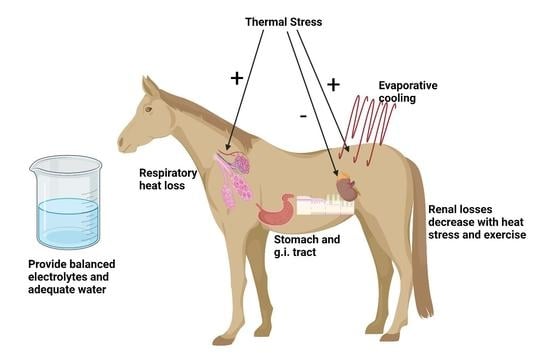
Oral Electrolyte and Water Supplementation in Horses
Abstract
Simple Summary
Abstract
1. Introduction
2. Electrolyte and Water Losses through Sweating
- Sweating rate in hot, humid conditions is higher than in hot, dry conditions due to the greater thermal stress for a given temperature arising from the decreased ability to evaporate sweat for cooling [14];
- High sweating rates can be sustained for more than two hours, especially when horses are adequately supplemented with effective electrolyte solutions;
- High sweating rates will result in dehydration when effective electrolyte supplementation is not provided;
- Cl− is the predominant ion lost in sweat, followed by Na+, K+, Mg2+ and Ca2+;
- Sweat Cl− losses are nearly equal to the combined losses of all cations;
- Sweating rates (and hence thermoregulatory cooling) decrease as horses become dehydrated.
- Electrolytes are required in body fluid compartments in order to retain the water in these compartments. Thus consuming only water to try to rehydrate will only dilute the body fluid compartments, resulting in renal water excretion together with more electrolytes. Water alone cannot rehydrate, and can further dehydrate [24].
3. Mineral Imbalance/Deficiency
4. Dehydration
5. Acid-Base Balance
6. Negative Clinical Effects of Excessive Electrolyte and Water Losses
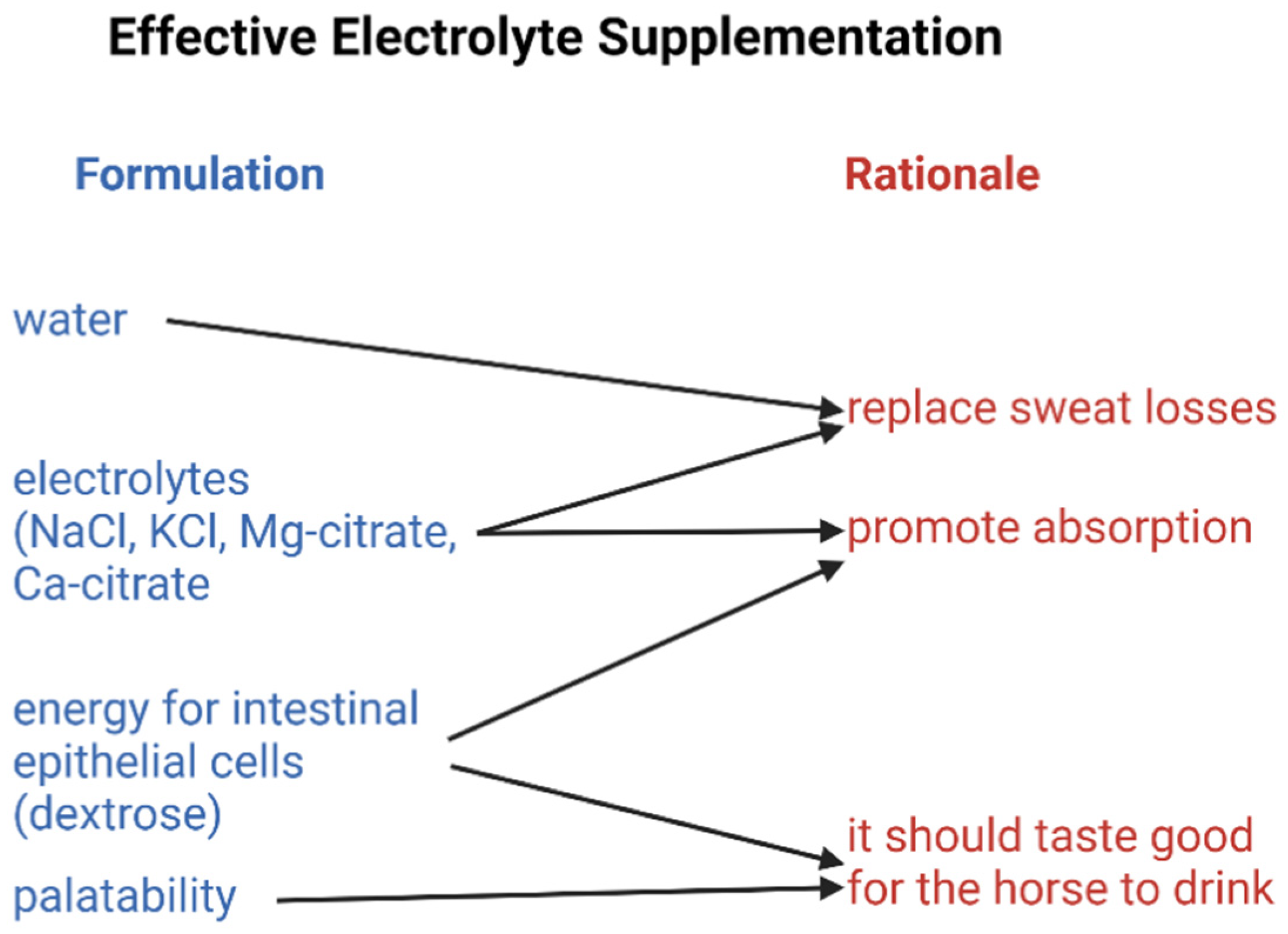
7. Strategies for Replacing Electrolyte and Water Losses
8. Requirements for an Effective Electrolyte Supplement
9. Biovailability
10. Taste and Training the Horse to Drink an Electrolyte Supplement
11. Pastes and Slurries
12. Electrolytes Top Dressed on Feed
13. Distribution of Ingested Electrolytes in the Body after ‘Absorption’ and Appearance in Sweat
14. What Happens If Too Much Electrolytes Are Given?
15. When to Administer—Timing of Electrolyte Supplementation
16. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Evans, C.L.; Smith, D.F.G. Sweating responses in the horse. Proc. R. Soc. London. Ser. B Boil. Sci. 1956, 145, 61–83. [Google Scholar] [CrossRef]
- Coenen, M. Exercise and stress: Impact on adaptive processes involving water and electrolytes. Livest. Prod. Sci. 2005, 92, 131–145. [Google Scholar] [CrossRef]
- Kronfeld, D.S. Body fluids and exercise: Physiological responses (part I). J. Equine Vet. Sci. 2001, 21, 312–322. [Google Scholar] [CrossRef]
- Johnson, P.J. Electrolyte and Acid-Base Disturbances in the Horse. Vet. Clin. North Am. Equine Pract. 1995, 11, 491–514. [Google Scholar] [CrossRef]
- Carlson, G. Hematology and Body Fluids in the Equine Athlete: A Review. In Proceedings of the Equine Exercise Physiology, San Diego, CA, USA, 7–11 August 1987. [Google Scholar]
- Friend, T.H. Dehydration, stress, and water consumption of horses during long-distance commercial transport. J. Anim. Sci. 2000, 78, 2568–2580. [Google Scholar] [CrossRef]
