Electrocardiographic Findings and Cardiac Troponin I Assay in Dogs with SIRS Diagnosis
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Study Group
2.3. Electrocardiographic Examination
2.4. Collection of Blood Samples and Laboratory Procedures
2.5. Statistical Analysis
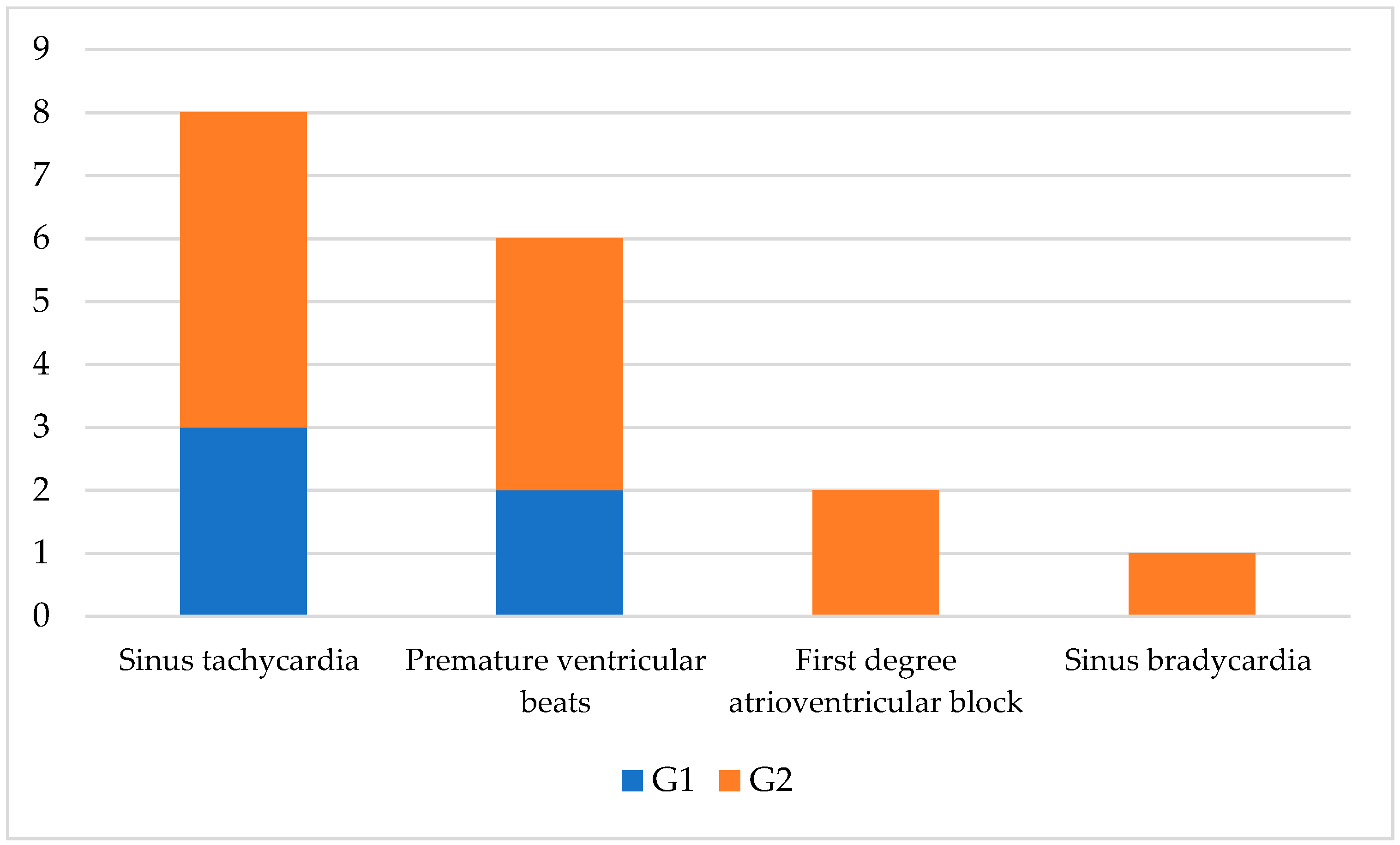
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- De Laforcade, A.M. Systemic inflammatory response syndrome. In Small Animal Critical Care Medicine, 2nd ed.; Silverstein, D.C., Hopper, K., Eds.; Saunders Elsevier: St. Louis, MO, USA, 2015; pp. 31–34. [Google Scholar]
- Chakraborty, R.K.; Burns, B. Systemic Inflammatory Response Syndrome. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Brady, C.A.; Otto, C.M. Systemic inflammatory response syndrome, sepsis, and multiple organ dysfunction. Vet. Clin. N. Am. Small Anim. Pract. 2001, 31, 1147–1162. [Google Scholar] [CrossRef] [PubMed]
- Lord, J.M.; Midwinter, M.J.; Chen, Y.F.; Belli, A.; Brohi, K.; Kovacs, E.J.; Koenderman, L.; Kubes, P.; Lilford, R.J. The systemic immune response to trauma: An overview of pathophysiology and treatment. Lancet 2014, 384, 1455–1465. [Google Scholar] [CrossRef] [PubMed]
- Binkowska, A.M.; Michalak, G.; Słotwiński, R. Current views on the mechanisms of immune responses to trauma and infection. Cent. Eur. J. Immunol. 2015, 40, 206–216. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, M. Acute pancreatitis as a model of SIRS. Front. Biosci. 2009, 14, 2042–2050. [Google Scholar] [CrossRef]
- Welzl, C.; Leisewitz, A.L.; Jacobson, L.S.; Vaughan-Scott, T.; Myburgh, E. Systemic inflammatory response syndrome and multiple-organ damage/dysfunction in complicated canine babesiosis. J. S. Afr. Vet. Assoc. 2001, 72, 158–162. [Google Scholar] [CrossRef] [PubMed]
- Beletić, A.; Janjić, F.; Radaković, M.; Spariosu, K.; Francuski Andrić, J.; Chandrashekar, R.; Tyrrell, P.; Radonjić, V.; Balint, B.; Ajtić, J.; et al. Systemic inflammatory response syndrome in dogs naturally infected with Babesia canis: Association with the parasite load and host factors. Vet. Parasitol. 2021, 291, 109366. [Google Scholar] [CrossRef] [PubMed]
- Mittleman Boller, E.; Otto, C.M. Sepsis. In Small Animal Critical Care Medicine, 2nd ed.; Silverstein, D.C., Hopper, K., Eds.; Saunders Elsevier: St. Louis, MO, USA, 2015; pp. 454–458. [Google Scholar]
- Merx, M.W.; Weber, C. Sepsis and the heart. Circulation 2007, 116, 793–802. [Google Scholar] [CrossRef] [PubMed]
- Rudiger, A.; Singer, M. Mechanisms of sepsis-induced cardiac dysfunction. Crit. Care Med. 2007, 35, 1599–1608. [Google Scholar] [CrossRef]
- Levy, R.J.; Piel, D.A.; Acton, P.D.; Zhou, R.; Ferrari, V.A.; Karp, J.S.; Deutschman, C.S. Evidence of myocardial hibernation in the septic heart. Crit. Care Med. 2005, 33, 2752–2756. [Google Scholar] [CrossRef]
