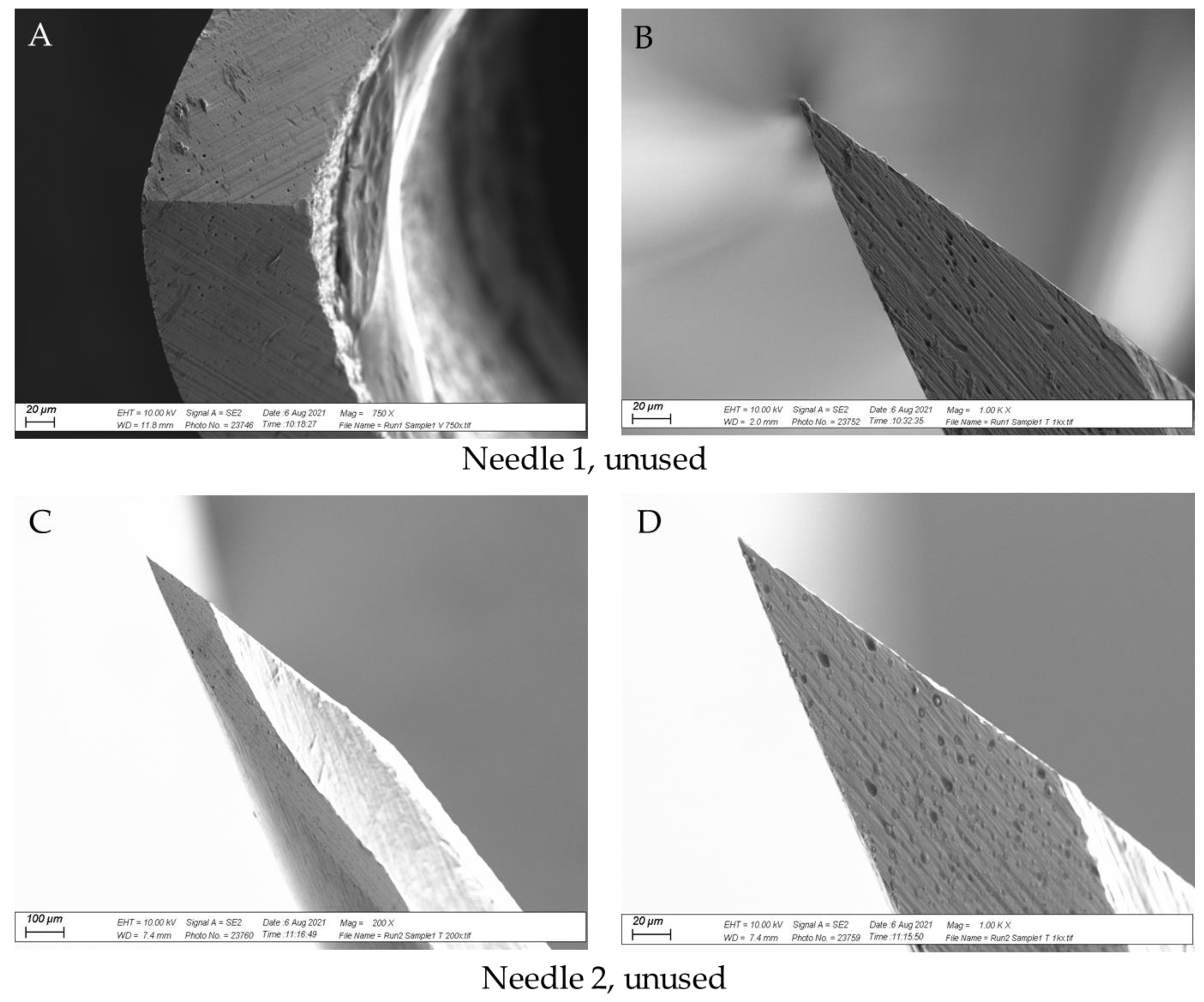
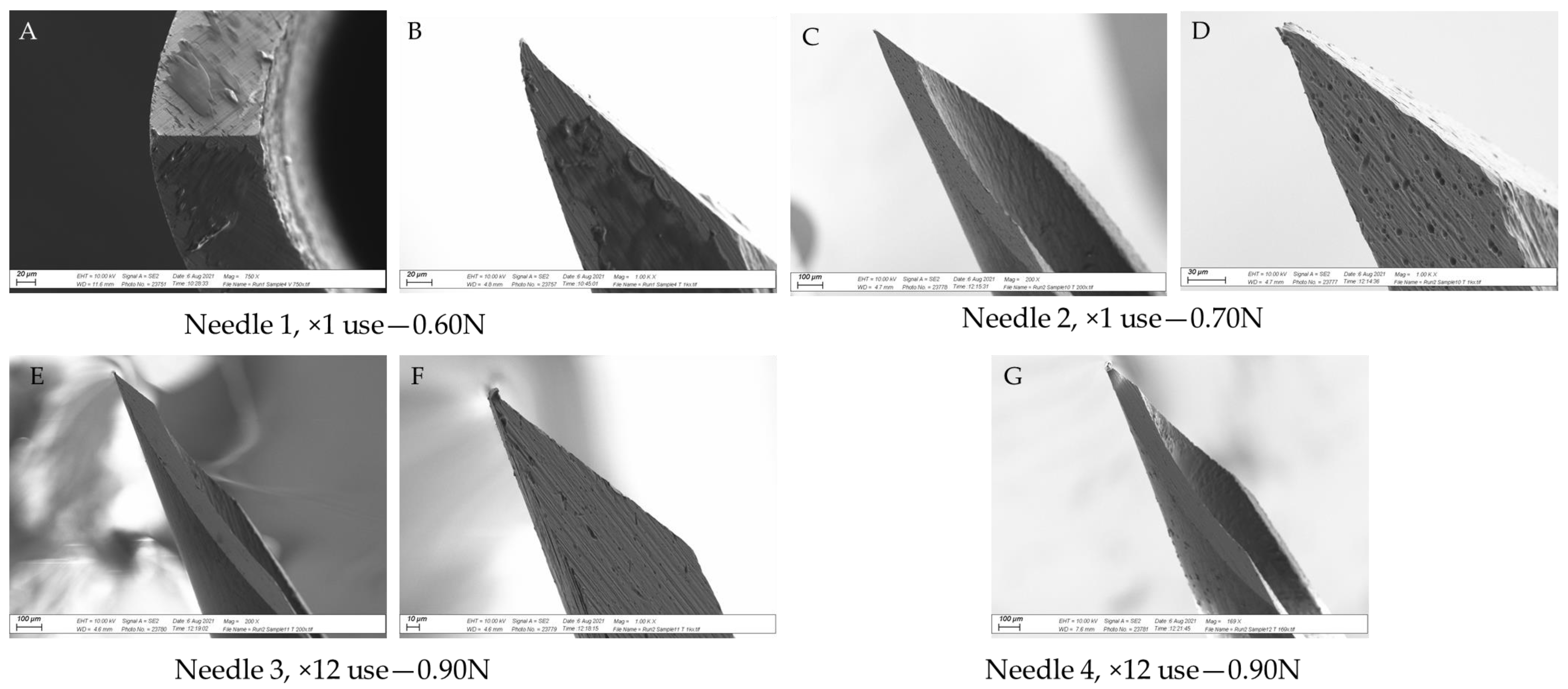
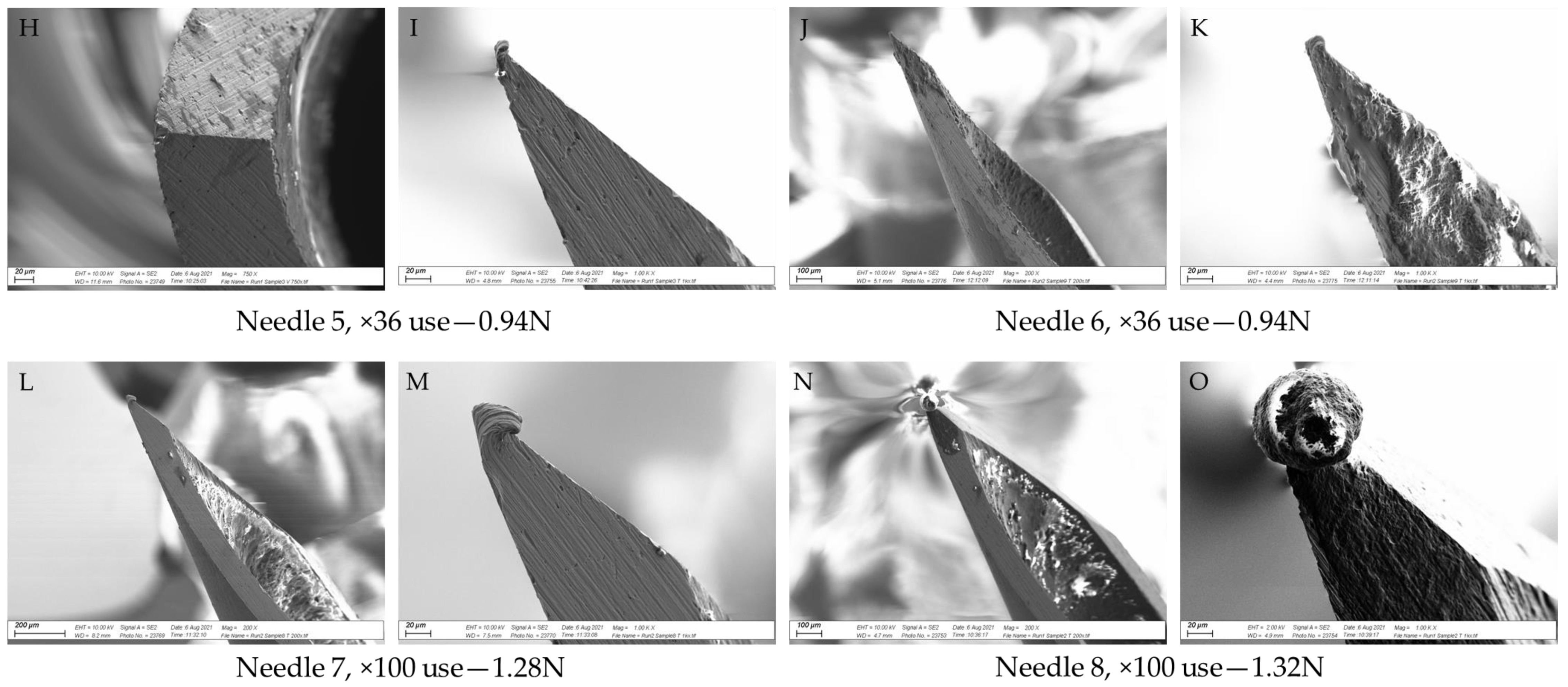
2.2.2. SEM Analysis
3. Results
3.1. Survey
3.2. Piglet Cadaver Studies
Variation across Piglet Cadavers
3.3. SEM Analysis
4. Discussion
4.1. Survey
4.2. Repeated Needle Use
4.3. SEM Assessment of Needle Tip Damage
4.4. Study Design
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- AHDB. UK Pig Numbers and Holdings. Available online: https://ahdb.org.uk/pork/uk-pig-numbers-and-holdings (accessed on 9 September 2021).