- van den Berg, J.S.; Guthrie, A.J.; Meintjes, R.A.; Nurton, J.P.; Adamson, D.A.; Travers, C.W.; Lund, R.J.; Mostert, H.J. Water and electrolyte intake and output in conditioned Thoroughbred horses transported by road. Equine Vet. J. 1998, 30, 316–323. [Google Scholar] [CrossRef]
- Kronfeld, D.S. Body fluids and exercise: Replacement strategies. J. Equine Vet. Sci. 2001, 21, 368–375. [Google Scholar] [CrossRef]
- Kronfeld, D.S. Body fluids and exercise: Influences of nutrition and feeding management. J. Equine Vet. Sci. 2001, 21, 417–428. [Google Scholar] [CrossRef]
- Gunn, H.M.M. Muscle, Bone and Fat Proportions and Muscle Distribution of Thoroughbreds and Other Horses. In Equine Exercise Physiology; ICEEP Publications: Davis, CA, USA, 1987; pp. 253–264. [Google Scholar]
- Hodgson, D.; Davis, R.; McConaghy, F. Thermoregulation in the horse in response to exercise. Br. Vet. J. 1994, 150, 219–235. [Google Scholar] [CrossRef]
- Kingston, J.K.; Geor, R.; McCutcheon, L.J. Use of dew-point hygrometry, direct sweat collection, and measurement of body water losses to determine sweating rates in exercising horses. Am. J. Vet. Res. 1997, 58, 175–181. [Google Scholar]
- Jenkinson, D.M.; Elder, H.Y.; Bovell, D.L. Equine sweating and anhidrosis Part 1. Equine sweating. Vet. Dermatol. 2006, 17, 361–392. [Google Scholar] [CrossRef]
- McCutcheon, L.J.; Geor, R.; Hare, M.J.; Ecker, G.L.; Lindinger, M. Sweating rate and sweat composition during exercise and recovery in ambient heat and humidity. Equine Vet. J. 1995, 27, 153–157. [Google Scholar] [CrossRef] [PubMed]
- McCutcheon, L.J.; Geor, R.J.; Ecker, G.L.; Lindinger, M.I. Equine sweating responses to submaximal exercise during 21 days of heat acclimation. J. Appl. Physiol. 1999, 87, 1843–1851. [Google Scholar] [CrossRef] [PubMed]
- Kerr, M.G.; Snow, D.H. Composition of sweat of the horse during prolonged epinephrine (adrenaline) infusion, heat exposure, and exercise. Am. J. Vet. Res. 1983, 44, 1571–1577. [Google Scholar]
- Carlson, G.; Ocen, P. Composition of equine sweat following exercise in high environmental temperatures and in response to intravenous epinephrine administration. J. Equine Med. Surg. 1979, 3, 27–32. [Google Scholar]
- McConaghy, F.F.; Hodgson, D.R.; Evans, D.L.; Rose, R.J. Equine sweat composition: Effects of adrenaline infusion, exercise and training. Equine Vet. J. 1995, 27, 158–164. [Google Scholar] [CrossRef]
- Geor, R.J.; McCutcheon, L.J.; Ecker, G.L.; Lindinger, M.I. Heat storage in horses during submaximal exercise before and after humid heat acclimation. J. Appl. Physiol. 2000, 89, 2283–2293. [Google Scholar] [CrossRef]
- Hodgson, D.R. Thermoregulation. In The Athletic Horse: Principles and Practice of Equine Sports Medicine, 2nd ed.; Elsevier Inc.: Blacksburg, VA, USA, 2014; pp. 108–124. ISBN 9780721600758. [Google Scholar]
- Naylor, J.R.; Bayly, W.M.; Gollnick, P.D.; Brengelmann, G.L.; Hodgson, D.R. Effects of dehydration on thermoregulatory responses of horses during low-intensity exercise. J. Appl. Physiol. 1993, 75, 994–1001. [Google Scholar] [CrossRef]
