Does TLS Exist in Canine Mammary Gland Tumours? Preliminary Results in Simple Carcinomas
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
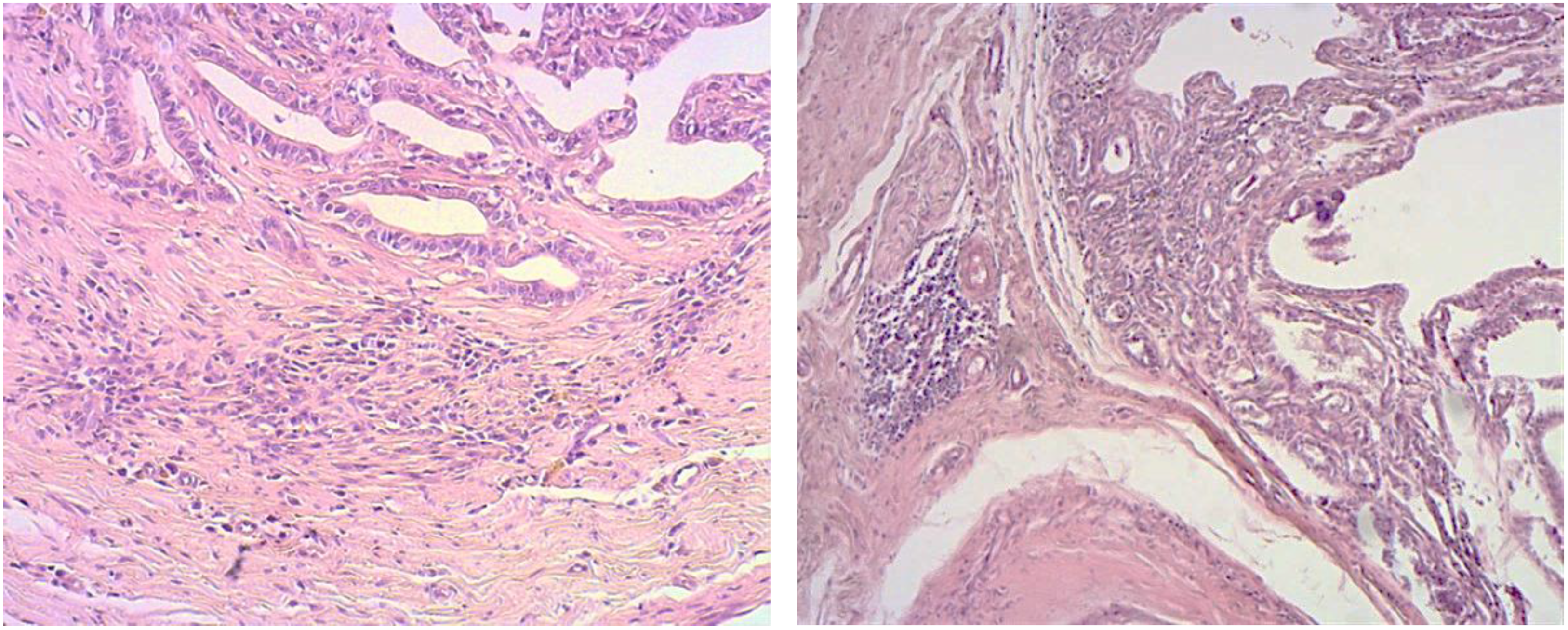
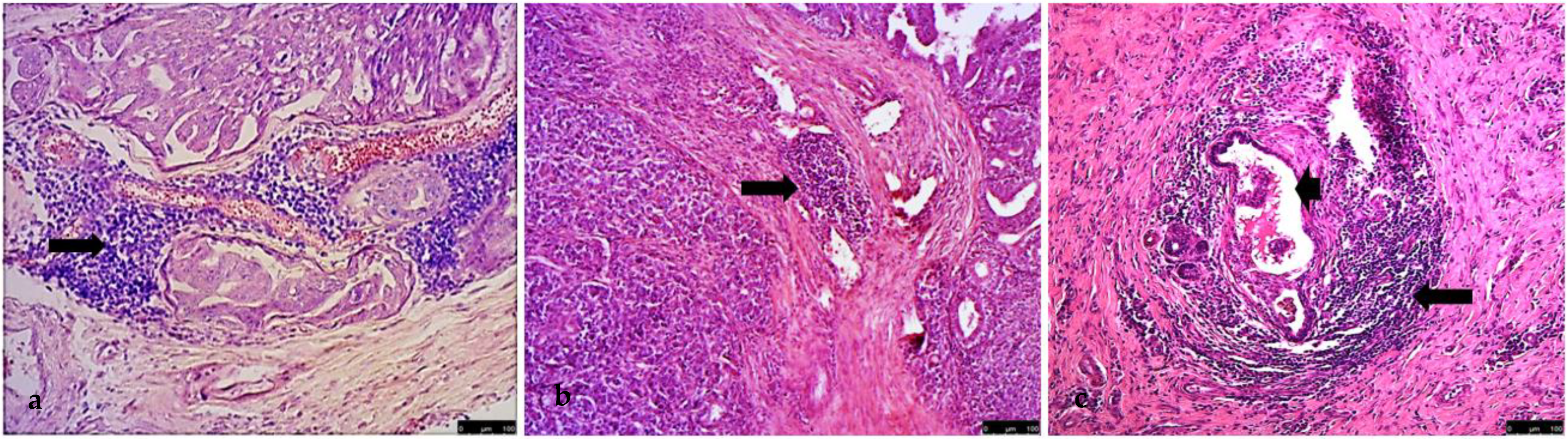
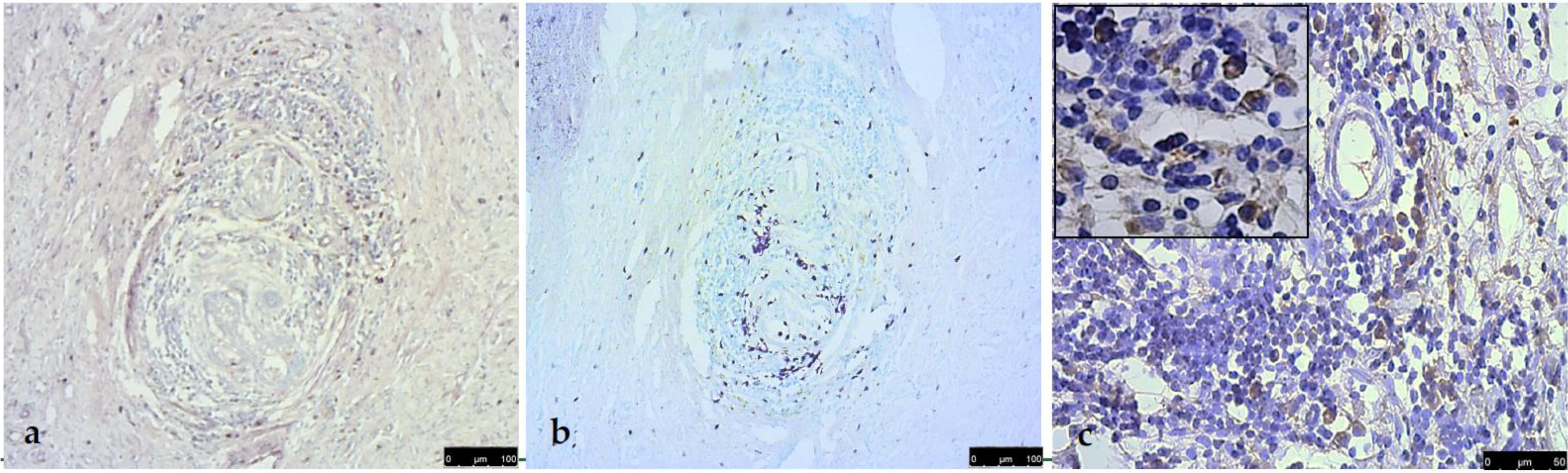
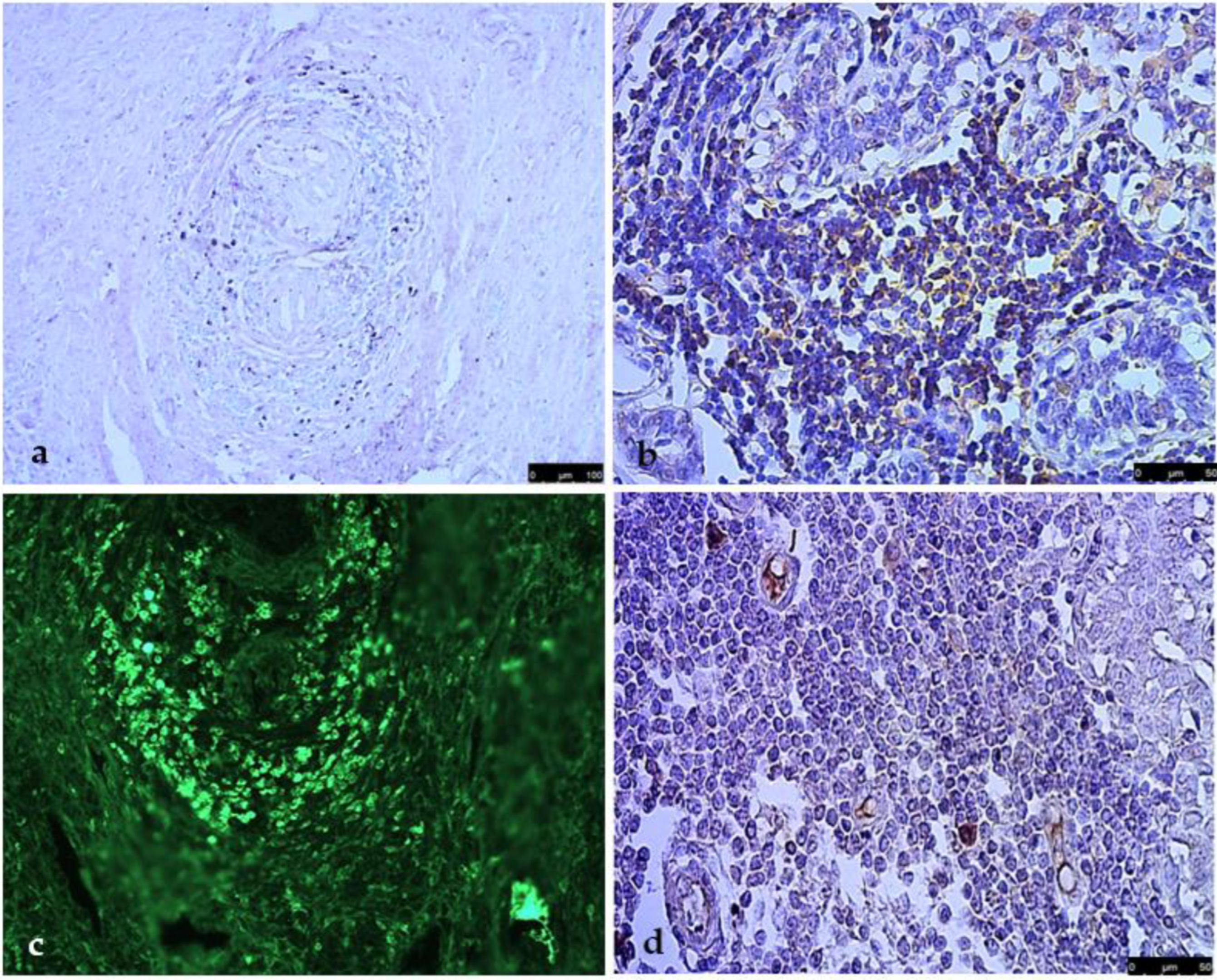
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mellman, I.; Coukos, G.; Dranoff, G. Cancer immunotherapy comes of age. Nature 2011, 480, 480–489. [Google Scholar] [CrossRef] [PubMed]
- Dieu-Nosjean, M.C.; Giraldo, N.A.; Kaplon, H.; Germain, C.; Fridman, W.H.; Sautès-Fridman, C. Tertiary lymphoid structures, drivers of the anti-tumor responses in human cancers. Immunol. Rev. 2016, 271, 260–275. [Google Scholar] [CrossRef]
- Lucchesi, D.; Bombardieri, M. The role of viruses in autoreactive B cell activation within tertiary lymphoid structures in autoimmune diseases. J. Leukoc. Biol. 2013, 94, 1191–1199. [Google Scholar] [CrossRef]
- Aloisi, F.; Pujol-Borrell, R. Lymphoid neogenesis in chronic inflammatory diseases. Nat. Rev. Immunol. 2006, 6, 205–217. [Google Scholar] [CrossRef] [PubMed]
- Martinet, L.; Garrido, I.; Filleron, T.; Le Guellec, S.; Bellard, E.; Fournie, J.J.; Rochaix, P.; Girard, J.P. Human solid tumors contain high endothelial venules: Association with T- and B-lymphocyte infiltration and favorable prognosis in breast cancer. Cancer Res. 2011, 71, 5678–5687. [Google Scholar] [CrossRef] [PubMed]
- Cipponi, A.; Mercier, M.; Seremet, T.; Baurain, J.F.; Théate, I.; van den Oord, J.; Stas, M.; Boon, T.; Coulie, P.G.; van Baren, N. Neogenesis of lymphoid structures and antibody responses occur in human melanoma metastases. Cancer Res. 2012, 72, 3997–4007. [Google Scholar] [CrossRef]
- Jones, G.W.; Hill, D.G.; Jones, S.A. Understanding Immune Cells in Tertiary Lymphoid Organ Development: It Is All Starting to Come Together. Front. Immunol. 2016, 7, 401. [Google Scholar] [CrossRef]
