Molecular Detection of Cryptosporidium cuniculus in Rabbits (Oryctolagus cuniculus) from Tenerife, Canary Islands, Spain
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Ethical Statement
2.3. Staining Method
2.4. DNA Extraction
2.5. PCR Amplification
2.6. Sequencing and Phylogenetic Analysis
3. Results
3.1. Staining and Molecular Results
3.2. Phylogenetic Analyses
3.2.1. 18S rRNA Gene Analysis
3.2.2. gp60 Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Banco de Datos de Biodiversidad de Canarias (EXOS). Available online: https://www.biodiversidadcanarias.es/exos/especie/V00215 (accessed on 23 December 2021).
- Ministerio de Agricultura, Pesca y Alimentación (MAPA). Caracterización del Sector Cunícola en España. Año 2020. Available online: https://www.mapa.gob.es/es/ganaderia/temas/produccion-y-mercados-ganaderos/caracterizacioncunicola_rev_tcm30-583128.pdf (accessed on 6 February 2022).
- Bello-Rodríguez, V.; Mateo, R.G.; Pellissier, L.; Cubas, J.; Cooke, B.; González-Mancebo, J.M. Forecast increase in invasive rabbit spread into ecosystems of an oceanic island (Tenerife) under climate change. Ecol. Appl. 2021, 31, e02206. [Google Scholar] [CrossRef] [PubMed]
- Foronda, P.R.; Figueruelo, E.O.; Ortega, A.R.; Abreu, N.A.; Casanova, J.C. Parasites (viruses, coccidia and helminths) of the wild rabbit (Oryctolagus cuniculus) introduced to Canary Islands from Iberian Peninsula. Acta Parasitol. 2005, 50, 80–84. [Google Scholar]
- Prediger, J.; Ježková, J.; Holubová, N.; Sak, B.; Konečný, R.; Rost, M.; McEvoy, J.; Rajský, D.; Kváč, M. Cryptosporidium sciurinum n. sp. (Apicomplexa: Cryptosporidiidae) in Eurasian Red Squirrels (Sciurus vulgaris). Microorganisms 2021, 9, 2050. [Google Scholar] [CrossRef] [PubMed]
- O’Donoghue, P.J. Cryptosporidium infections in man, animals, birds and fish. Aust. Vet. J. 1985, 62, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Fayer, R.; Morgan, U.; Upton, S.J. Epidemiology of Cryptosporidium: Transmission, detection and identification. Int. J. Parasitol. 2000, 30, 1305–1322. [Google Scholar] [CrossRef]
- Daraei, H.; Conti, G.O.; Sahlabadi, F.; Thai, V.N.; Gholipour, S.; Turki, H.; Fakhri, Y.; Ferrante, M.; Moradi, A.; Khaneghah, A.M. Prevalence of Cryptosporidium spp. in water: A global systematic review and meta-analysis. Environ. Sci. Pollut. Res. Int. 2020, 28, 9498–9507. [Google Scholar] [CrossRef]
- Tyzzer, E.E. Cryptosporidium parvum (sp. nov.) a coccidium found in the small intestine of the common mouse. Arch. Protistenkd. 1912, 26, 394–412. [Google Scholar]
- Inman, L.R.; Takeuchi, A. Spontaneous cryptosporidiosis in an adult female rabbit. Vet. Pathol. 1979, 16, 89–95. [Google Scholar] [CrossRef]
- Robinson, G.; Wright, S.; Elwin, K.; Hadfield, S.J.; Katzer, F.; Bartley, P.M.; Hunter, P.R.; Nath, M.; Innes, E.A.; Chalmers, R.M. Re-description of Cryptosporidium cuniculus Inman and Takeuchi, 1979 (Apicomplexa: Cryptosporidiidae): Morphology, biology and phylogeny. Int. J. Parasitol. 2010, 40, 1539–1548. [Google Scholar] [CrossRef]
- Xiao, L.; Sulaiman, I.M.; Ryan, U.M.; Zhou, L.; Atwill, E.R.; Tischler, M.L.; Zhang, X.; Fayer, R.; Lal, A.A. Host adaptation and host-parasite co-evolution in Cryptosporidium: Implications for taxonomy and public health. Int. J. Parasitol. 2002, 32, 1773–1785. [Google Scholar] [CrossRef]
- Shi, K.; Jian, F.; Lv, C.; Ning, C.; Zhang, L.; Ren, X.; Dearen, T.K.; Li, N.; Qi, M.; Xiao, L. Prevalence, Genetic Characteristics, and Zoonotic Potential of Cryptosporidium Species Causing Infections in Farm Rabbits in China. J. Clin. Microbiol. 2010, 48, 3263–3266. [Google Scholar] [CrossRef]
- Zhang, W.; Shen, Y.; Wang, R.; Liu, A.; Ling, H.; Li, Y.; Cao, J.; Zhang, X.; Shu, J.; Zhang, L. Cryptosporidium cuniculus and Giardia duodenalis in rabbits: Genetic diversity and possible zoonotic transmission. PLoS ONE 2012, 7, e31262. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, X.; Zhong, Z.; Chen, W.; Deng, J.; Niu, L.; Wang, Q.; Peng, G. New subtype of Cryptosporidium cuniculus isolated from rabbits by sequencing the Gp60 gene. J. Parasitol. 2014, 100, 532–536. [Google Scholar] [CrossRef]