- Matsuda, N. Alert cell strategy in SIRS-induced vasculitis: Sepsis and endothelial cells. J. Intensive Care 2016, 4, 21. [Google Scholar] [CrossRef] [PubMed]
- Rich, M.M.; McGarvey, M.L.; Teener, J.W.; Frame, L.H. ECG changes during septic shock. Cardiology 2002, 97, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Wasserstrum, Y.; Lotan, D.; Itelman, E.; Barbarova, I.; Kogan, M.; Klempfner, R.; Dagan, A.; Segal, G. Corrected QT interval anomalies are associated with worse prognosis among patients suffering from sepsis. Intern. Med. J. 2016, 46, 1204–1211. [Google Scholar] [CrossRef] [PubMed]
- Walkey, A.J.; McManus, D. When rhythm changes cause the blues: New-onset atrial fibrillation during sepsis. Am. J. Respir. Crit. Care Med. 2017, 195, 152–154. [Google Scholar] [CrossRef] [PubMed]
- Leibovici, L.; Gafter-Gvili, A.; Paul, M.; Almanasreh, N.; Tacconelli, E.; Andreassen, S.; Nielsen, A.D.; Frank, U.; Cauda, R. Relative tachycardia in patients with sepsis: An independent risk factor for mortality. QJM 2007, 100, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Babuin, L.; Vasile, V.C.; Rio Perez, J.A.; Alegria, J.R.; Chai, H.S.; Afessa, B.; Jaffe, A.S. Elevated cardiac troponin is an independent risk factor for short- and long-term mortality in medical intensive care unit patients. Crit. Care Med. 2008, 36, 759–765. [Google Scholar] [CrossRef] [PubMed]
- Filatov, V.L.; Katrukha, A.G.; Bulargina, T.V.; Gusev, N.B. Troponin: Structure, properties, and mechanism of functioning. Biochemistry 1999, 64, 969–985. [Google Scholar]
- Adams, J.E.; Bodor, G.S.; Dávila-Román, V.G.; Delmez, J.A.; Apple, F.S.; Ladenson, J.H.; Jaffe, A.S. Cardiac troponin I. A marker with high specificity for cardiac injury. Circulation 1993, 88, 101–106. [Google Scholar] [CrossRef]
- Langhorn, R.; Willesen, J.L. Cardiac troponins in dogs and cats. J. Vet. Intern. Med. 2016, 30, 36–50. [Google Scholar] [CrossRef]
- Dickinson, A.; Rozanski, E.; Rush, J. Reversible myocardial depression associated with sepsis in a dog. J. Vet. Intern. Med. 2007, 21, 1117–1120. [Google Scholar] [CrossRef]
- Nelson, O.; Thompson, P. Cardiovascular dysfunction in dogs associated with critical illnesses. J. Am. Anim. Hosp. Assoc. 2006, 42, 344–349. [Google Scholar] [CrossRef]
- Gommeren, K.; Desmas, I.; Garcia, A.; Clercx, C.; Mc Entee, K.; Merveille, A.C.; Peeters, D. Cardiovascular biomarkers in dogs with systemic inflammatory response syndrome. J. Vet. Emerg. Crit. Care. 2019, 29, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Werdan, K.; Schmidt, H.; Ebelt, H.; Zorn-Pauly, K.; Koidl, B.; Hoke, R.S.; Heinroth, K.; Müller-Werdan, U. Impaired regulation of cardiac function in sepsis, SIRS, and MODS. Can. J. Physiol. Pharmacol. 