- Di Caro, G.; Bergomas, F.; Grizzi, F.; Doni, A.; Bianchi, P.; Malesci, A.; Laghi, L.; Allavena, P.; Mantovani, A.; Marchesi, F. Occurrence of tertiary lymphoid tissue is associated with T-cell infiltration and predicts better prognosis in early-stage colorectal cancers. Clin. Cancer Res. 2014, 20, 2147–2158. [Google Scholar] [CrossRef]
- Goc, J.; Germain, C.; Vo-Bourgais, T.K.; Lupo, A.; Klein, C.; Knockaert, S.; de Chaisemartin, L.; Ouakrim, H.; Becht, E.; Alifano, M.; et al. Dendritic cells in tumor-associated tertiary lymphoid structures signal a Th1 cytotoxic immune contexture and license the positive prognostic value of infiltrating CD8+ T cells. Cancer Res. 2014, 74, 705–715. [Google Scholar] [CrossRef]
- Montfort, A.; Pearce, O.; Maniati, E.; Vincent, B.G.; Bixby, L.; Böhm, S.; Dowe, T.; Wilkes, E.H.; Chakravarty, P.; Thompson, R.; et al. A Strong B-cell Response Is Part of the Immune Landscape in Human High-Grade Serous Ovarian Metastases. Clin. Cancer Res. 2017, 23, 250–262. [Google Scholar] [CrossRef]
- Sautès-Fridman, C.; Petitprez, F.; Calderaro, J.; Fridman, W.H. Tertiary lymphoid structures in the era of cancer immunotherapy. Nat. Rev. Cancer 2019, 19, 307–325. [Google Scholar] [CrossRef] [PubMed]
- Figenschau, S.L.; Fismen, S.; Fenton, K.A.; Fenton, C.; Mortensen, E.S. Tertiary lymphoid structures are associated with higher tumor grade in primary operable breast cancer patients. BMC Cancer 2015, 15, 101. [Google Scholar] [CrossRef] [PubMed]
- Figenschau, S.L.; Knutsen, E.; Urbarova, I.; Fenton, C.; Elston, B.; Perander, M.; Mortensen, E.S.; Fenton, K.A. ICAM1 expression is induced by proinflammatory cytokines and associated with TLS formation in aggressive breast cancer subtypes. Sci. Rep. 2018, 8, 11720. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Tsang, J.Y.S.; Hlaing, T.; Hu, J.; Ni, Y.B.; Chan, S.K.; Cheung, S.Y.; Tse, G.M. Distinct Tertiary Lymphoid Structure Associations and Their Prognostic Relevance in HER2 Positive and Negative Breast Cancers. Oncologist 2017, 22, 1316–1324. [Google Scholar] [CrossRef] [PubMed]