- AHDB. GB Estimated Pig Slaughterings. Available online: https://ahdb.org.uk/pork/gb-estimated-pig-slaughterings (accessed on 9 September 2021).
- Gov.UK. FAWC Report on the Welfare of Pigs Kept Outdoors. Available online: https://www.gov.uk/government/publications/fawc-report-on-the-welfare-of-pigs-kept-outdoors (accessed on 9 September 2021).
- Svoboda, M.V.; Berlinska, J. Parenteral iron administration in suckling piglets—A review. Acta Vet. Brno 2017, 86, 249–261. [Google Scholar] [CrossRef]
- Heidbüchel, K.; Raabe, J.; Baldinger, L.; Hagmüller, W.; Bussemas, R. One iron injection is not enough-Iron status and growth of suckling piglets on an organic farm. Animals 2019, 9, 651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cresswell, E.; Remnant, J.; Butterworth, A.; Wapenaar, W. Injection-site lesion prevalence and potential risk factors in UK beef cattle. Vet. Rec. 2017, 180, 70. [Google Scholar] [CrossRef] [PubMed]
- Hall, L.E. Survey on Vaccination Technique in UK Sheep Farmers and the Impact of Training; Royal Veterinary College: London, UK, 2021. [Google Scholar]
- AHDB. Using Medicines for Better Returns. Available online: https://projectblue.blob.core.windows.net/media/Default/Beef%20&%20Lamb/UsingMedsCorrectly2370_190204_WEB.pdf (accessed on 9 September 2021).