2009, 87, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Kenney, E.M.; Rozanski, E.A.; Rush, J.E.; deLaforcade-Buress, A.M.; Berg, J.R.; Silverstein, D.C.; Montealegre, C.D.; Jutkowitz, L.A.; Adamantos, S.; Ovbey, D.H.; et al. Association between outcome and organ system dysfunction in dogs with sepsis: 114 cases (2003–2007). J. Am. Vet. Med. Assoc. 2010, 236, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Sharkey, L.C.; Berzina, I.; Ferasin, L.; Tobias, A.H.; Lulich, J.P.; Hegstad-Davies, R.L. Evaluation of serum cardiac troponin I concentration in dogs with renal failure. J. Am. Vet. Med. Assoc. 2009, 15, 767–770. [Google Scholar] [CrossRef]
- Santilli, R.; Sidney Moïse, N.; Pariaut, R.; Perego, M. Electrocardiography of the Dog and Cat. Diagnosis of Arrhythmias, 2nd ed.; Edra: Milano, Italy, 2018. [Google Scholar]
- Santilli, R.A.; Vázquez, D.M.P.; Gerou-Ferriani, M.; Lombardo, S.F.; Perego, M. Development and assessment of a novel precordial lead system for accurate detection of right atrial and ventricular depolarization in dogs with various thoracic conformations. Am. J. Vet. Res. 2019, 80, 358–368. [Google Scholar] [CrossRef] [PubMed]
- Kraus, M.; Moïse, N.S.; Rishniw, M.; Dykes, N.; Erb, H.N. Morphology of ventricular arrhythmias in the boxer as measured by 12-lead electrocardiography with pace-mapping comparison. J. Vet. Intern. Med. 2002, 16, 153–158. [Google Scholar] [CrossRef]
- Tilley, L.P. The approach to the electrocardiogram. In Essentials of Canine and Feline Electrocardiography, 3rd ed.; Tilley, L.P., Ed.; Lea & Febiger: Philadelphia, PA, USA, 1994. [Google Scholar]
- Hamlin, R.L.; Kijtawornrat, A.; Keene, B.W. How many cardiac cycles must be measured to permit accurate RR, QT, and QTc estimates in conscious dogs? J. Pharmacol. Toxicol. Methods 2004, 50, 103–108. [Google Scholar] [CrossRef]
- Oyama, M.A.; Sisson, D.D. Cardiac troponin-I concentration in dogs with cardiac disease. J. Vet. Intern. Med. 2004, 18, 831–839. [Google Scholar] [CrossRef]
- Payne, E.E.; Roberts, B.K.; Schroeder, N.; Burk, R.L.; Schermerhorn, T. Assessment of a point-of-care cardiac troponin I test to differentiate cardiac from noncardiac causes of respiratory distress in dogs. J. Vet. Emerg. Crit. Care 2011, 21, 217–225. [Google Scholar] [CrossRef]
- Schober, K.E.; Cornand, C.; Kirbach, B.; Aupperle, H.; Oechtering, G. Serum cardiac troponin I and cardiac troponin T concentrations in dogs with gastric dilatation-volvulus. J. Am. Vet. Med. Assoc. 2002, 221, 381–388. [Google Scholar] [CrossRef]
- Langhorn, R.; Thawley, V.; Oyama, M.A.; King, L.G.; Machen, M.C.; Trafny, D.J.; Willesen, J.L.; Tarnow, I.; Kjelgaard-Hansen, M. Prediction of long-term outcome by measurement of serum concentration of cardiac troponins in critically ill dogs with systemic inflammation. J. Vet. Intern. Med. 2014, 28, 1492–1497. [Google Scholar] [CrossRef] [PubMed]