- Finkin, S.; Yuan, D.; Stein, I.; Taniguchi, K.; Weber, A.; Unger, K.; Browning, J.L.; Goossens, N.; Nakagawa, S.; Gunasekaran, G.; et al. Ectopic lymphoid structures function as microniches for tumor progenitor cells in hepatocellular carcinoma. Nat. Immunol. 2015, 16, 1235–1244. [Google Scholar] [CrossRef]
- Zappulli, V.; Pena, L.; Rasotto, R.; Goldschmidt, M.H.; Gama, A.; Scruggs, J.L.; Kiupel, M. Surgical Pathology of Tumors of Domestic Animals. Volume 2: Mammary Tumors; Davis-Thompson Foundation: Gurnee, IL, USA, 2018. [Google Scholar]
- Peña, L.; De Andrés, P.J.; Clemente, M.; Cuesta, P.; Pérez-Alenza, M.D. Prognostic value of histological grading in noninflammatory canine mammary carcinomas in a prospective study with two-year follow-up: Relationship with clinical and histological characteristics. Vet. Pathol. 2013, 50, 94–105. [Google Scholar] [CrossRef]
- Sfacteria, A.; Napoli, E.; Rifici, C.; Commisso, D.; Giambrone, G.; Mazzullo, G.; Marino, G. Immune Cells and Immunoglobulin Expression in the Mammary Gland Tumors of Dog. Animals 2021, 11, 1189. [Google Scholar] [CrossRef] [PubMed]
- Estrela-Lima, A.; Araújo, M.S.; Costa-Neto, J.M.; Teixeira-Carvalho, A.; Barrouin-Melo, S.M.; Cardoso, S.V.; Martins-Filho, O.A.; Serakides, R.; Cassali, G.D. Immunophenotypic features of tumor infiltrating lymphocytes from mammary carcinomas in female dogs associated with prognostic factors and survival rates. BMC Cancer 2010, 10, 256. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, M.I.; Pires, I.; Prada, J.; Queiroga, F.L. A role for T-lymphocytes in human breast cancer and in canine mammary tumors. BioMed Res. Int. 2014, 2014, 130894. [Google Scholar] [CrossRef]
