High Concentration of FBS Can Save mTOR Down-Regulation Caused by Mycoplasmas bovis Infection
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Treatment
2.2. Cell Viability Assay
2.3. Transcriptional Profiling
2.4. Quantitative Real-Time PCR (qPCR) Analysis
2.5. Western Blotting Analysis
2.6. Statistical Analysis
3. Results
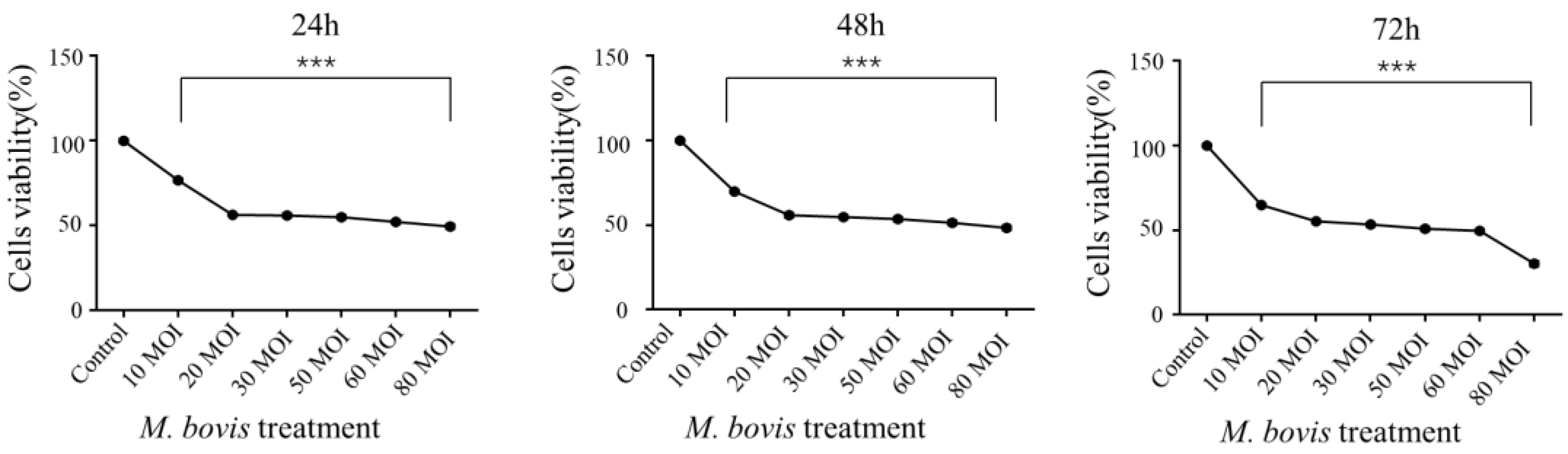
3.1. M. bovis Infection Inhibits EBL Growth
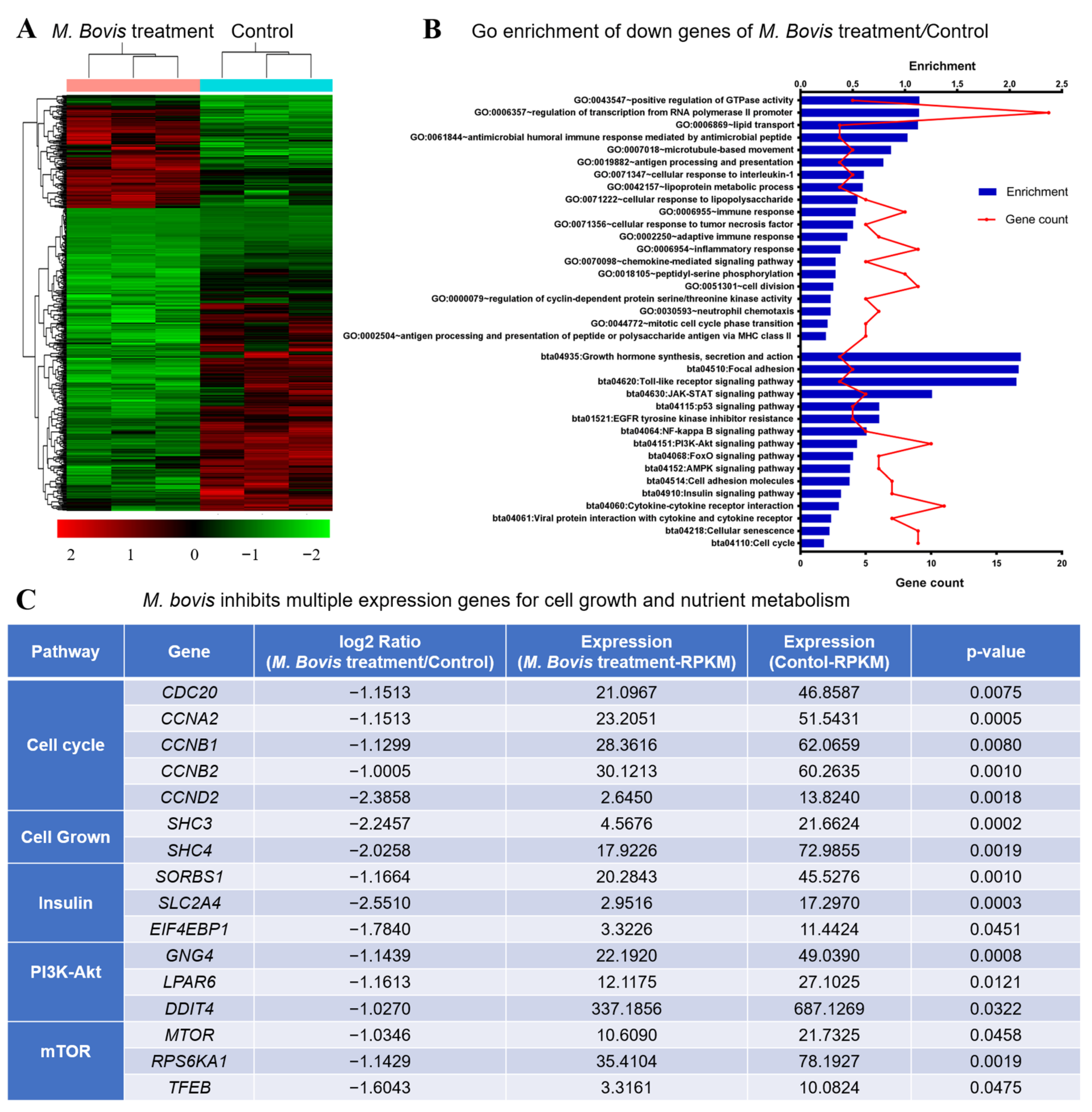
3.2. M. bovis Inhibits Multiple Expression Genes for Cell Growth and Nutrient Metabolism
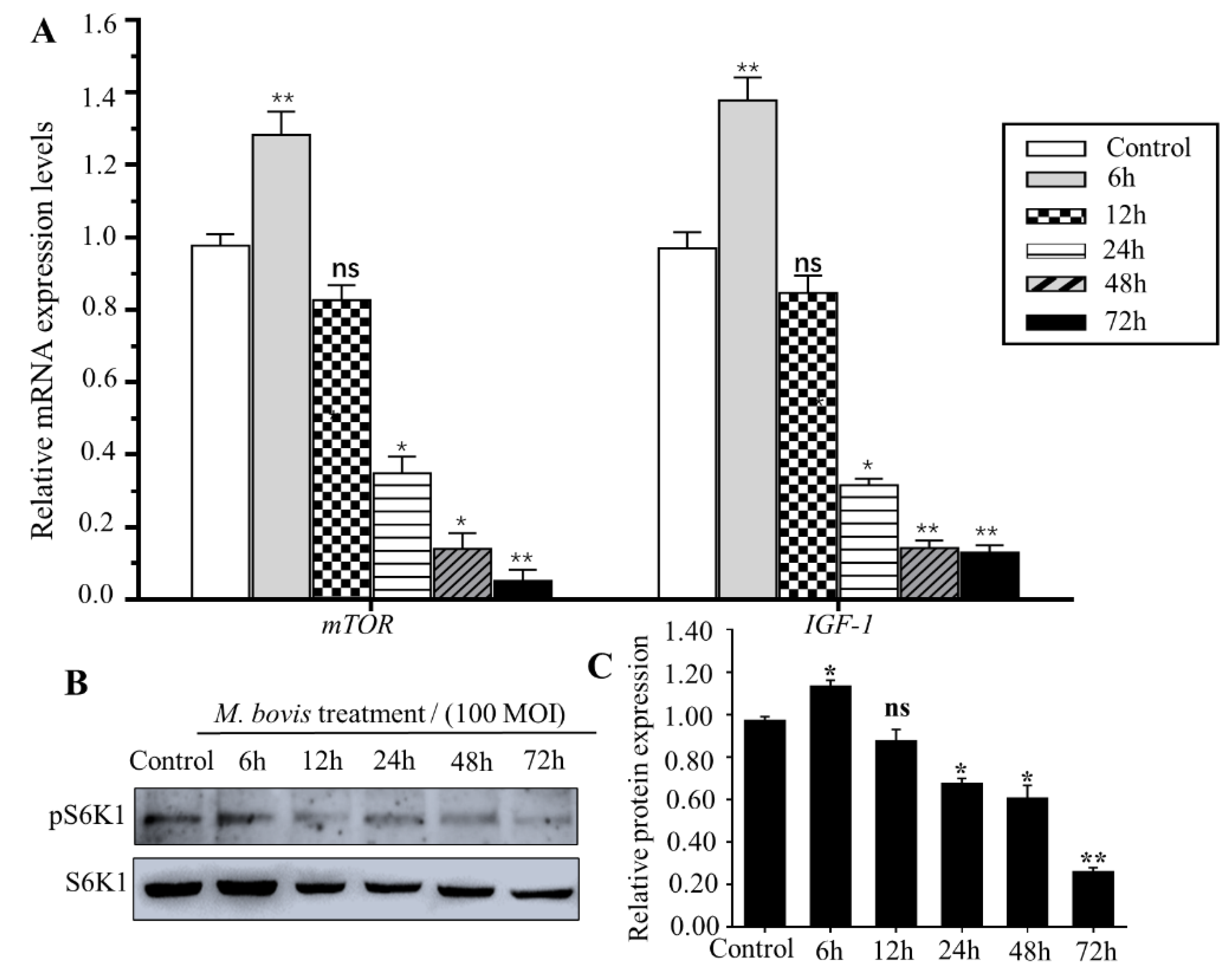
3.3. M. bovis Inhibit mTOR Signaling Pathway in EBL Cells
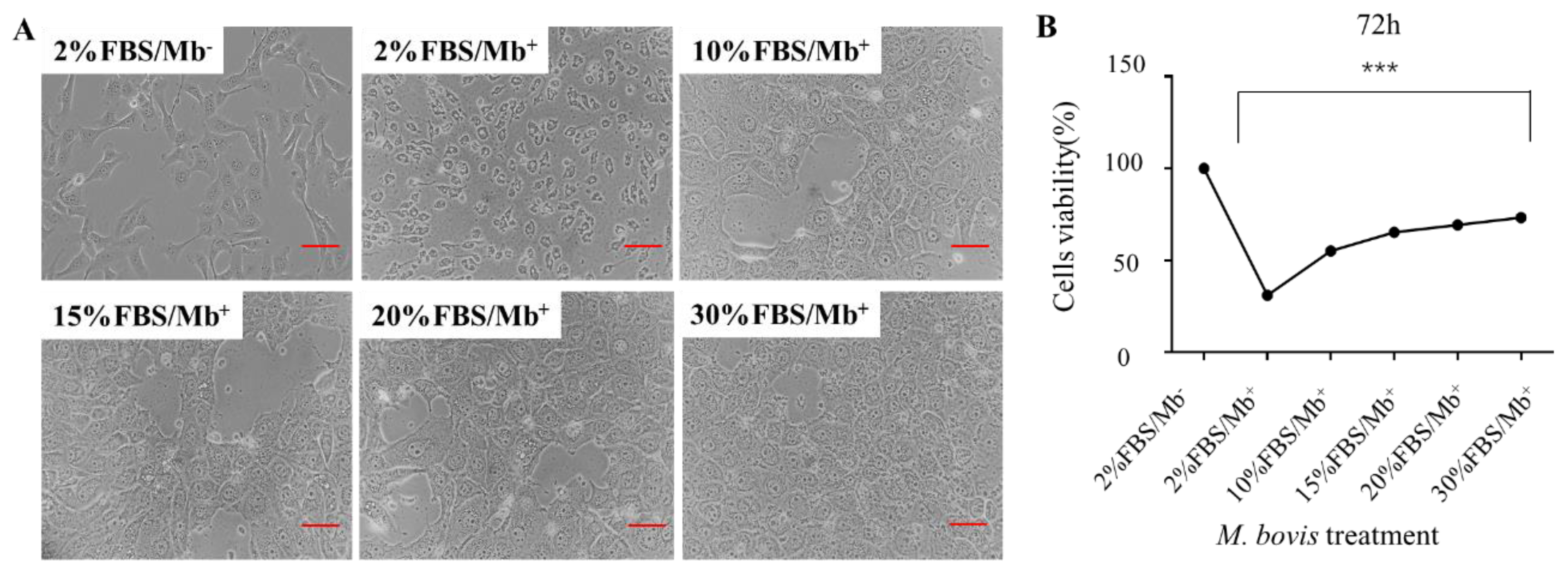
3.4. High Concentration of FBS Can Save M. bovis-Induced Cell Damage
3.5. High Concentration of FBS Rescued mTOR Signaling
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dudek, K.; Nicholas, R.A.J.; Szacawa, E.; Bednarek, D. Mycoplasma bovis Infections-Occurrence, Diagnosis and Control. Pathogens 2020, 9, 640. [Google Scholar] [CrossRef] [PubMed]
- Maunsell, F.P.; Chase, C. Mycoplasma bovis: Interactions with the Immune System and Failure to Generate an Effective Immune Response. Vet. Clin. North Am. Food Anim. Pract. 2019, 35, 471–483. [Google Scholar] [CrossRef] [PubMed]