- Hamacher, L.; Dörfelt, R.; Müller, M.; Wess, G. Serum cardiac troponin I concentrations in dogs with systemic inflammatory response syndrome. J. Vet. Intern. Med. 2015, 29, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Langhorn, R.; Oyama, M.A.; King, L.G.; Machen, M.C.; Trafny, D.J.; Thawley, V.; Willesen, J.L.; Tarnow, I.; Kjelgaard-Hansen, M. Prognostic importance of myocardial injury in critically ill dogs with systemic inflammation. J. Vet. Intern. Med. 2013, 27, 895–903. [Google Scholar] [CrossRef]
- Parker, M.; Shelhamer, J.; Bacharach, S.; Green, M.V.; Natanson, C.; Frederick, T.M.; Damske, B.A.; Parrillo, J.E. Profound but reversible myocardial depression in patients with septic shock. Ann. Intern. Med. 1984, 100, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Court, O.; Kumar, A.; Parrillo, J.E.; Kumar, A. Clinical review: Myocardial depression in sepsis and septic shock. Crit. Care 2002, 6, 500–508. [Google Scholar] [CrossRef] [PubMed]
- Natanson, C.; Fink, M.P.; Ballantyne, H.K.; MacVittie, T.J.; Conklin, J.J.; Parrillo, J.E. Gram-negative bacteremia produces both severe systolic and diastolic cardiac dysfunction in a canine model that simulates human septic shock. J. Clin. Investig. 1986, 78, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Hussain, N. Elevated cardiac troponins in setting of systemic inflammatory response syndrome, sepsis, and septic shock. ISRN Cardiol. 2013, 2013, 723435. [Google Scholar] [CrossRef]
- Favory, R.; Neviere, R. Bench-to-bedside review: Significance and interpretation of elevated troponin in septic patients. Crit. Care 2006, 10, 224. [Google Scholar] [CrossRef]
- Shahreyar, M.; Fahhoum, R.; Akinseye, O.; Bhandari, S.; Dang, G.; Khouzam, R.N. Severe sepsis and cardiac arrhythmias. Ann. Transl. Med. 2018, 6, 6. [Google Scholar] [CrossRef]
- Klein Klouwenberg, P.M.; Frencken, J.F.; Kuipers, S.; Ong, D.S.; Peelen, L.M.; van Vught, L.A.; Schultz, M.J.; van der Poll, T.; Bonten, M.J.; Cremer, O.L.; et al. Incidence, predictors, and outcomes of new-onset atrial fibrillation in critically ill patients with sepsis. A cohort study. Am. J. Respir. Crit. Care Med. 2017, 15, 205–211. [Google Scholar] [CrossRef]
| Variables | 0 (Normal Range) | 1 (Out of Normal Range) |
|---|---|---|
| Body temperature | >37.7 °C or <39.7 °C | <37.7 °C or >39.7 °C |
| Pulse rate | <160 bpm | >160 bpm |
| Respiratory rate | <40 brpm | >40 brpm |
| White blood cells | <12,000/mm3 or >4000/mm3 | >12,000/mm3 or <4000/mm3 |
| Banded leukocyte | <10% | >10 |
| G1 (n.) | G2 (n.) | |
|---|---|---|
| Male | 6 | 8 |
| Female | 7 | 3 |
| Crossbreed | 10 | 6 |
| German Shepherd | 3 | 1 |