- Colbeck, E.J.; Ager, A.; Gallimore, A.; Jones, G.W. Tertiary Lymphoid Structures in Cancer: Drivers of Antitumor Immunity, Immunosuppression, or Bystander Sentinels in Disease? Front. Immunol. 2017, 8, 1830. [Google Scholar] [CrossRef]
- Engelhard, V.H.; Rodriguez, A.B.; Mauldin, I.S.; Woods, A.N.; Peske, J.D.; Slingluff, C.L., Jr. Immune Cell Infiltration and Tertiary Lymphoid Structures as Determinants of Antitumor Immunity. J. Immunol. 2018, 200, 432–442. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, T.N.; Thommen, D.S. Tertiary lymphoid structures in cancer. Science 2022, 375, eabf9419. [Google Scholar] [CrossRef] [PubMed]
- Nerviani, A.; Pitzalis, C. Role of chemokines in ectopic lymphoid structures formation in autoimmunity and cancer. J. Leukoc. Biol. 2018, 104, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Ma, C.; Chen, Z.; Yi, W.; McNutt, M.A.; Wang, Y.; Korteweg, C.; Gu, J. Correlation of immunoglobulin G expression and histological subtype and stage in breast cancer. PLoS ONE 2013, 8, e58706. [Google Scholar] [CrossRef] [PubMed]
- Daëron, M. Innate myeloid cells under the control of adaptive immunity: The example of mast cells and basophils. Curr. Opin. Immunol. 2016, 38, 101–108. [Google Scholar] [CrossRef]
- Groot Kormelink, T.; Powe, D.G.; Kuijpers, S.A.; Abudukelimu, A.; Fens, M.H.; Pieters, E.H.; Kassing van der Ven, W.W.; Habashy, H.O.; Ellis, I.O.; Blokhuis, B.R.; et al. Immunoglobulin free light chains are biomarkers of poor prognosis in basal-like breast cancer and are potential targets in tumor-associated inflammation. Oncotarget 2014, 5, 3159–3167. [Google Scholar] [CrossRef][Green Version]
- Lochner, M.; Ohnmacht, C.; Presley, L.; Bruhns, P.; Si-Tahar, M.; Sawa, S.; Eberl, G. Microbiota-induced tertiary lymphoid tissues aggravate inflammatory disease in the absence of RORgamma t and LTi cells. J. Exp. Med. 2011, 208, 125–134. [Google Scholar] [CrossRef]