- Demina, I.A.; Serebryakova, M.V.; Ladygina, V.G.; Rogova, M.A.; Zgoda, V.G.; Korzhenevskyi, D.A.; Govorun, V.M. Proteome of the bacterium Mycoplasma gallisepticum. Biochem. Biokhimiia 2009, 74, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Adamu, J.Y.; Wawegama, N.K.; Browning, G.F.; Markham, P.F. Membrane proteins of Mycoplasma bovis and their role in pathogenesis. Res. Vet. Sci. 2013, 95, 321–325. [Google Scholar] [CrossRef] [PubMed]
- Perez-Casal, J.; Prysliak, T.; Maina, T.; Suleman, M.; Jimbo, S. Status of the development of a vaccine against Mycoplasma bovis. Vaccine 2017, 35, 2902–2907. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C.W.; Kanci, A.; Citti, C.; Rosengarten, R.; Chiu, C.J.; Chen, Z.H.; Geary, S.J.; Browning, G.F.; Markham, P.F. MalF is essential for persistence of Mycoplasma gallisepticum in vivo. Microbiology 2013, 159, 1459–1470. [Google Scholar] [CrossRef]
- Perez-Casal, J. Pathogenesis and Virulence of Mycoplasma bovis. Vet. Clin. N. Am. Food Anim. Pract. 2020, 36, 269–278. [Google Scholar] [CrossRef]
- Wu, X.; Zhao, L.; Chen, Z.; Ji, X.; Qiao, X.; Jin, Y.; Liu, W. FLCN Maintains the Leucine Level in Lysosome to Stimulate mTORC1. PLoS ONE 2016, 11, e0157100. [Google Scholar] [CrossRef]
- Albert, V.; Hall, M.N. mTOR signaling in cellular and organismal energetics. Curr. Opin. Cell Biol. 2015, 33, 55–66. [Google Scholar] [CrossRef]
