First Study to Describe the Prevalence of Porcine Reproductive and Respiratory Syndrome Virus and Porcine Circovirus Type 2 among the Farmed Pig Population in the Hong Kong Special Administrative Region
Abstract
1. Introduction
2. Materials and Methods
2.1. The Study Population and Pig Production System
2.2. Pig Samples
2.3. Blood Sample Processing and Testing
2.4. Statistical Analysis
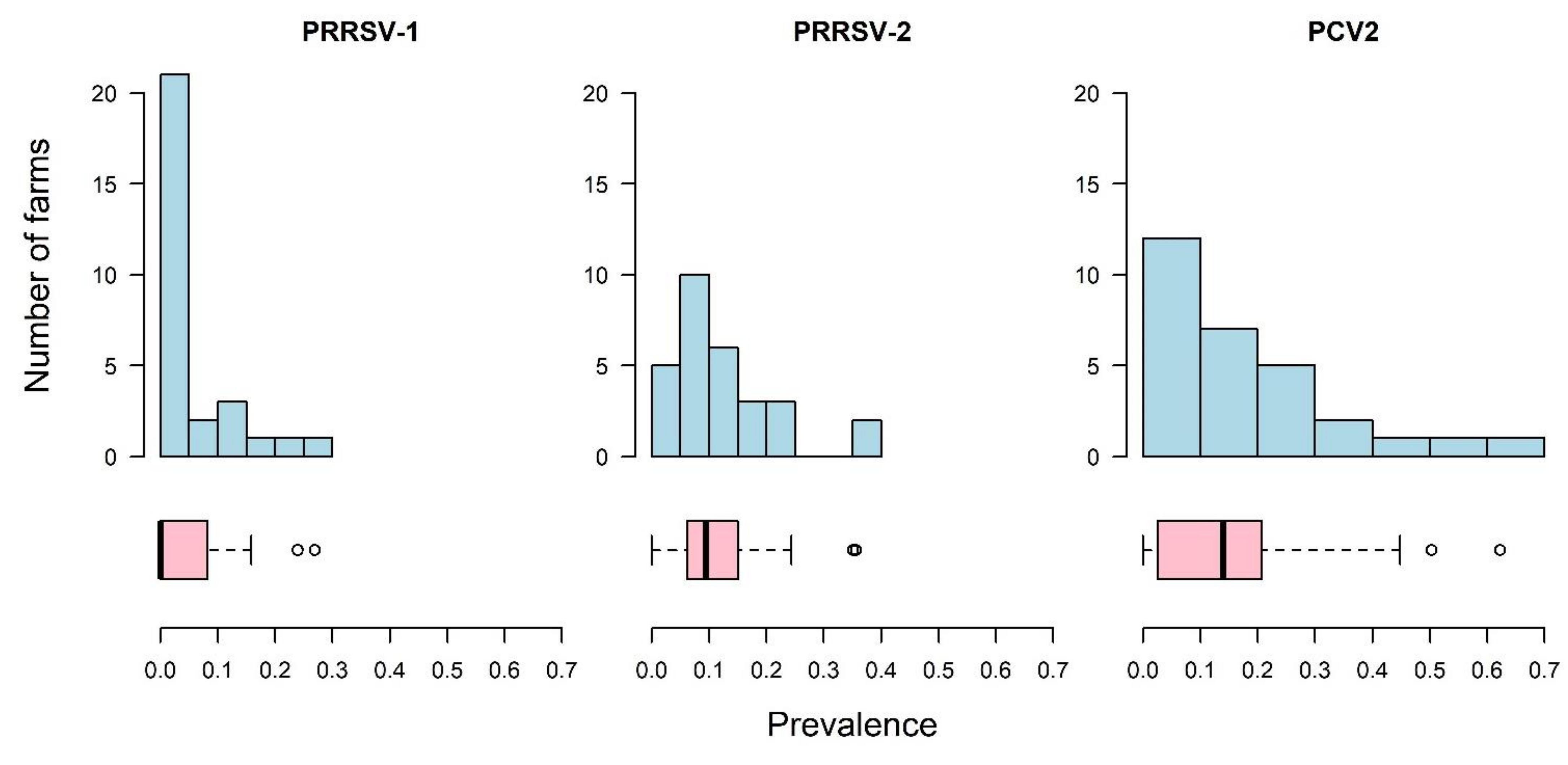
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cleveland-Nielsen, A.; Nielsen, E.O.; Ersbøll, A.K. Chronic pleuritis in Danish slaughter pig herds. Prev. Vet. Med. 2002, 55, 121–135. [Google Scholar] [CrossRef]
- Nathues, H.; Alarcon, P.; Rushton, J.; Jolie, R.; Fiebig, K.; Jimenez, M.; Geurts, V.; Nathues, C. Cost of porcine reproductive and respiratory syndrome virus at individual farm level—An economic disease model. Prev. Vet. Med. 2017, 142, 16–29. [Google Scholar] [CrossRef] [PubMed]
- Sassu, E.L.; Bossé, J.T.; Tobias, T.J.; Gottschalk, M.; Langford, P.R.; Hennig-Pauka, I. Update on Actinobacillus pleuropneumoniae—Knowledge, gaps and challenges. Transbound. Emerg. Dis. 2018, 65, 72–90. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, C.S.; Christiansen, M.G.; Pedersen, K.; Larsen, L.E. Production losses five months after outbreak with a recombinant of two PRRSV vaccine strains in 13 Danish sow herds. Porc. Health Manag. 2020, 6, 26. [Google Scholar] [CrossRef] [PubMed]
- Fablet, C.; Rose, N.; Grasland, B.; Robert, N.; Lewandowski, E.; Gosselin, M. Factors associated with the growing-finishing performances of swine herds: An exploratory study on serological and herd level indicators. Porc. Health Manag. 2018, 4, 6. [Google Scholar] [CrossRef]
- Chantziaras, I.; De Meyer, D.; Vrielinck, L.; Van Limbergen, T.; Pineiro, C.; Dewulf, J.; Kyriazakis, I.; Maes, D. Environment-, health-, performance- and welfare-related parameters in pig barns with natural and mechanical ventilation. Prev. Vet. Med. 2020, 183, 105150. [Google Scholar] [CrossRef]
- Hälli, O.; Haimi-Hakala, M.; Oliviero, C.; Heinonen, M. Herd-level risk factors for chronic pleurisy in finishing pigs: A case-control study. Porc. Health Manag. 2020, 6, 21. [Google Scholar] [CrossRef]
- Sanhueza, J.M.; Stevenson, M.A.; Vilalta, C.; Kikuti, M.; Corzo, C.A. Spatial relative risk and factors associated with porcine reproductive and respiratory syndrome outbreaks in United States breeding herds. Prev. Vet. Med. 2020, 183, 105128. [Google Scholar] [CrossRef]