- Guedj, K.; Khallou-Laschet, J.; Clement, M.; Morvan, M.; Gaston, A.T.; Fornasa, G.; Dai, J.; Gervais-Taurel, M.; Eberl, G.; Michel, J.B.; et al. M1 macrophages act as LTβR-independent lymphoid tissue inducer cells during atherosclerosis-related lymphoid neogenesis. Cardiovasc. Res. 2014, 101, 434–443. [Google Scholar] [CrossRef]
- Sofopoulos, M.; Fortis, S.P.; Vaxevanis, C.K.; Sotiriadou, N.N.; Arnogiannaki, N.; Ardavanis, A.; Vlachodimitropoulos, D.; Perez, S.A.; Baxevanis, C.N. The prognostic significance of peritumoral tertiary lymphoid structures in breast cancer. Cancer Immunol. Immunother. 2019, 68, 1733–1745. [Google Scholar] [CrossRef]


| Case | Breed | Age | Histotype | Grade |
|---|---|---|---|---|
| 1 | Sicilian hound | 7 | Tubular carcinoma | I |
| 2 | Crossbreed | n.a. | Tubular carcinoma | I |
| 3 | Crossbreed | 12 | Tubular carcinoma | I |
| 4 | Jack russel | 9 | Tubular carcinoma | I |
| 5 | Crossbreed | n.a. | Tubular carcinoma | I |
| 6 | Crossbreed | n.a. | Tubular carcinoma | I |
| 7 | Chihuahua | 8 | Tubular carcinoma | I |
| 8 | Beagle | 10 | Tubular carcinoma | II |
| 9 | Crossbreed | 11 | Tubular carcinoma | II |
| 10 | Yorkshire terrier | 7 | Tubulopapillary carcinoma | I |
| 11 | Crossbreed | 5 | Tubulopapillary carcinoma | I |
| 12 | German shepherd | 11 | Tubulopapillary carcinoma | I |
| 13 | Yorkshire terrier | 10 | Tubulopapillary carcinoma | I |
| 14 | Beagle | 8 | Tubulopapillary carcinoma | I |
| 15 | Yorkshire terrier | 4 | Tubulopapillary carcinoma | II |
| 16 | Cocker spaniel | 15 | Tubulopapillary carcinoma | II |
| 17 | Cocker spaniel | 10 | Tubulopapillary carcinoma | II |
| 18 | Rottweiler | 9 | Tubulopapillary carcinoma | II |
| 19 | Crossbreed | 9 | Tubulopapillary carcinoma | II |