- Weichhart, T. mTOR as Regulator of Lifespan, Aging, and Cellular Senescence: A Mini-Review. Gerontology 2018, 64, 127–134. [Google Scholar] [CrossRef]
- Saxton, R.A.; Sabatini, D.M. mTOR Signaling in Growth, Metabolism, and Disease. Cell 2017, 168, 960–976. [Google Scholar] [CrossRef]
- Marsan, E.; Baulac, S. Review: Mechanistic target of rapamycin (mTOR) pathway, focal cortical dysplasia and epilepsy. Neuropathol Appl. Neurobiol. 2018, 44, 6–17. [Google Scholar] [CrossRef]
- Vanden Bush, T.J.; Rosenbusch, R.F. Mycoplasma bovis induces apoptosis of bovine lymphocytes. FEMS Immunol. Med. Microbiol. 2002, 32, 97–103. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, S.; Long, C.; An, Z.; Xing, X.; Wen, F.; Bao, S. Mycoplasmas bovis P48 induces apoptosis in EBL cells via an endoplasmic reticulum stress-dependent signaling pathway. Vet. Microbiol. 2021, 255, 109013. [Google Scholar] [CrossRef]
- Jimbo, S.; Suleman, M.; Maina, T.; Prysliak, T.; Mulongo, M.; Perez-Casal, J. Effect of Mycoplasma bovis on bovine neutrophils. Vet. Immunol. Immunopathol. 2017, 188, 27–33. [Google Scholar] [CrossRef]
- Liu, Y.; Deng, Z.; Xu, S.; Liu, G.; Lin, Y.; Khan, S.; Gao, J.; Qu, W.; Kastelic, J.P.; Han, B. Mycoplasma bovis subverts autophagy to promote intracellular replication in bovine mammary epithelial cells cultured in vitro. Vet. Res. 2021, 52, 130. [Google Scholar] [CrossRef]