- Yang, Z.; Zhao, W.; Shen, Y.; Zhang, W.; Shi, Y.; Ren, G.; Yang, D.; Ling, H.; Yang, F.; Liu, A.; et al. Subtyping of Cryptosporidium cuniculus and genotyping of Enterocytozoon bieneusi in rabbits in two farms in Heilongjiang Province, China. Parasite 2016, 23, 52. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Qi, M.; Jing, B.; Yu, F.; Wu, Y.; Chang, Y.; Zhao, A.; Wei, Z.; Dong, H.; Zhang, L. Molecular Characterization of Cryptosporidium spp., Giardia duodenalis and Enterocytozoon bieneusi in Rabbits in Xinjiang, China. J. Eukaryot. Microbiol. 2018, 65, 854–859. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Liu, X.; Liu, J.; Tang, X.; Zhu, G.; Striepen, B.; Suo, X. Immunocompetent rabbits infected with Cryptosporidium cuniculus as an animal model for anti-cryptosporidial drug testing. Int. J. Parasitol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Ryan, U.; Xiao, L.; Carolyn, R.; Zhou, L.; Lal, A.A.; Pavlasek, I. Identification of novel Cryptosporidium genotypes from the Czech Republic. Appl. Environ. Microbiol. 2003, 69, 4302–4307. [Google Scholar] [CrossRef]
- Learmonth, J.; Ionas, G.; Ebbett, K.A.; Kwan, E.S. Genetic characterization and transmission cycles of Cryptosporidium species isolated from humans in New Zealand. Appl. Environ. Microbiol. 2004, 70, 973–978. [Google Scholar] [CrossRef]
- Chalmers, R.M.; Robinson, G.; Elwin, K.; Hadfield, S.J.; Xiao, L.; Ryan, U.M.; Modha, D.; Mallaghan, C. Cryptosporidium sp. rabbit genotype, a newly identified human pathogen. Emerg. Infect. Dis. 2009, 15, 829–830. [Google Scholar] [CrossRef]
- Nolan, M.J.; Jex, A.R.; Haydon, S.R.; Stevens, M.A.; Gasser, R.B. Molecular detection of Cryptosporidium cuniculus in rabbits in Australia. Infect. Genet. Evol. 2010, 10, 1179–1187. [Google Scholar] [CrossRef]
- Nolan, M.J.; Jex, A.R.; Koehler, A.V.; Haydon, S.R.; Stevens, M.A.; Gasser, R.B. Molecular-based investigation of Cryptosporidium and Giardia from animals in water catchments in southeastern Australia. Water Res. 2013, 47, 1726–1740. [Google Scholar] [CrossRef] [PubMed]
- Koehler, A.V.; Haydon, S.R.; Jex, A.R.; Gasser, R.B. Cryptosporidium and Giardia taxa in faecal samples from animals in catchments supplying the city of Melbourne with drinking water (2011 to 2015). Parasit. Vectors 2016, 9, 315. [Google Scholar] [CrossRef] [PubMed]
- Zahedi, A.; Monis, P.; Aucote, S.; King, B.; Paparini, A.; Jian, F.; Yang, R.; Oskam, C.; Ball, A.; Robertson, I.; et al. Zoonotic Cryptosporidium Species in Animals Inhabiting Sydney Water Catchments. PLoS ONE 2016, 11, e0168169. [Google Scholar] [CrossRef] [PubMed]
- Zahedi, A.; Monis, P.; Gofton, A.W.; Oskam, C.L.; Ball, A.; Bath, A.; Bartkow, M.; Robertson, I.; Ryan, U. Cryptosporidium species and subtypes in animals inhabiting drinking water catchments in three states across Australia. Water Res. 2018, 134, 327–340. [Google Scholar] [CrossRef] [PubMed]
- Kaupke, A.; Kwit, E.; Chalmers, R.M.; Michalski, M.M.; Rzeżutka, A. An outbreak of massive mortality among farm rabbits associated with Cryptosporidium infection. Res. Vet. Sci. 2014, 97, 85–87. [Google Scholar] [CrossRef] [PubMed]
- Heker, M.M.; Nakamura, A.A.; Meireles, M.V. Molecular characterization of Cryptosporidium spp. in Brazilian rabbit farms. Arq. Bras. Med. Vet. Zootec. 2016, 68, 977–982. [Google Scholar] [CrossRef]
- Naguib, D.; Roellig, D.M.; Arafat, N.; Xiao, L. Genetic Characterization of Cryptosporidium cuniculus from Rabbits in Egypt. Pathogens 2021, 10, 775. [Google Scholar] [CrossRef]
- Ayinmode, A.B.; Agbajelola, V.I. Molecular identification of Cryptosporidium parvum in rabbits (Oryctolagus cuniculus) in Nigeria. Ann. Parasitol. 2019, 65, 237–243. [Google Scholar]
- Koehler, A.V.; Whipp, M.J.; Haydon, S.R.; Gasser, R.B. Cryptosporidium cuniculus–new records in human and kangaroo in Australia. Parasit. Vectors 2014, 7, 492. [Google Scholar] [CrossRef]
- Koehler, A.V.; Rashid, M.H.; Zhang, Y.; Vaughan, J.L.; Gasser, R.B.; Jabbar, A. First cross-sectional, molecular epidemiological survey of Cryptosporidium, Giardia and Enterocytozoon in alpaca (Vicugna pacos) in Australia. Parasit. Vectors 2018, 11, 498. [Google Scholar] [CrossRef]
- Puleston, R.L.; Mallaghan, C.M.; Modha, D.E.; Hunter, P.R.; Nguyen-Van-Tam, J.S.; Regan, C.M.; Nichols, G.L.; Chalmers, R.M. The first recorded outbreak of cryptosporidiosis due to Cryptosporidium cuniculus (formerly rabbit genotype), following a water quality incident. J. Water Health 2014, 12, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Xiao, L.; Wang, L.; Zhao, S.; Zhao, X.; Duan, L.; Guo, M.; Liu, L.; Feng, Y. Molecular surveillance of Cryptosporidium spp., Giardia duodenalis, and Enterocytozoon bieneusi by genotyping and subtyping parasites in wastewater. PLoS Negl. Trop. Dis. 2012, 6, e1809. [Google Scholar] [CrossRef] [PubMed]