| 20 | Crossbreed | n.a. | Tubulopapillary carcinoma | II |
| 21 | Breton | 8 | Micropapillary carcinoma | II |
| 22 | Shih-Tzu | 10 | Micropapillary carcinoma | II |
| 23 | German shepherd | 5 | Micropapillary carcinoma | II |
| 24 | Jack russel | 5 | Micropapillary carcinoma | II |
| 25 | Jack russel | 7 | Micropapillary carcinoma | II |
| 26 | German shepherd | 10 | Micropapillary carcinoma | II |
| 27 | Yorkshire terrier | 7 | Micropapillary carcinoma | III |
| 28 | Dalmation dog | 8 | Micropapillary carcinoma | III |
| 29 | Crossbreed | n.a. | Micropapillary carcinoma | III |
| 30 | Pitbull | 9 | Micropapillary carcinoma | III |
| 31 | Golden retriever | 8 | Solid carcinoma | II |
| 32 | Crossbreed | 10 | Solid carcinoma | II |
| 33 | Crossbreed | 14 | Solid carcinoma | II |
| 34 | Crossbreed | 12 | Solid carcinoma | II |
| 35 | Shar pei | 6 | Solid carcinoma | II |
| 36 | Chihuahua | 9 | Solid carcinoma | II |
| 37 | Jack russel | 10 | Solid carcinoma | II |
| 38 | Crossbreed | n.a. | Solid carcinoma | III |
| 39 | Crossbreed | 8 | Solid carcinoma | III |
| 40 | Rottweiler | 9 | Anaplastic carcinoma | III |
| 41 | Pomeranian | 13 | Hyperplasia/Dysplasia | |
| 42 | Crossbreed | 9 | Hyperplasia/Dysplasia | |
| 43 | German shepherd | n.a. | Hyperplasia/Dysplasia | |
| 44 | Crossbreed | 6 | Hyperplasia/Dysplasia | |
| 45 | Yorkshire terrier | 5 | Hyperplasia/Dysplasia | |
| 46 | Poodle | 10 | Hyperplasia/Dysplasia | |
| 47 | Crossbreed | n.a. | Lobular Hyperplasia with Atypia | |
| 48 | Siberian husky | 10 | Hyperplasia/Dysplasia | |
| 49 | Poodle | 6 | Lobular Hyperplasia with Atypia | |
| 50 | Siberian husky | 8 | Hyperplasia/Dysplasia |
| Antibody I | Clone | Specificity | Dilution | Brand |
| Mast cell Tryptase | 10D11 | Mast cells | 1:100 | Santa Cruz Biotechnology |
| Macrophage Marker | MAC/387 | Macrophages | 1:200 | Santa Cruz Biotechnology |