- Xu, M.; Liu, Y.; Mayinuer, T.; Lin, Y.; Wang, Y.; Gao, J.; Wang, D.; Kastelic, J.P.; Han, B. Mycoplasma bovis inhibits autophagy in bovine mammary epithelial cells via a PTEN/PI3K-Akt-mTOR-dependent pathway. Front. Microbiol. 2022, 13, 935547. [Google Scholar] [CrossRef]
- Zhang, H.; Lu, D.; Zhang, Y.; Zhao, G.; Raheem, A.; Chen, Y.; Chen, X.; Hu, C.; Chen, H.; Yang, L.; et al. Annexin A2 regulates Mycoplasma bovis adhesion and invasion to embryo bovine lung cells affecting molecular expression essential to inflammatory response. Front. Immunol. 2022, 13, 974006. [Google Scholar] [CrossRef]
- Hegde, S.; Hegde, S.; Spergser, J.; Brunthaler, R.; Rosengarten, R.; Chopra-Dewasthaly, R. In vitro and in vivo cell invasion and systemic spreading of Mycoplasma agalactiae in the sheep infection model. Int. J. Med. Microbiol. 2014, 304, 1024–1031. [Google Scholar] [CrossRef]
- Gao, Y.; Wu, X.; Zhao, S.; Zhang, Y.; Ma, H.; Yang, Z.; Yang, W.; Zhao, C.; Wang, L.; Zhang, Q. Melatonin receptor depletion suppressed hCG-induced testosterone expression in mouse Leydig cells. Cell. Mol. Biol. Lett. 2019, 24, 21. [Google Scholar] [CrossRef]