- Xiao, D.; Lyu, Z.; Chen, S.; Huo, Y.; Fan, W.; Huo, M. Tracking Cryptosporidium in urban wastewater treatment plants in a cold region: Occurrence, species and infectivity. Front. Environ. Sci. Eng. 2022, 16, 1–14. [Google Scholar] [CrossRef]
- Ulloa-Stanojlović, F.M.; Aguiar, B.; Jara, L.M.; Zanoli-Sato, M.I.; Arzola-Guerrero, J.; Hachich, E.; Rogério-Matté, G.; Dropa, M.; Matté, M.H.; de Araújo, R.S. Occurrence of Giardia intestinalis and Cryptosporidium sp. in wastewater samples from São Paulo State, Brazil, and Lima, Peru. Environ. Sci. Pollut. Res. Int. 2016, 23, 22197–22205. [Google Scholar] [CrossRef]
- Ramo, A.; del Cacho, E.; Sánchez-Acedo, C.; Quílez, J. Occurrence and genetic diversity of Cryptosporidium and Giardia in urban wastewater treatment plants in north-eastern Spain. Sci. Total Environ. 2017, 598, 628–638. [Google Scholar] [CrossRef]
- Zahedi, A.; Gofton, A.W.; Greay, T.; Monis, P.; Oskam, C.; Ball, A.; Bath, A.; Watkinson, A.; Robertson, I.; Ryan, U. Profiling the diversity of Cryptosporidium species and genotypes in wastewater treatment plants in Australia using next generation sequencing. Sci. Total Environ. 2018, 644, 635–648. [Google Scholar] [CrossRef] [PubMed]
- Zahedi, A.; Greay, T.L.; Paparini, A.; Linge, K.L.; Joll, C.A.; Ryan, U.M. Identification of eukaryotic microorganisms with 18S rRNA next-generation sequencing in wastewater treatment plants, with a more targeted NGS approach required for Cryptosporidium detection. Water Res. 2019, 158, 301–312. [Google Scholar] [CrossRef]
- Hu, Y.; Feng, Y.; Huang, C.; Xiao, L. Occurrence, source, and human infection potential of Cryptosporidium and Enterocytozoon bieneusi in drinking source water in Shanghai, China, during a pig carcass disposal incident. Environ. Sci. Technol. 2014, 48, 14219–14227. [Google Scholar] [CrossRef]
- Swaffer, B.; Vial, H.M.; King, B.J.; Daly, R.; Frizenschaf, J.; Monis, P.T. Investigation source water Cryptosporidium concentration, species and infectivity rates during rainfall-runoff in a multi-use catchment. Water Res. 2014, 67, 310–320. [Google Scholar] [CrossRef]
- Mphephu, M.G.; Ekwanzala, M.D.; Momba, M.N.B. Cryptosporidium species and subtypes in river water and riverbed sediment using next-generation sequencing. Int. J. Parasitol. 2021, 51, 339–351. [Google Scholar] [CrossRef]
- Swaffer, B.; Abbott, H.; King, B.; van der Linden, L.; Monis, P. Understanding human infectious Cryptosporidium risk in drinking water supply catchments. Water Res. 2018, 138, 282–292. [Google Scholar] [CrossRef] [PubMed]
- Zahedi, A.; Ryan, U.; Rawlings, V.; Greay, T.; Hancock, S.; Bruce, M.; Jacobson, C. Cryptosporidium and Giardia in dam water on sheep farms—An important source of transmission? Vet. Parasitol. 2020, 288, 109281. [Google Scholar] [CrossRef] [PubMed]
- Cacciò, S.M.; Chalmers, R.M. Human cryptosporidiosis in Europe. Clin. Microbiol. Infect. 2016, 22, 471–480. [Google Scholar] [CrossRef] [PubMed]
- Cieloszyk, J.; Goñi, P.; García, A.; Remacha, M.A.; Sánchez, E.; Clavel, A. Two cases of zoonotic cryptosporidiosis in Spain by the unusual species Cryptosporidium ubiquitum and Cryptosporidium felis. Enferm. Infecc. Microbiol. Clin. 2012, 30, 549–551. [Google Scholar] [CrossRef] [PubMed]
- Robinson, G.; Elwin, K.; Chalmers, R.M. Unusual Cryptosporidium Genotypes in Human Cases of Diarrhea. Emerg. Infect. Dis. 2008, 14, 1800–1802. [Google Scholar] [CrossRef] [PubMed]
- Chalmers, R.M.; Elwin, K.; Hadfield, S.J.; Robinson, G. Sporadic Human Cryptosporidiosis Caused by Cryptosporidium cuniculus, United Kingdom, 2007–2008. Emerg. Infect. Dis. 2011, 17, 536–538. [Google Scholar] [CrossRef] [PubMed]
- McKerr, C.; Chalmers, R.M.; Elwin, K.; Ayres, H.; Vivancos, R.; O’Brien, S.J.; Christley, R.M. Cross-sectional household transmission study of Cryptosporidium shows tht C. hominis infections are a key risk factor for spread. BMC Infect. Dis. 2022, 22, 114. [Google Scholar] [CrossRef]
- ANOFEL Cryptosporidium National Network. Laboratory based surveillance for Cryptosporidium in France, 2006–2009. Euro. Surveill. 2010, 15, 19642. [Google Scholar]
- Costa, D.; Razakandrainibe, R.; Sautour, M.; Valot, S.; Basmaciyan, L.; Gargala, G.; Lemeteil, D.; French National Network on Surveillance of Human Cryptosporidiosis. Human Cryptosporidiosis in immunodeficient patients in France (2015–2017). Exp. Parasitol. 2018, 192, 108–112. [Google Scholar] [CrossRef]