| CD3 | T lymphocytes | 1:50 | Santa Cruz Biotechnology | |
| CD20 | D-10 | B lymphocytes | 1:200 | Santa Cruz Biotechnology |
| CD21 | A-3 | Follicular dendritic cells (FDCs) | 1:200 | Santa Cruz Biotechnology |
| MECA-79 | MECA-79 | High endothelial venules (HEVs) | 1:200 | Santa Cruz Biotechnology |
| Sheep Anti-canine IgG:FITC | OBT4041F | Immunoglobulin G dog | 1:50 | Oxford Biotechnology |
| Mouse Anti-human IgG:FITC | HP-6017 | ImmunoglobulinG human | 1:50 | Sigma |
| Antibody II | Clone | Specificity | Dilution | Brand |
| Goat anti-mouse IgG-B | Mouse IgG | 1:100 | BioSpa, Milan | |
| Donkey anti-goat IgG-B | Goat IgG | 1:200 | Santa Cruz Biotechnology |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giambrone, G.; Di Giorgio, S.; Vullo, C.; Marino, G.; Puleio, R.; Mariotti, F.; Mazzullo, G.; Sfacteria, A. Does TLS Exist in Canine Mammary Gland Tumours? Preliminary Results in Simple Carcinomas. Vet. Sci. 2022, 9, 628. https://doi.org/10.3390/vetsci9110628
Giambrone G, Di Giorgio S, Vullo C, Marino G, Puleio R, Mariotti F, Mazzullo G, Sfacteria A. Does TLS Exist in Canine Mammary Gland Tumours? Preliminary Results in Simple Carcinomas. Veterinary Sciences. 2022; 9(11):628. https://doi.org/10.3390/vetsci9110628
Chicago/Turabian StyleGiambrone, Giada, Stefania Di Giorgio, Cecilia Vullo, Gabriele Marino, Roberto Puleio, Francesca Mariotti, Giuseppe Mazzullo, and Alessandra Sfacteria. 2022. "Does TLS Exist in Canine Mammary Gland Tumours? Preliminary Results in Simple Carcinomas" Veterinary Sciences 9, no. 11: 628. https://doi.org/10.3390/vetsci9110628
APA StyleGiambrone, G., Di Giorgio, S., Vullo, C., Marino, G., Puleio, R., Mariotti, F., Mazzullo, G., & Sfacteria, A. (2022). Does TLS Exist in Canine Mammary Gland Tumours? Preliminary Results in Simple Carcinomas. Veterinary Sciences, 9(11), 628. https://doi.org/10.3390/vetsci9110628