- van der Valk, J. Fetal bovine serum-a cell culture dilemma. Science 2022, 375, 143–144. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Liu, J.L. mTOR-S6K1 pathway mediates cytoophidium assembly. J. Genet. Genomics. 2019, 46, 65–74. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, X.; Ma, J.; Jia, S.; Zhang, X.; Zhang, X.; An, Z.; Wei, Y.; Xing, X.; Wen, F.; Gao, Y.; et al. High Concentration of FBS Can Save mTOR Down-Regulation Caused by Mycoplasmas bovis Infection. Vet. Sci. 2022, 9, 630. https://doi.org/10.3390/vetsci9110630
Wu X, Ma J, Jia S, Zhang X, Zhang X, An Z, Wei Y, Xing X, Wen F, Gao Y, et al. High Concentration of FBS Can Save mTOR Down-Regulation Caused by Mycoplasmas bovis Infection. Veterinary Sciences. 2022; 9(11):630. https://doi.org/10.3390/vetsci9110630
Chicago/Turabian StyleWu, Xiaochun, Jinrui Ma, Shangdong Jia, Xudong Zhang, Xinlan Zhang, Zhen An, Yanquan Wei, Xiaoyong Xing, Fengqin Wen, Yuan Gao, and et al. 2022. "High Concentration of FBS Can Save mTOR Down-Regulation Caused by Mycoplasmas bovis Infection" Veterinary Sciences 9, no. 11: 630. https://doi.org/10.3390/vetsci9110630
APA StyleWu, X., Ma, J., Jia, S., Zhang, X., Zhang, X., An, Z., Wei, Y., Xing, X., Wen, F., Gao, Y., & Bao, S. (2022). High Concentration of FBS Can Save mTOR Down-Regulation Caused by Mycoplasmas bovis Infection. Veterinary Sciences, 9(11), 630. https://doi.org/10.3390/vetsci9110630