- Costa, D.; Razakandrainibe, R.; Valor, S.; Vannier, M.; Sautour, M.; Basmaciyan, L.; Gargala, G.; Viller, V.; Lemeteil, D.; Ballet, J.J.; et al. Epidemiology of Cryptosporidiosis in France from 2017 to 2019. Microorganisms 2020, 8, 1358. [Google Scholar] [CrossRef]
- Molloy, S.F.; Smith, H.V.; Kirwan, P.; Nichols, R.A.B.; Asaolu, S.O.; Connelly, L.; Holland, C.V. Identification of a high diversity of Cryptosporidium species genotypes and subtypes in a pediatric population in Nigeria. Am. J. Trop. Med. Hyg. 2010, 82, 608–613. [Google Scholar] [CrossRef] [PubMed]
- García-R, J.C.; French, N.; Pita, A.; Velathanthiri, N.; Shrestha, R.; Hayman, D. Local and global genetic diversity of protozoan parasites: Spatial distribution of Cryptosporidium and Giardia genotypes. PLoS Negl. Trop. Dis. 2017, 11, e0005736. [Google Scholar] [CrossRef] [PubMed]
- García-R, J.C.; Pita, A.B.; Velathanthiri, N.; French, N.P.; Hayman, D.T.S. Species and genotypes causing human cryptosporidiosis in New Zealand. Parasitol. Res. 2020, 119, 2317–2326. [Google Scholar] [CrossRef]
- Knox, M.A.; García-R, J.C.; Ogbuigwe, P.; Pita, A.; Velathanthiri, N.; Hayman, D.T.S. Absence of Cryptosporidium hominis and dominance of zoonotic Cryptosporidium species in patients after Covid-19 restrictions in Auckland, New Zealand. Parasitology 2021, 148, 1288–1292. [Google Scholar] [CrossRef] [PubMed]
- Guy, R.A.; Yanta, C.A.; Muchaal, P.K.; Rankin, M.A.; Thivierge, K.; Lau, R.; Boggild, A.K. Molecular characterization of Cryptosporidium isolates from humans in Ontario, Canada. Parasit. Vectors 2021, 14, 69. [Google Scholar] [CrossRef]
- Lebbad, M.; Winiecka-Krusnell, J.; Stensvold, C.R.; Beser, J. High Diversity of Cryptosporidium Species and Subtypes Identified in Cryptosporidiosis Acquired in Sweden and Abroad. Pathogens 2021, 10, 523. [Google Scholar] [CrossRef]
- Martínez-Ruiz, R.; de Lucio, A.; Fuentes, I.; Carmena, D. Autochthonous Cryptosporidium cuniculus infection in Spain: First report in a symptomatic paediatric patient from Madrid. Enferm. Infecc. Microbiol. Clin. 2016, 34, 532–534. [Google Scholar] [CrossRef]
- Abreu-Acosta, N.; Quispe, M.A.; Foronda-Rodríguez, P.; Alcoba-Florez, J.; Lorenzo-Morales, J.; Ortega-Rivas, A.; Valladares, B. Cryptosporidium in patients with diarrhea, on Tenerife, Canary Islands, Spain. Ann. Trop. Med. Parasitol. 2007, 101, 539–545. [Google Scholar] [CrossRef]
- Novo-Veleiro, I.; Martín-Sánchez, A.M.; Elcuaz-Romano, R.; Afonso, O.; García-Bardeci, D.; Bordes-Benítez, A.; Carranza-Rodríguez, C.; Hernández-Cabrera, M.; Alvela-Suárez, M.L.; Pérez-Arellano, J.L.; et al. Parasitosis en Gran Canaria (España) Estudio Prospectivo Multicéntrico Durante Un Año. Rev. Ibero-Latinoam. Parasitol. 2012, 71, 34–41. [Google Scholar]
- Sistema EDO de la Red Canaria de Vigilancia Epidemiológica. Available online: https://www3.gobiernodecanarias.org/sanidad/scs/contenidoGenerico.jsp?idDocument=72483736-0d7a-11de-9de1-998efb13096d&idCarpeta=0f67aaf7-9d88-11e0-b0dc-e55e53ccc42c (accessed on 22 December 2021).
- Rodríguez, F.; Orós, J.; Rodríguez, J.L.; González, J.; Castro, P.; Fernández, A. Intestinal cryptosporidiosis in pigeons (Columba livia). Avian Dis. 1997, 41, 748–750. [Google Scholar] [CrossRef]
- Abreu-Acosta, N.; Foronda-Rodríguez, P.; López, M.; Valladares, B. Occurrence of Cryptosporidium hominis in pigeons (Columba livia). Acta Parasitol. 2009, 54, 1–5. [Google Scholar] [CrossRef]
- Feliu, C.; López, M.; Gómez, M.S.; Torres, J.; Sánchez, S.; Miquel, J.; Abreu-Acosta, N.; Segovia, J.M.; Martín-Alonso, A.; Montoliu, I.; et al. Parasite fauna of rodents (Murinae) from El Hierro (Canary Islands, Spain): A multidisciplinary approach. Acta Parasitol. 2012, 57, 171–178. [Google Scholar] [CrossRef] [PubMed]
- García-Livia, K.; Martín-Alonso, A.; Foronda, P. Diversity of Cryptosporidium spp. in wild rodents from the Canary Islands, Spain. Parasit. Vectors 2020, 13, 445. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo-Rodríguez, E.; Martín-Carrillo, N.; Valladares, B.; Foronda, P. Study of Zoonotic Enteric Pathogens of Atelerix algirus in Tenerife, Canary Islands, Spain. Front. Vet. Sci 2020, 7, 579602. [Google Scholar] [CrossRef]