| Rottweiler | 0 | 2 |
| Jack Russel | 0 | 1 |
| Beagle | 0 | 1 |
| G1 (n.) | G2 (n.) | |
|---|---|---|
| Gastroenteritis | 4 | 2 |
| Pneumonia | 4 | 1 |
| Closed cervix pyometra | 2 | 2 |
| Trauma | 1 | 3 |
| Pancreatitis | 1 | 2 |
| Intestinal foreign body | 1 | 1 |
| Clinical Variables | G1 | G2 | CTR |
|---|---|---|---|
| Temperature (°C) | 38.2 ± 1.78 | 39.0 ± 1.82 b | 38.0 ± 1.24 b |
| Pulse rate (bpm) | 130 ± 14.1 a | 153.3 ± 72.1 b | 90 ± 15 ab |
| Respiratory rate (brpm) | 37 ± 9.9 a | 40.7 ± 14.5 b | 20.1 ± 4.2 ab |
| Leukocytes (103/μL) | 10.53 ± 1.8 a | 17.44 ± 8.84 b | 8.20 ± 3.7 ab |
| Band neutrophils (%) | 6.6 ± 3.5 a | 9.1 ± 4.6 b | 2.1 ± 0.6 ab |
| Variables | G1 | G2 | CTR | Normal Values [21] |
|---|---|---|---|---|
| Clinical Score | 2.2 ± 0.43 ab | 3 ±0.7 ab | 0.9 ±0.3 ab | - |
| cTnI (ng/mL) | 88.2 ± 151 a | 1794.7 ± 1853 b | 0.10 ± 0.02 ab | 0.00–0.11 |
| Electrocardiographic Variables | G1 | G2 | CTR |
|---|---|---|---|
| Heart rate (bpm) | 148.3 ± 72.2 a | 188 ± 122.1 b | 88.2 ± 12.8 ab |
| MEA (degree) | 57.8 ± 38.7 | 64.6 ± 13.7 b | 56.1 ± 14 b |
| P duration (ms) | 40 ± 0 a | 32 ± 17.8 | 34.8 ± 0.22 a |
| P amplitude (mV) | 0.14 ± 0.05 a | 0.12 ± 0.08 b | 0.22 ± 0.05 ab |
| PR duration (ms) | 73.3 ± 10 a | 88 ± 76.9 b | 101 ± 10.1 ab |
| QRS duration (ms) | 40 ± 0 | 45 ± 12.9 b | 40.8 ± 2.6 b |
| R amplitude (mV) | 0.83 ± 0.57 | 1.0 ± 0.2 b | 0.74 ± 0.41 b |
| ST deviation (mV) | 0 | 0.01 ± 0.003 | 0 |
| T amplitude (mV) | 0.25 ± 0.26 | 0.32 ± 0.38 b | 0.20 ± 0.17 b |
| QT duration (ms) | 176 ± 60.6 a | 228 ± 50.4 b | 194 ± 22 ab |
| Corrected QT (ms) | 178 ± 55 a | 230 ± 48 | 215 ± 19 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pugliese, M.; La Maestra, R.; Ragusa, M.; Or, M.E.; Merola, G.; Napoli, E.; Passantino, A. Electrocardiographic Findings and Cardiac Troponin I Assay in Dogs with SIRS Diagnosis. Vet. Sci. 2022, 9, 655. https://doi.org/10.3390/vetsci9120655
Pugliese M, La Maestra R, Ragusa M, Or ME, Merola G, Napoli E, Passantino A. Electrocardiographic Findings and Cardiac Troponin I Assay in Dogs with SIRS Diagnosis. Veterinary Sciences. 2022; 9(12):655. https://doi.org/10.3390/vetsci9120655
Chicago/Turabian StylePugliese, Michela, Rocky La Maestra, Monica Ragusa, Mehmet Erman Or, Giordana Merola, Ettore Napoli, and Annamaria Passantino. 2022. "Electrocardiographic Findings and Cardiac Troponin I Assay in Dogs with SIRS Diagnosis" Veterinary Sciences 9, no. 12: 655. https://doi.org/10.3390/vetsci9120655
APA StylePugliese, M., La Maestra, R., Ragusa, M., Or, M. E., Merola, G., Napoli, E., & Passantino, A. (2022). Electrocardiographic Findings and Cardiac Troponin I Assay in Dogs with SIRS Diagnosis. Veterinary Sciences, 9(12), 655. https://doi.org/10.3390/vetsci9120655