- Lindinger, M.I.; Waller, A.P. Tracing oral Na+ and K+ in sweat during exercise and recovery in horses. Exp. Physiol. 2021, 106, EP089232. [Google Scholar] [CrossRef]
- McCutcheon, L.J.; Geor, R.J. Influence of training on sweating responses during submaximal exercise in horses. J. Appl. Physiol. 2000, 89, 2463–2471. [Google Scholar] [CrossRef]
- Maughan, R.J.; Owen, J.H.; Shirreffs, S.M.; Leiper, J.B. Post-exercise rehydration in man: Effects of electrolyte addition to ingested fluids. Eur. J. Appl. Physiol. Occup. Physiol. 1994, 69, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Waller, A.P.; Lindinger, M.I. Pre-loading large volume oral electrolytes: Tracing fluid and ion fluxes in horses during rest, exercise and recovery. J. Physiol. 2021, 599, 3879–3896. [Google Scholar] [CrossRef] [PubMed]
- Gottlieb-Vedi, M.; Dahlborn, K.; Jansson, A.; Wroblewski, R. Elemental composition of muscle at rest and potassium levels in muscle, plasma and sweat of horses exercising at 20 °C and 35 °C. Equine Vet. J. 1996, 22, 35–41. [Google Scholar] [CrossRef]
- Lindinger, M.I.; Cairns, S.P. Regulation of muscle potassium: Exercise performance, fatigue and health implications. Eur. J. Appl. Physiol. 2021, 121, 721–748. [Google Scholar] [CrossRef]
- Cheuvront, S.N.; Kenefick, R.W. Dehydration: Physiology, Assessment, and Performance Effects. Compr. Physiol. 2014, 4, 257–285. [Google Scholar] [CrossRef] [PubMed]
- Adan, A. Cognitive Performance and Dehydration. J. Am. Coll. Nutr. 2012, 31, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Snow, D.; Kerr, M.; Nimmo, M.; Abbott, E. Alterations in blood, sweat, urine and muscle composition during prolonged exercise in the horse. Vet. Rec. 1982, 110, 377–384. [Google Scholar] [CrossRef]
- Ecker, G.L.; Lindinger, M.I. Effects of terrain, speed, temperature and distance on water and ion losses. Equine Vet. J. 1995, 27, 298–305. [Google Scholar] [CrossRef]
- Lund, R.J.; Guthrie, A.J. Measurement of maximal oxygen consumption of thoroughbred horses at an altitude of 1250 m using open-circuit flow-through calorimetry. J. South Afr. Vet. Assoc. 1995, 66, 239–243. [Google Scholar]
- Thomas, D.R.; Cote, T.R.; Lawhorne, L.; Levenson, S.A.; Rubenstein, L.; Smith, D.A.; Stefanacci, R.G.; Tangalos, E.G.; Morley, J.E.; Dehydration Council. Understanding Clinical Dehydration and Its Treatment. J. Am. Med. Dir. Assoc. 2008, 9, 292–301. [Google Scholar] [CrossRef]
- Waller, A.; Lindinger, M.I. Time course and magnitude of fluid and electrolyte shifts during recovery from high-intensity exercise in Standardbred racehorses. Equine Comp. Exerc. Physiol. 2005, 2, 77–87. [Google Scholar] [CrossRef]
- Sneddon, J.; Argenzio, R. Feeding strategy and water homeostasis in equids: The role of the hind gut. J. Arid Environ. 1998, 38, 493–509. [Google Scholar] [CrossRef]
- Argenzio, R.A.; Lowe, J.E.; Pickard, D.W.; Stevens, C.E. Digesta passage and water exchange in the equine large intestine. Am. J. Physiol.-Leg. Content 1974, 226, 1035–1042. [Google Scholar] [CrossRef] [PubMed]