- Sanchez-Vazquez, M.J.; Strachan, W.D.; Armstrong, D.; Nielen, M.; Gunn, G.J. Papers: The British pig health schemes: Integrated systems for large-scale pig abattoir lesion monitoring. Vet. Rec. 2011, 169, 413. [Google Scholar] [CrossRef]
- Rodrigues da Costa, M.; Fitzgerald, R.M.; Manzanilla, E.G.; O’Shea, H.; Moriarty, J.; McElroy, M.C.; Leonard, F.C. A cross-sectional survey on respiratory disease in a cohort of Irish pig farms. Ir. Vet. J. 2020, 73, 24. [Google Scholar] [CrossRef]
- Duinhof, T.; Van Schaik, G.; Van Esch, E.; Wellenberg, G. Detection of PRRSV circulation in herds without clinical signs of PRRS: Comparison of five age groups to assess the preferred age group and sample size. Vet. Microbiol. 2011, 150, 180–184. [Google Scholar] [CrossRef] [PubMed]
- Neumann, E.J.; Kliebenstein, J.B.; Johnson, C.D.; Mabry, J.W.; Bush, E.J.; Seitzinger, A.H.; Green, A.L.; Zimmerman, J.J. Assessment of the economic impact of porcine reproductive and respiratory syndrome on swine production in the United States. J. Am. Vet. Med. Assoc. 2005, 227, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Thomann, B.; Rushton, J.; Schuepbach-Regula, G.; Nathues, H. Modeling Economic Effects of Vaccination Against Porcine Reproductive and Respiratory Syndrome: Impact of Vaccination Effectiveness, Vaccine Price, and Vaccination Coverage. Front. Vet. Sci. 2020, 7, 500. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, J.J.; Karriker, L.A.; Ramirez, A.; Schwartz, K.J.; Stevenson, G.W.; Zhang, J. Diseases of Swine, 11th ed.; John Wiley & Sons, Incorporated: Newark, NJ, USA, 2019. [Google Scholar]
- Renken, C.; Nathues, C.; Swam, H.; Fiebig, K.; Weiss, C.; Eddicks, M.; Ritzmann, M.; Nathues, H. Application of an economic calculator to determine the cost of porcine reproductive and respiratory syndrome at farm-level in 21 pig herds in Germany. Porc. Health Manag. 2021, 7, 3. [Google Scholar] [CrossRef] [PubMed]
- Holtkamp, D.J.; Kliebenstein, J.B.; Neumann, E.J.; Zimmerman, J.J.; Rotto, H.F.; Yoder, T.K.; Wang, C.; Yeske, P.E.; Mowrer, C.L.; Haley, C.A. Assessment of the economic impact of porcine reproductive and respiratory syndrome virus on United States pork producers. J. Swine Health Prod. 2013, 21, 72–84. [Google Scholar]
- Alarcon, P.; Rushton, J.; Nathues, H.; Wieland, B. Economic efficiency analysis of different strategies to control post-weaning multi-systemic wasting syndrome and porcine circovirus type 2 subclinical infection in 3-weekly batch system farms. Prev. Vet. Med. 2013, 110, 103–118. [Google Scholar] [CrossRef]
- Pham, H.; Antoine-Moussiaux, N.; Grosbois, V.; Moula, N.; Truong, B.D.; Phan, T.D.; Vu, T.; Trinh, T.Q.; Vu, C.; Rukkwamsuk, T. Financial impacts of priority swine diseases to pig farmers in Red River and Mekong River Delta, Vietnam. Transbound. Emerg. Dis. 2017, 64, 1168–1177. [Google Scholar] [CrossRef]