- Red_Tractor. Good Needle Practice—Everyones Responsibility. Available online: https://assurance.redtractor.org.uk/contentfiles/Farmers-7020.pdf?_=636969832572197880 (accessed on 9 September 2021).
- McCrindle, C. Injecting Pigs the Correct Way. Available online: https://www.farmersweekly.co.za/farm-basics/how-to-livestock/injecting-pigs-the-correct-way/ (accessed on 9 September 2021).
- Uniferon. How to Adminster Iron for Piglets. Available online: http://www.uniferon.com/learning-center/how-to-administer-iron-for-piglets.aspx (accessed on 9 September 2021).
- Zoetis. Dosing Guns, Injectors and Maintenance. Available online: https://www.zoetis.co.uk/livestock-farming/useful-resources/pdfs-and-images/VPS_applicator_and_Injector_Guide_2020.pdf (accessed on 9 September 2021).
- Otake, S.; Dee, S.A.; Rossow, K.D.; Joo, H.S.; Deen, J.; Molitor, T.W.; Pijoan, C. Transmission of porcine reproductive and respiratory syndrome virus by needles. Vet. Rec. 2002, 150, 114–115. [Google Scholar] [CrossRef] [PubMed]
- Darpel, K.E.; Barber, J.; Hope, A.; Wilson, A.J.; Gubbins, S.; Henstock, M.; Frost, L.; Batten, C.; Veronesi, E.; Moffat, K.; et al. Using shared needles for subcutaneous inoculation can transmit bluetongue virus mechanically between ruminant hosts. Sci. Rep. 2016, 6, 20627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marchant-Forde, J.; Lay, D.; McMunn, K.; Cheng, H.; Pajor, E.; Marchant-Forde, R. Postnatal piglet husbandry practices and well-being: The effects of alternative techniques delivered separately. J. Anim. Sci. 2009, 87, 1479–1492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arendt-Nielsen, L.; Egekvist, H.; Bjerring, P. Pain following controlled cutaneous insertion of needles with different diameters. Somatosens. Mot. Res. 2006, 23, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Egekvist, H.; Bjerring, P.; Arendt-Nielsen, L. Pain and mechanical injury of human skin following needle insertions. Eur. J. Pain 1999, 3, 41–49. [Google Scholar] [CrossRef]
- Whitfield, L.; Robinson, S. Reuse of Needles: Is this an Indicator of a Culture of Care? Available online: https://www.nc3rs.org.uk/news/reuse-needles-indicator-culture-care (accessed on 9 September 2021).
- Hoff, S.J.; Sundberg, P. Breakage and deformation characteristics of hypodermic devices under static and dynamic loading. Am. J. Vet. Res. 1999, 60, 292–298. [Google Scholar] [PubMed]
- Daniels, C.; Funk, J. Prevalence of Carcass Defects in Sows at Harvest. Available online: https://core.ac.uk/download/pdf/38897968.pdf (accessed on 9 September 2021).