- Abreu-Acosta, N.; Martín-Delgado, M.; Ortega-Rivas, A.; del Castillo-Remiro, A.; Aguiar-González, E.; Valladares, B. Giardia lamblia and Cryptosporidium spp. presence in treated wastewater reutilised for irrigation in Tenerife Island, Spain. Long-distance transport effects in the reutilised water quality. Rev. Salud Ambient. 2002, 2, 2–7. [Google Scholar]
- Abreu-Acosta, N.; Vera, L. Occurrence and removal of parasites, enteric bacteria and faecal contamination indicators in wastewater natural reclamation systems in Tenerife-Canary Islands, Spain. Ecol. Eng. 2011, 37, 496–503. [Google Scholar] [CrossRef]
- Manore, A.J.W.; Harper, S.L.; Aguilar, B.; Weese, J.S.; Shapiro, K. Comparison of freeze-thaw cycles for nucleic acid extraction and molecular detection of Cryptosporidium parvum and Toxoplasma gondii oocysts in environmental matrices. J. Microbiol. Methods 2019, 156, 1–4. [Google Scholar] [CrossRef]
- Zhao, G.H.; Ren, W.X.; Gao, M.; Bian, Q.Q.; Hu, B.; Cong, M.M.; Lin, Q.; Wang, R.J.; Qi, M.; Qi, M.Z.; et al. Genotyping Cryptosporidium andersoni in cattle in Shaanxi Province, Northwestern China. PLoS ONE 2013, 8, e60112. [Google Scholar] [CrossRef]
- Alves, M.; Xiao, L.; Sulaiman, I.; Lal, A.A.; Matos, O.; Antunes, F. Subgenotype analysis of Cryptosporidium isolates from humans, cattle, and zoo rumiants in Portugal. J. Clin. Microbiol. 2003, 41, 2744–2747. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [PubMed]
- Tamura, K.; Nei, M. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol. Biol. Evol. 1993, 10, 512–526. [Google Scholar] [PubMed]
- Shiibashi, T.; Imai, T.; Sato, Y.; Abe, N.; Yukawa, M.; Nogami, S. Cryptosporidium infection in juvenile pet rabbits. J. Vet. Med. Sci. 2006, 68, 281–282. [Google Scholar] [CrossRef]
- Da Silva, A.S.; Ceolin, L.V.; Monteiro, S.G. Endoparasitoses de coelhos criados em diferentes sistemas de manejo. Rev. FZVA 2006, 13, 127–136. [Google Scholar]
- Elshahawy, I.; Elgoniemy, A. An Epidemiological Study on Endoparasites of Domestic Rabbits (Oryctolagus cuniculus) in Egypt with Special Reference to Their Health Impact. Sains Malays. 2018, 47, 9–18. [Google Scholar] [CrossRef]
- Smith, R.P.; Chalmers, R.M.; Elwin, K.; Clifton-Hadley, F.A.; Mueller-Doblies, D.; Watkins, J.; Paiba, G.A.; Giles, M. Investigation of the Role of Companion Animals in the Zoonotic Transmission of Cryptosporidiosis. Zoonoses Public Health 2009, 56, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Akinkuotu, O.A.; Akinkuotu, A.C.; Oseni, O.T. Prevalence of Cryptosporidium Infection in a Rabbitory in Abeokuta, Nigeria. Nig. Vet. J. 2016, 37, 243–246. [Google Scholar]
- Respaldiza-Cardeñosa, E.; Simón-Palacios, M.C.; Respaldiza-Fernández, E. Estudio de un Foco de Criptosporidiosis en Conejo. XIV Symposium de Cunicultura. Manresa. Boletín De Cunicult. 1989, 46, 47–49. [Google Scholar]
- Del Águila, C.; Izquierdo, F.; Navajas, R.; Pieniazek, N.J.; Miró, G.; Alonso, A.I.; Da Silva, A.J.; Fenoy, S. Enterocytozoon bieneusi in animals: Rabbits and dogs as new hosts. J. Eukaryot. Microbiol. 1999, 46, 8–9. [Google Scholar]
- Cox, P.; Griffith, M.; Angles, M.; Deere, D.; Ferguson, C. Concentrations of pathogens and indicators in animal feces in the Sydney watershed. Appl. Environ. Microbiol. 2005, 71, 5929–5934. [Google Scholar] [CrossRef]
- Robinson, G.; Chalmers, R.M. The European rabbit (Oryctolagus cuniculus), a source of zoonotic cryptosporidiosis. Zoonoses Public Health 2010, 57, 13. [Google Scholar] [CrossRef] [PubMed]
- Marhoon, I.A.; Mattar, K.T.; Mohammad, F.I. Parasitic Infection in Wild Rabbits Oryctolagus cuniculus. Eurasian. J. Anal. Chem. 2018, 13, 55. [Google Scholar] [CrossRef]
- González-Ramírez, L.C.; Vázquez, C.J.; Chimbaina, M.B.; Djabayan-Djibeyan, P.; Prato-Moreno, J.G.; Trelis, M.; Fuentes, M.V. Ocurrence of enteroparasites with zoonotic potential in animals of the rural area of San Andres, Chimborazo, Ecuador. Vet. Parasitol. Reg. Stud. 2021, 26, 100630. [Google Scholar] [CrossRef] [PubMed]
- Sturdee, A.P.; Chalmers, R.M.; Bull, S.A. Detection of Cryptosporidium oocysts in wild mammals of mainland Britain. Vet. Parasitol. 1999, 80, 273–280. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control (ECDC). Available online: http://atlas.ecdc.europa.eu/public/index.aspx?Dataset=27&HealthTopic=15 (accessed on 22 December 2012).
- Instituto de Salud Carlos III (ISCIII). Available online: https://www.isciii.es/QueHacemos/Servicios/VigilanciaSaludPublicaRENAVE/EnfermedadesTransmisibles/Documents/INFORMES/INFORMES%20RENAVE/ultimo%20informe.pdf (accessed on 22 December 2012).