- Tornimbene, B.; Frossard, J.-P.; Chhim, V.; Sorn, S.; Guitian, J.; Drew, T. Emergence of highly pathogenic porcine reproductive and respiratory syndrome (HP-PRRS) in medium-scale swine farms in southeastern Cambodia. Prev. Vet. Med. 2015, 118, 93–103. [Google Scholar] [CrossRef]
- Lv, N.; Zhu, L.; Li, W.; Li, Z.; Qian, Q.; Zhang, T.; Liu, L.; Hong, J.; Zheng, X.; Wang, Y.; et al. Molecular epidemiology and genetic variation analyses of porcine circovirus type 2 isolated from Yunnan Province in China from 2016–2019. BMC Vet. Res. 2020, 16, 96. [Google Scholar] [CrossRef]
- Wang, Y.; Noll, L.; Lu, N.; Porter, E.; Stoy, C.; Zheng, W.; Liu, X.; Peddireddi, L.; Niederwerder, M.; Bai, J. Genetic diversity and prevalence of porcine circovirus type 3 (PCV3) and type 2 (PCV2) in the Midwest of the USA during 2016–2018. Transbound. Emerg. Dis. 2020, 67, 1284–1294. [Google Scholar] [CrossRef]
- Thangthamniyom, N.; Sangthong, P.; Poolperm, P.; Thanantong, N.; Boonsoongnern, A.; Hansoongnern, P.; Semkum, P.; Petcharat, N.; Lekcharoensuk, P. Genetic diversity of porcine circovirus type 2 (PCV2) in Thailand during 2009–2015. Vet. Microbiol. 2017, 208, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Weigel, R.; Firkins, L.; Scherba, G. Prevalence and risk factors for infection with Porcine Reproductive and Respiratory Syndrome Virus (PRRSV) in swine herds in Illinois (USA). Vet. Res. 2000, 31, 87–88. [Google Scholar] [CrossRef]
- Cheon, D.S.; Chae, C.; Lee, Y.S. Seroprevalence of antibody to porcine reproductive and respiratory syndrome virus using enzyme-linked immunosorbent assay in selected herds in Korea. J. Vet. Diagn. Investig. 1997, 9, 434–436. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Gong, Q.-L.; Nie, L.-B.; Wang, Q.; Ge, G.-Y.; Li, D.-L.; Ma, B.-Y.; Sheng, C.-Y.; Su, N.; Zong, Y.; et al. Prevalence of porcine circovirus 2 throughout China in 2015–2019: A systematic review and meta-analysis. Microb. Pathog. 2020, 149, 104490. [Google Scholar] [CrossRef]
- Cheong, Y.; Oh, C.; Lee, K.; Cho, K.H. Survey of porcine respiratory disease complex-associated pathogens among commercial pig farms in Korea via oral fluid method. J. Vet. Sci. 2017, 18, 283–289. [Google Scholar] [CrossRef]
- Anonymous. The Government of the HKSAR Press Releases LCQ14: Measures to Alleviate the Impacts of African Swine Fever. Available online: https://www.info.gov.hk/gia/general/201911/20/P2019112000356.htm (accessed on 23 June 2021).