- Plummer, A.E. Impactions of the Small and Large Intestines. Vet. Clin. North Am. Equine Pract. 2009, 25, 317–327. [Google Scholar] [CrossRef]
- Lindinger, M.I.; Waller, A.P. Physicochemical analysis of mixed venous and arterial blood acid-base state in horses at core temperature during and after moderate-intensity exercise. Animals 2022, 12, 1875. [Google Scholar] [CrossRef]
- Meyer, N.D.; Bayly, W.M.; Sides, R.H.; Wardrop, K.J.; Slinker, B.K. Changes in arterial, mixed venous and intraerythrocytic ion concentrations during prolonged exercise. Equine Vet. J. 2010, 42, 185–190. [Google Scholar] [CrossRef]
- Lindinger, M.I.; Ecker, G.L. Gastric emptying, intestinal absorption of electrolytes and exercise performance in electrolyte-supplemented horses. Exp. Physiol. 2013, 98, 193–206. [Google Scholar] [CrossRef]
- Koterba, A.; Carlson, G.P. Acid-base and electrolyte alterations in horses with exertional rhabdomyolysis. J. Am. Vet. Med. Assoc. 1982, 180, 303–306. [Google Scholar]
- Waller, A.; Lindinger, M.I. Hydration of exercised Standardbred racehorses assessed noninvasively using multi-frequency bioelectrical impedance analysis. Equine Vet. J. 2006, 38, 285–290. [Google Scholar] [CrossRef]
- Lundvall, J.; Mellander, S.; Westling, H.; White, T. C 12: Dynamics of fluid transfer between the intra- and extravascular compartments during exercise. Acta Physiol. Scand. 1970, 80, 31A–32A. [Google Scholar] [CrossRef]
- Thomas, D.P.; Fregin, G.F. Cardiorespiratory drift during exercise in the horse. Equine Vet. J. 1990, 22, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Lindinger, M. Determining dehydration and its compartmentation in horses at rest and with exercise: A concise review and focus on multi-frequency bioelectrical impedance analysis. Comp. Exerc. Physiol. 2014, 10, 3–11. [Google Scholar] [CrossRef]
- Lindinger, M.; McKelvie, R.S.; Heigenhauser, G.J. K+ and Lac− distribution in humans during and after high-intensity exercise: Role in muscle fatigue attenuation? J. Appl. Physiol. 1995, 78, 765–777. [Google Scholar] [CrossRef] [PubMed]
- Lester, G.; Merritt, A.; Kuck, H.; Burrow, J. Systemic, renal, and colonic effects of intravenous and enteral rehydration in horses. J. Vet. Intern. Med. 2013, 27, 554–566. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Ohmura, H.; Mukai, K.; Shiose, T.; Takahashi, T. A comparison of five cooling methods in hot and humid environments in thoroughbred horses. J. Equine Vet. Sci. 2020, 91, 103130. [Google Scholar] [CrossRef]
- Jenkinson, D.M.; Elder, H.Y.; Bovell, D.L. Equine sweating and anhidrosis Part 2: Anhidrosis. Vet. Dermatol. 2007, 18, 2–11. [Google Scholar] [CrossRef]
- Shi, X.; Summers, R.W.; Schedl, H.P.; Chang, R.T.; Lambert, G.P.; Gisolfi, C.V. Effects of solution osmolality on absorption of select fluid replacement solutions in human duodenojejunum. J. Appl. Physiol. 1994, 77, 1178–1184. [Google Scholar] [CrossRef] [PubMed]