- Robertson, L.J.; Björkman, C.; Axén, C.; Fayer, R. Cryptosporidiosis in Farmed Animals. In Cryptosporidium: Parasite and Disease; Cacciò, S.M., Widmer, G., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 149–236. [Google Scholar]
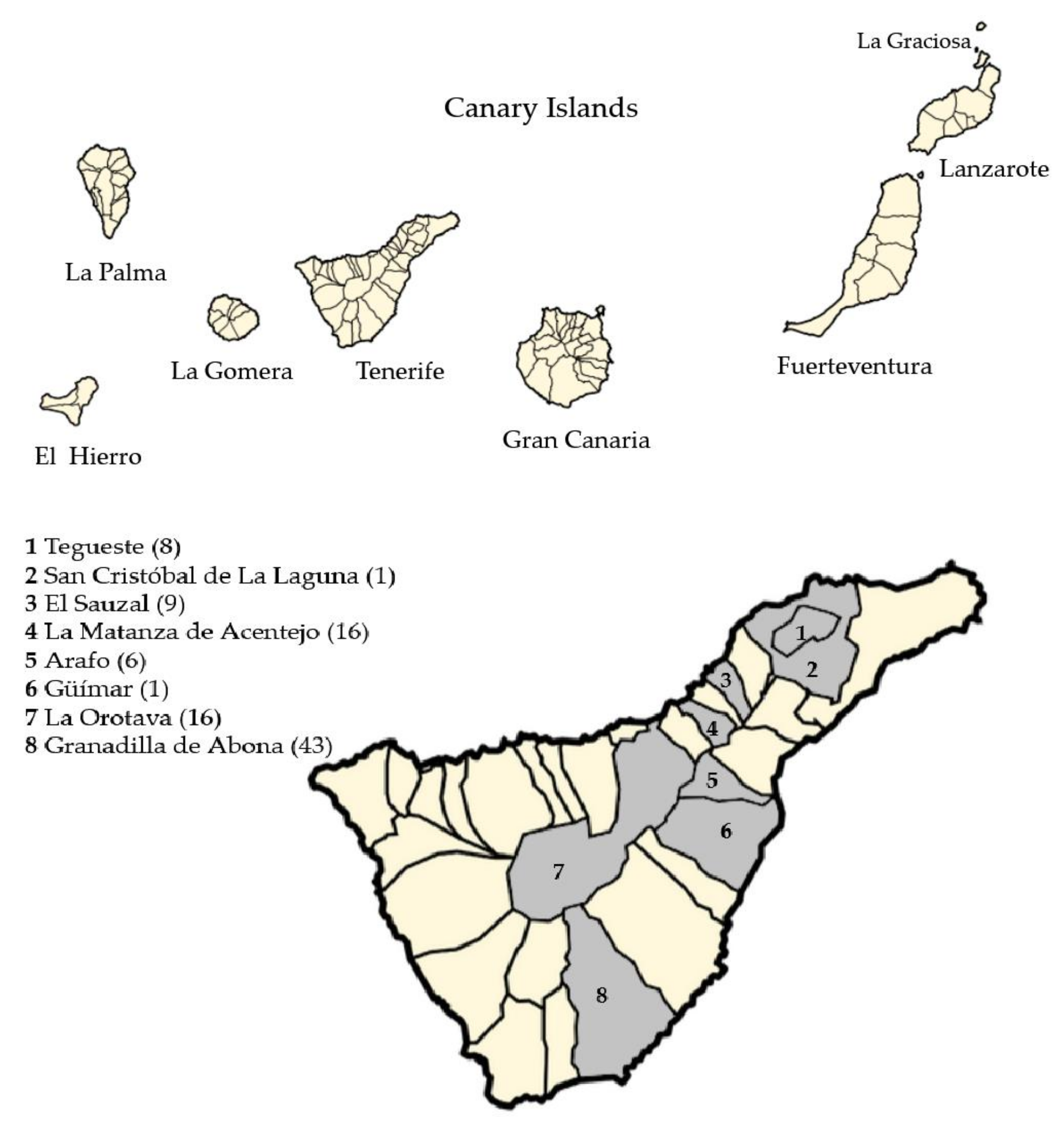
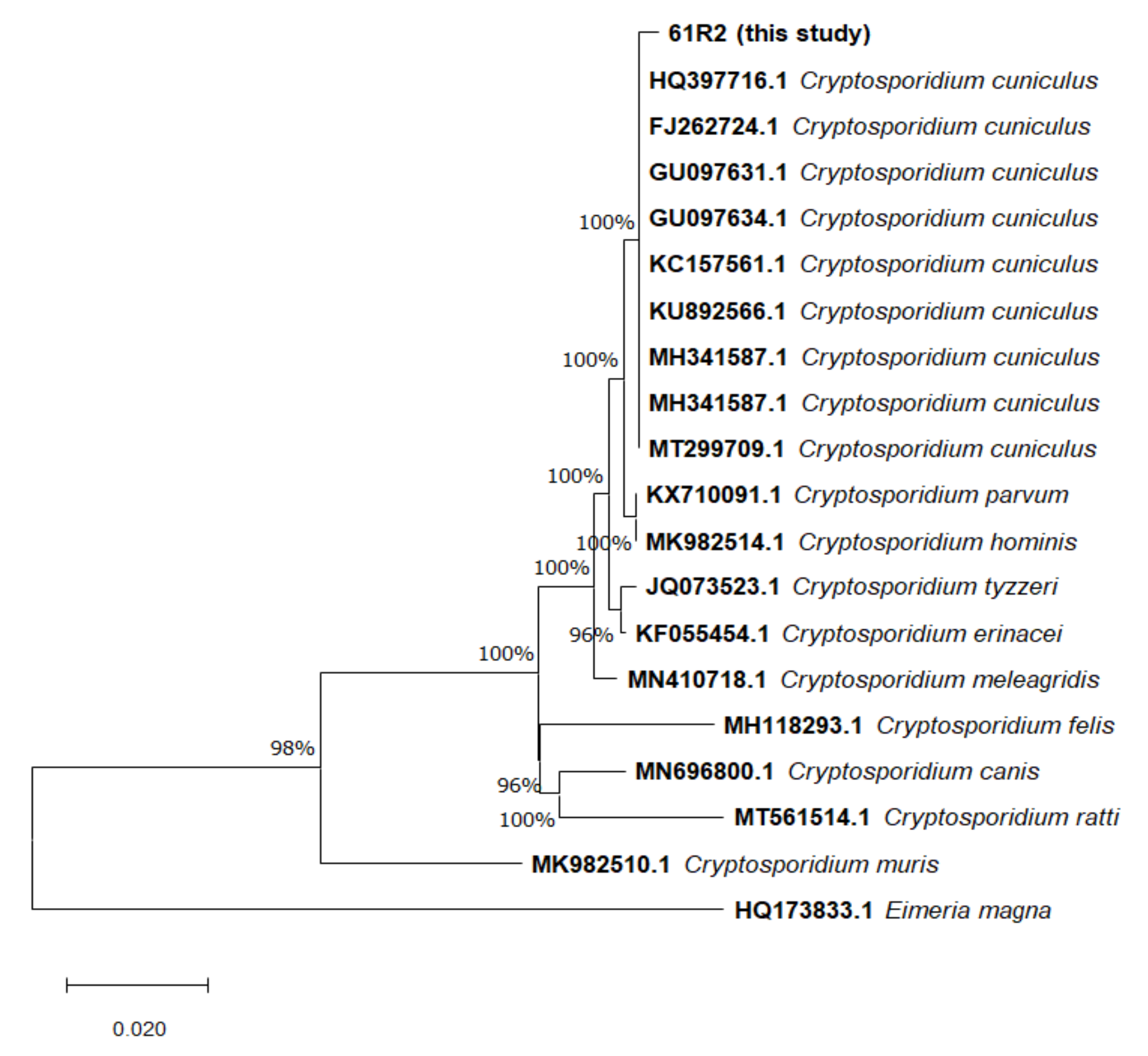
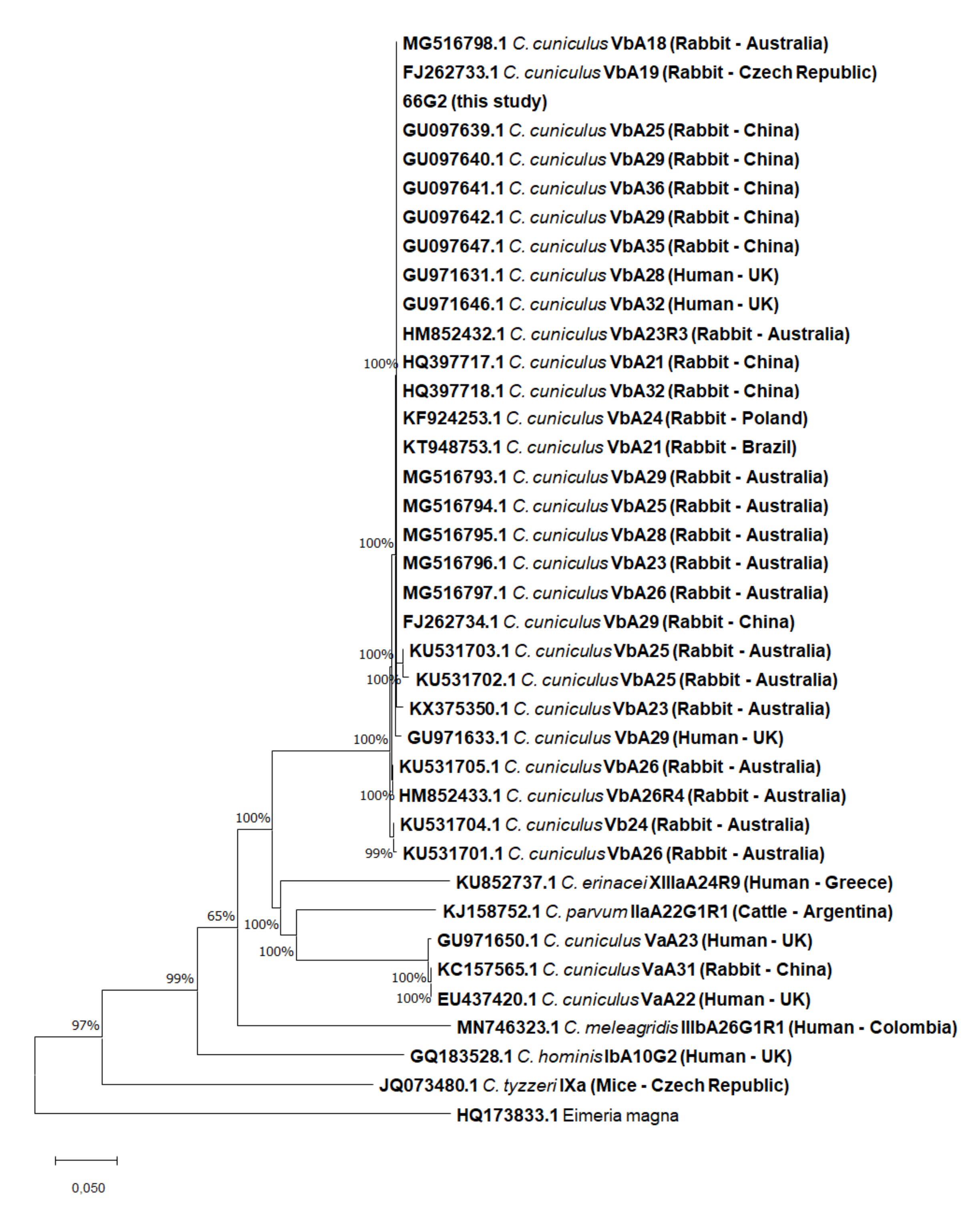
| Country | Subtype (n) | Period | Case/Prevalence (%) (nº Positive Cases/Total) | Reference |
|---|---|---|---|---|
| UK | VaA22 (1) | 2007 | 1 case * | [21,47] |
| UK | VbA11 (1), VbA20 (1), VbA22 (1), VbA23 (1), VbA25 (1), VbA26 (1), VbA28 (1), VbA29(2), VbA30 (1), VbA32 (1), VbA33 (1), VbA34 (1), VbA36 (1), VbA37 (1), VaA9 (1), VaA18 (1) VaA19 (1), VaA21 (1) VaA22 (1) | 2007–2008 | 1.2% (37/3,030) | [48] |
| UK | VaA18 (23) | 2008 | 23 cases (422 estimated) | [21,33] |
| Nigeria | - | 2006–2007 | 6.5% (5/77) | [53] |
| France | - | 2006–2009 | 0.3% (1/310) | [50] |
| Australia | VbA25 (1) | 2009 | 1 case * | [31] |
| Spain | VbA34 (1) | 2015 | 1 case * | [59] |
| New Zealand | VbA22 (2) VbA25 (1) VbA27 (1) | 2009–2015 | 0.7% (4/579) | [54] |
| France | - | 2015–2017 | 1% (1/87) | [51] |
| France | - | 2017–2019 | >1% (no data) | [52] |
| New Zealand | VbA13 (2), VbA15 (1) VbA17 (4), VbA22 (3) VbA23 (2), VbA24 (5) VbA25 (8), VbA26 (3) VbA27 (2), VbA28 (3) | 2009–2019 | 1.3% (33/2,598) | [55] |
| New Zealand | Vb | 2015–2021 | 1.9% (28/1,502) | [56] |
| Canada | VbA38 (1) | 2008–2017 | 0.8% (1/129) | [57] |
| Sweden | VaA19 (1) VbA20R2 (1) VbA29R4 (1) | 2013–2014 | 1.3% (5/379) | [58] |
| Spain | VbA25R3 (1) | |||
| Greece | VbA31R4 (1) | |||
| UK | - | 2018–2020 | 2.8% (3/109) | [49] |
| Target Gene | Primer | Primer Sequences (5′-3′) | Expected Size (bp) | PCR Conditions 1 |
|---|---|---|---|---|