- Anonymous. Food and Environmental Hygiene Department of the Government of the HKSAR, Slaughterhouses, Monthly Average Daily Supply and Auction Prices of Live Pigs in the Past 12 Months. Available online: https://www.fehd.gov.hk/english/sh/data/supply_avg_tw.html (accessed on 23 June 2021).
- Brar, M.S.; Shi, M.; Hui, R.K.-H.; Leung, F.C.-C. Genomic evolution of porcine reproductive and respiratory syndrome virus (PRRSV) isolates revealed by deep sequencing. PLoS ONE 2014, 9, e88807. [Google Scholar]
- Anonymous. Livestock Keeping Licence. Available online: https://www.afcd.gov.hk/english/agriculture/agr_live/agr_live.html (accessed on 29 December 2021).
- Anonymous. Slaughterhouses and Food Animals Monitoring. Available online: https://www.cfs.gov.hk/english/import/import_smi.html (accessed on 29 December 2021).
- Anonymous. Quantification of Porcine Reproductive and Respiratory Syndrome Virus Genomes Genesig Advanced Kit Handbook HB10.05.10. Available online: https://www.genesig.com/assets/files/prrsv.pdf (accessed on 10 January 2022).
- Anonymous. Quantification of Porcine Circovirus 2 Genomes Genesig Advanced Kit Handbook HB10.03.11. Available online: https://www.genesig.com/assets/files/pcv2.pdf (accessed on 10 January 2022).
- Yang, D.A.; Laven, R.A. Design-based approach for analysing survey data in veterinary research. Vet. Sci. 2021, 8, 105. [Google Scholar] [CrossRef]
- Lumley, T. Analysis of complex survey samples. J. Stat. Softw. 2004, 9, 1–19. [Google Scholar] [CrossRef]
- Korn, E.L.; Graubard, B.I. Confidence intervals for proportions with small expected number of positive counts estimated from survey data. Surv. Methodol. 1998, 24, 193–201. [Google Scholar]
- Holtkamp, D.J.; Polson, D.D.; Torremorell, M.; Morrison, B.; Classen, D.M.; Becton, L.; Henry, S.; Rodibaugh, M.T.; Rowland, R.R.; Snelson, H.; et al. Terminology for classifying swine herds by porcine reproductive and respiratory syndrome virus status. J. Swine Health Prod. 2011, 19, 44–56. [Google Scholar]
- Khophloiklang, V.; Sariya, L.; Nara-Arj, P.; Poomikasemsak, M.; Areekit, K.; Kamyun, N.; Sangpoom, S.; Chamkasem, A.; Laohasinnarong, D. Prevalence of porcine circovirus type 2 (PCV-2) in smallholder pig farms in Thung-Yai, Nakhon Si Thammarat, Thailand. Walailak J. Sci. Technol. 2021, 18, 1–10. [Google Scholar] [CrossRef]
- Kim, S.C.; Nazki, S.; Kwon, S.; Juhng, J.H.; Mun, K.H.; Jeon, D.Y.; Jeong, C.G.; Khatun, A.; Kang, S.J.; Kim, W.I. The prevalence and genetic characteristics of porcine circovirus type 2 and 3 in Korea. BMC Vet. Res. 2018, 14, 294. [Google Scholar] [CrossRef] [PubMed]
- Qu, T.; Li, R.; Yan, M.; Luo, B.; Yang, T.; Yu, X. High prevalence of PCV2d in Hunan province, China: A retrospective analysis of samples collected from 2006 to 2016. Arch. Virol. 2018, 163, 1897–1906. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Ruan, H.; Qiao, S.; Deng, R.; Zhang, G. Co-infection status of porcine circoviruses (PCV2 and PCV3) and porcine epidemic diarrhea virus (PEDV) in pigs with watery diarrhea in Henan province, central China. Microb. Pathog. 2020, 142, 104047. [Google Scholar] [CrossRef]