- Fayet-Moore, F.; Wibisono, C.; Carr, P.; Duve, E.; Petocz, P.; Lancaster, G.; McMillan, J.; Marshall, S.; Blumfield, M. An analysis of the mineral composition of pink salt available in australia. Foods 2020, 9, 1490. [Google Scholar] [CrossRef] [PubMed]
- Porath, A.; Mosseri, M.; Harman, I.; Ovsyshcher, I.; Keynan, A. Dead Sea water poisoning. Ann. Emerg. Med. 1989, 18, 187–191. [Google Scholar] [CrossRef]
- Waller, A.P.; Lindinger, M.I. Tracing acid-base variables in exercising horses: Effects of pre-loading oral electrolytes. Animals 2022. [Google Scholar]
- Gisolfi, C.V.; Summers, R.W.; Lambert, G.P.; Xia, T. Effect of beverage osmolality on intestinal fluid absorption during exercise. J. Appl. Physiol. 1998, 85, 1941–1948. [Google Scholar] [CrossRef]
- Monreal, L.; Garzón, N.; Espada, Y.; Ruiz-Gopegui, R.; Homedes, J. Electrolyte vs. glucose-electrolyte isotonic solutions for oral rehydration therapy in horses. Equine Vet. J. 1999, 30, 425–429. [Google Scholar] [CrossRef] [PubMed]
- Sampieri, F.; Schott, H.C.; Hinchcliff, K.; Geor, R.; Jose-Cunilleras, E. Effects of oral electrolyte supplementation on endurance horses competing in 80 km rides. Equine Vet. J. 2006, 38, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Stone, M.S.; Martyn, L.; Weaver, C.M. Potassium intake, bioavailability, hypertension, and glucose control. Nutrients 2016, 8, 444. [Google Scholar] [CrossRef]
- Melikian, A.P.; Cheng, L.K.; Wright, G.J.; Cohen, A.; Bruce, R.E. Bioavailability of potassium from three dosage forms: Suspension, capsule, and solution. J. Clin. Pharmacol. 1988, 28, 1046–1050. [Google Scholar] [CrossRef]
- Turnlund, J.R. Mineral bioavailability and metabolism determined by using stable isotope tracers1. J. Anim. Sci. 2006, 84, E73–E78. [Google Scholar] [CrossRef]
- De Baaij, J.H.F.; Hoenderop, J.G.J.; Bindels, R.J. Magnesium in man: Implications for health and disease. Physiol. Rev. 2015, 95, 1–46. [Google Scholar] [CrossRef]
- Heffernan, S.M.; Horner, K.; De Vito, G.; Conway, G.E. The role of mineral and trace element supplementation in exercise and athletic performance: A systematic review. Nutrients 2019, 11, 696. [Google Scholar] [CrossRef]
- Mansmann, R.A.; Carlson, G.P.; White, N.A., 2nd; Milne, D.W. Synchronous diaphragmatic flutter in horses. J. Am. Vet. Med. Assoc. 1974, 165, 265–270. [Google Scholar] [PubMed]
- Pardo, M.R.; Vilar, E.G.; Martín, I.S.M.; Martín, M.A.C. Bioavailability of magnesium food supplements: A systematic review. Nutrition 2021, 89, 111294. [Google Scholar] [CrossRef]
- Van Diest, T.J.; Kogan, C.J.; Kopper, J.J. The effect of water flavor on voluntary water intake in hospitalized horses. J. Equine Vet. Sci. 2021, 98, 103361. [Google Scholar] [CrossRef] [PubMed]
- Randall, R.P.; Schurg, W.A.; Church, D.C. Response of horses to sweet, salty, sour and bitter solutions. J. Anim. Sci. 1978, 47, 51–55. [Google Scholar] [CrossRef] [PubMed]