18S rRNA | 18SF1 18SR1 | CCCATTTCCTTCGAAACAGGA TTCTAGAGCTAATACATGCG | 830 | 94 °C—45 s 55 °C—45 s 72 °C—1 min For 35 cycles |
| 18SF2 18SR2 | AAGGAGTAAGGAACAACCTCCA GGAAGGGTTGTATTATTAGATAAAG | 94 °C—45 s 58 °C—45 s 72 °C—1 min For 35 cycles | ||
gp60 | AL3531 AL3535 | ATAGTCTCCGCTGTATTC GGAAGGAACGATGTATCT | 800–850 | 94 °C—45 s 50 °C—45 s 72 °C—1 min For 35 cycles (both steps) |
| AL3532 AL3534 | TCCGCTGTATTCTCAGCC GCAGAGGAACCAGCATC |
| Farmed Rabbits | ||||
| Country | Subtype (n) | Period | Case/Prevalence (%) (nº Positive Cases/Total) | Reference |
| Czech Republic | VbA19 (1) | - | 2 cases * | [19,21] |
| China | VbA29 (18), VbA35 (4), VbA36 (8) | 2007–2008 | 3.4% (37/1,081) | [13] |
| China | VbA32 (3), VbA21 (6) | 2008–2010 | 2.38% (9/378) | [14] |
| Poland | VbA24 (-) | 2012 | 300 cases * | [27] |
| Brazil | VbA21 (7) | 2012 | 12.73% (7/55) | [28] |
| China | VbA28 (2) VbA29 (16) VbA32 (3) | 2015–2016 | 11.2% (24/215) | [16] |
| China | VbA24 (5) | 2015–2017 | 3.4% (11/321) | [17] |
| Egypt | VbA19 (1) VbA33 (15) | 2015–2016 | 11.9% (28/235) | [29] |
| China | VbA24 (1) VbA29 (2) VbA31 (2) VbA33 (1) | - | 6 isolates* | [18] |
| Wild Rabbits | ||||
| Country | Subtype (n) | Period | Case/Prevalence (%) (nº Positive Cases/Total) | Reference |
| New Zealand | - | 2000–2003 | 1 case * | [20] |
| UK | VaA18 (1) | 2008 | 1 case * | [21] |
| Australia | VbA23R3 (11), VbA26R4 (1) | - | 6.8% (12/176) | [22] |
| Australia | VbA22R4 (-) VbA23R3 (-) VbA24R3 (-) VbA25R4 (-) VbA26R4 (-) | 2009–2011 | 8.4% (22/263) | [23] |
| Australia | VbA25 (2) VbA26 (1) VbA26 novel (3) VbA24 novel (1) | 2011–2015 | 2.18% (7/321) | [24] |
| Australia | VbA23 (9) | 2013–2015 | 13.2% (14/106) | [25] |
| Australia | VbA18 (12) VbA23 (46) VbA25 (16) VbA26 (8) VbA28 (2) VbA29 (5) | 2013–2015 | 14.3% (96/672) | [26] |