- Done, S.; Paton, D.; White, M. Porcine reproductive and respiratory syndrome (PRRS): A review, with emphasis on pathological, virological and diagnostic aspects. Br. Vet. J. 1996, 152, 153–174. [Google Scholar] [CrossRef]
- Wellenberg, G.; Bouwkamp, F.; Wolf, P.; Swart, W.; Mombarg, M.; De Gee, A. A study on the severity and relevance of porcine circovirus type 2 infections in Dutch fattening pigs with respiratory diseases. Vet. Microbiol. 2010, 142, 217–224. [Google Scholar] [CrossRef][Green Version]


| PRRSV-1 | PRRSV-2 | PCV2 | |
|---|---|---|---|
| 1 | 3 (3.9%) | 4 (5.2%) | 3 (7.8%) |
| 2 | 12 (24.0%) | 6 (12.7%) | 0 (0.0%) |
| 3 | 2 (3.5%) | 12 (35.6%) | 12(14.0%) |
| 4 | 9 (15.9%) | 3 (7.6%) | 10 (24.1%) |
| 5 | 0 (0.0%) | 3 (6.2%) | 18 (39.9%) |
| 6 | 0 (0.0%) | 8 (9.5%) | 1 (1.2%) |
| 7 | 0 (0.0%) | 0 (0.0%) | 4 (14.1%) |
| 8 | 5 (12.4%) | 0 (0.0%) | 18 (44.9%) |
| 9 | 13 (26.9%) | 3 (6.8%) | 0 (0.0%) |
| 10 | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| 11 | 0 (0.0%) | 12 (35.1%) | 8 (20.8%) |
| 12 | 0 (0.0%) | 9 (12.5%) | 9 (20.4%) |
| 13 | 0 (0.0%) | 11 (18.8%) | 4 (14.1%) |
| 14 | 7 (13.1%) | 0 (0.0%) | 24 (62.3%) |
| 15 | 0 (0.0%) | 5 (8.7%) | 14 (28.1%) |
| 16 | 0 (0.0%) | 4 (7.1%) | 4 (10.2%) |
| 17 | 5 (9.5%) | 1 (1.9%) | 22 (50.3%) |
| 18 | 0 (0.0%) | 8 (14.2%) | 10 (11.0%) |
| 19 | 1 (2.1%) | 9 (20.9%) | 8 (20.5%) |
| 20 | 2 (1.8%) | 9 (24.4%) | 15 (19.0%) |
| 21 | 0 (0.0%) | 8 (13.5%) | 5 (7.4%) |
| 22 | 0 (0.0%) | 6 (11.1%) | 0 (0.0%) |
| 23 | 6 (11.8%) | 8 (17.7%) | 4 (8.5%) |
| 24 | 4 (8.2%) | 6 (12.3%) | 8 (16.7%) |
| 25 | 0 (0.0%) | 7 (15.0%) | 1 (8.2%) |
| 26 | 2 (3.6%) | 5 (8.9%) | 0 (0.0%) |
| 27 | 2 (4.1%) | 4 (7.3%) | 1 (2.5%) |
| 28 | 0 (0.0%) | 10 (21.1%) | 0 (0.0%) |
| 29 | 0 (0.0%) | 4 (6.1%) | 21 (39.0%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flay, K.J.; Yang, D.A.; Choi, S.C.; Ip, J.; Lee, S.H.; Pfeiffer, D.U. First Study to Describe the Prevalence of Porcine Reproductive and Respiratory Syndrome Virus and Porcine Circovirus Type 2 among the Farmed Pig Population in the Hong Kong Special Administrative Region. Vet. Sci. 2022, 9, 80. https://doi.org/10.3390/vetsci9020080
Flay KJ, Yang DA, Choi SC, Ip J, Lee SH, Pfeiffer DU. First Study to Describe the Prevalence of Porcine Reproductive and Respiratory Syndrome Virus and Porcine Circovirus Type 2 among the Farmed Pig Population in the Hong Kong Special Administrative Region. Veterinary Sciences. 2022; 9(2):80. https://doi.org/10.3390/vetsci9020080
Chicago/Turabian StyleFlay, Kate J., Dan A. Yang, Sze Chun Choi, Joyce Ip, Song H. Lee, and Dirk U. Pfeiffer. 2022. "First Study to Describe the Prevalence of Porcine Reproductive and Respiratory Syndrome Virus and Porcine Circovirus Type 2 among the Farmed Pig Population in the Hong Kong Special Administrative Region" Veterinary Sciences 9, no. 2: 80. https://doi.org/10.3390/vetsci9020080
APA StyleFlay, K. J., Yang, D. A., Choi, S. C., Ip, J., Lee, S. H., & Pfeiffer, D. U. (2022). First Study to Describe the Prevalence of Porcine Reproductive and Respiratory Syndrome Virus and Porcine Circovirus Type 2 among the Farmed Pig Population in the Hong Kong Special Administrative Region. Veterinary Sciences, 9(2), 80. https://doi.org/10.3390/vetsci9020080