- King, D.; Painter, T.; Holtkamp, D.; DuBois, P.; Wang, C. Effect of injection tool on incidence of head and neck abscesses at slaughter. J. Swine Health Prod. 2010, 18, 290–293. [Google Scholar]
- Imeah, B.; Penz, E.; Rana, M.; Trask, C. Economic analysis of new workplace technology including productivity and injury: The case of needle-less injection in swine. PLoS ONE 2020, 15, e0233599. [Google Scholar] [CrossRef] [PubMed]
- Majcher, K.; Eichorn, D.; Waldner, C.; Johnston, J.; Clark, C.; Jelinski, M. Assessing the sharpness of hypodermic needles after repeated use. Can. Vet. J. 2018, 59, 1112–1114. [Google Scholar] [PubMed]
- Thacker, J.; Rodeheaver, G.; Towler, M.; Edlich, R. Surgical needle sharpness. Am. J. Surg. 1989, 157, 334–339. [Google Scholar] [CrossRef]
- Scollo, A.; Minervini, S.; Galli, M.; Cevidalli, A.; Bortoletto, G.; Romano, G.; Gottardo, F. Evaluation of pain and stress in three-week old piglets in relation to route of vaccine administration. Livest. Sci. 2020, 233, 103939. [Google Scholar] [CrossRef]
- Temple, D.; Jimenez, M.; Escribano, D.; Martin-Valls, G.; Diaz, I.; Manteca, X. Welfare benefits of intradermal vaccination of piglets. Animals 2020, 10, 1898. [Google Scholar] [CrossRef] [PubMed]
- Mackenzie, R.; Skuse, N.; Lethlean, K. Fibre function and perception during cutaneous nerve block. J. Neurol. Neurosurg. Psychiatry 1975, 38, 865–873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ko, E.; Cho, J.; Cho, J.H.; Jo, K.; Lee, S.; Chung, Y.; Jung, S. Reduction in lesion incidence in pork carcass using transdermal needle-free injection of foot and mouth disease vaccine. Korean J. Food Sci. Anim. Resour. 2018, 38, 1155–1159. [Google Scholar] [CrossRef] [PubMed]




| Parameter | Category | Frequency | Percentage |
|---|---|---|---|
| Was iron supplementation of piglets conducted? (n = 31) | Yes (Injection) | 28 | 90.3% |
| Yes (Oral) | 1 | 3.2% | |
| No | 2 | 6.5% | |
| Injection Site (n = 31) | Neck | 18 | 58.1% |
| Leg | 9 | 29.0% | |
| Neck or Leg | 1 | 3.2% | |
| Not applicable | 3 * | 9.7% | |
| Volume of iron dextran injected ** (n = 30) | 1 mL 200 mg/mL | 26 | 86.7% |
| 2 mL 100 mg/mL | 1 | 3.3% | |
| Not applicable | 3 * | 10.0% | |
| Use of automated injector ** (n = 20) | Yes | 17 | 85.0% |
| No | 3 | 15.0% | |
| Size of needle (n = 31) | 21 G 5/8” | 21 | 67.7% |
| 21 G 1/2” | 1 | 3.2% | |
| 21 G 3/8” | 1 | 3.2% | |
| 19 G 1” | 1 | 3.2% | |
| 20 G 3/8” | 1 | 3.2% | |
| 23 G 1/1/4” | 1 | 3.2% | |
| No injection | 4 * | 12.9% | |
| Unclear | 1 * | 3.2% | |
| Frequency of needle change (n = 31) | Every litter (10–12 piglets) | 8 | 25.8% |
| Every litter or earlier if blunt | 4 | 12.9% | |
| Every other litter | 1 | 3.2% | |
| Every 3 litters | 5 | 16.1% | |
| When blunt/changing bottle/after injection session/damaged | 7 | 22.6% | |
| Not applicable | 4 * | 12.9% | |
| Unclear | 2 * | 6.5% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Owen, K.; Blackie, N.; Gibson, T.J. The Effect of Needle Reuse on Piglet Skin Puncture Force. Vet. Sci. 2022, 9, 90. https://doi.org/10.3390/vetsci9020090
Owen K, Blackie N, Gibson TJ. The Effect of Needle Reuse on Piglet Skin Puncture Force. Veterinary Sciences. 2022; 9(2):90. https://doi.org/10.3390/vetsci9020090
Chicago/Turabian StyleOwen, Kathryn, Nicola Blackie, and Troy John Gibson. 2022. "The Effect of Needle Reuse on Piglet Skin Puncture Force" Veterinary Sciences 9, no. 2: 90. https://doi.org/10.3390/vetsci9020090
APA StyleOwen, K., Blackie, N., & Gibson, T. J. (2022). The Effect of Needle Reuse on Piglet Skin Puncture Force. Veterinary Sciences, 9(2), 90. https://doi.org/10.3390/vetsci9020090