| Others | ||||
| Country | Subtype (n) | Period | Case/Prevalence (%) (nº Positive Cases/Total) | Reference |
| China (unknown origin) | VbA29 (1) | - | 2 cases * | [12,21] |
| China (animal house) | VaA31 (3) | - | 1.03% (3/290) | [15] |
| Year | Cases | Island (n) | Notification Rate (per 100,000 Population) | Reference |
|---|---|---|---|---|
| 2015 | 4 | Gran Canaria (4) | 0.2 | [62] |
| 2016 | 4 | Gran Canaria (2) Lanzarote (2) | 0.2 | |
| 2017 | 26 | Gran Canaria (25) Lanzarote (1) | 1.2 | |
| 2018 | 10 | Gran Canaria (9) La Palma (1) | 0.5 | |
| 2019 | 7 | Gran Canaria (7) | 0.3 | |
| 2020 | 3 | Gran Canaria (3) | 0.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baz-González, E.; Martín-Carrillo, N.; García-Livia, K.; Foronda, P. Molecular Detection of Cryptosporidium cuniculus in Rabbits (Oryctolagus cuniculus) from Tenerife, Canary Islands, Spain. Vet. Sci. 2022, 9, 91. https://doi.org/10.3390/vetsci9020091
Baz-González E, Martín-Carrillo N, García-Livia K, Foronda P. Molecular Detection of Cryptosporidium cuniculus in Rabbits (Oryctolagus cuniculus) from Tenerife, Canary Islands, Spain. Veterinary Sciences. 2022; 9(2):91. https://doi.org/10.3390/vetsci9020091
Chicago/Turabian StyleBaz-González, Edgar, Natalia Martín-Carrillo, Katherine García-Livia, and Pilar Foronda. 2022. "Molecular Detection of Cryptosporidium cuniculus in Rabbits (Oryctolagus cuniculus) from Tenerife, Canary Islands, Spain" Veterinary Sciences 9, no. 2: 91. https://doi.org/10.3390/vetsci9020091
APA StyleBaz-González, E., Martín-Carrillo, N., García-Livia, K., & Foronda, P. (2022). Molecular Detection of Cryptosporidium cuniculus in Rabbits (Oryctolagus cuniculus) from Tenerife, Canary Islands, Spain. Veterinary Sciences, 9(2), 91. https://doi.org/10.3390/vetsci9020091