- León, L.A.S.; Hodgson, D.R.; Carlson, G.P.; Rose, R.J. Effects of concentrated electrolytes administered via a paste on fluid, electrolyte, and acid base balance in horses. Am. J. Vet. Res. 1998, 59, 898–903. [Google Scholar]
- Ii, H.C.S.; Axiak, S.M.; Woody, K.A.; Eberhart, S.W. Effect of oral administration of electrolyte pastes on rehydration of horses. Am. J. Vet. Res. 2002, 63, 19–27. [Google Scholar] [CrossRef]
- Düsterdieck, K.F.; Ii, H.C.S.; Eberhart, S.W.; Woody, K.A.; Coenen, M. Electrolyte and glycerol supplementation improve water intake by horses performing a simulated 60 km endurance ride. Equine Vet. J. 1999, 30, 418–424. [Google Scholar] [CrossRef]
- Costill, D.L.; Saltin, B. Factors limiting gastric emptying during rest and exercise. J. Appl. Physiol. 1974, 37, 679–683. [Google Scholar] [CrossRef]
- Barker, G.R.; Cochrane, G.M.; Corbett, G.A.; Hunt, J.N.; Roberts, S.K. Actions of glucose and potassium chloride on osmoreceptors slowing gastric emptying. J. Physiol. 1974, 237, 183–186. [Google Scholar] [CrossRef]
- Holbrook, T.C.; Simmons, R.D.; Payton, M.E.; MacAllister, C.G. Effect of repeated oral administration of hypertonic electrolyte solution on equine gastric mucosa. Equine Vet. J. 2005, 37, 501–504. [Google Scholar] [CrossRef]
- Ryan, A.J.; Bleiler, T.L.; Carter, J.E.; Gisolfi, C.V. Gastric emptying during prolonged cycling exercise in the heat. Med. Sci. Sports Exerc. 1989, 21, 51–58. [Google Scholar] [CrossRef]
- Muñoz, A.; Riber, C.; Trigo, P.; Castejón, F.M.; Lucas, R.G.; Palacio, J. The effects of hypertonic dehydration changes on renal function and arginine vasopressin in the horse during pulling exercises. Vet. J. 2011, 189, 83–88. [Google Scholar] [CrossRef]
- Muñoz, A.; Riber, C.; Trigo, P.; Castejón, F.M.; Castejón-Riber, C. Dehydration, electrolyte imbalances and renin-angiotensin-aldosterone-vasopressin axis in successful and unsuccessful endurance horses. Equine Vet. J. 2010, 42, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Jeffcott, L.; Kohn, C.W. Contributions of equine exercise physiology research to the success of the 1996 Equestrian Olympic Games: A review. Equine Vet. J. 1999, 30, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Lindinger, M.I.; Marlin, D.J. Heat stress and acclimation in the performance horse: Where we are and where we are going. Equine Vet. Educ. 1995, 7, 256–262. [Google Scholar] [CrossRef]
| Parameter | Horse | Human |
|---|---|---|
| Body mass—BM (kg) | 500 | 80 |
| Contracting muscle (kg) a | 200 | 16 |
| Surface area (SA) for cooling (m2) b | 5.09 | 1.8 |
| BM: SA ratio | 100 | 40 |
| Sweating rate (mL·m−2·min−1) c | 50 | 30 |
| % sweat used for cooling d | 25–30 | 30–50 |
| Total sweat [ion]s] (mmol/L) e | 200 | 50 |
| Sweat [Na+] (mmol/L) e | 120 | 40 |
| Sweat [K+] (mmol/L) e | 60 | 4 |
| Sweat [Cl−] (mmol/L) e | 180 | 50 |
| Sweat [Ca2+] (mmol/L) f | 3 to 7 | 40 |
| Sweat [Mg2+] (mmol/L) f | 3 to 6 | 4 |
| Sweating rate (L/h) e | 10–15 | 2–3 |
| Source | NaCl | Ca | Mg | K |
|---|---|---|---|---|
| % Dry Weight | ||||
| Redmond–Rock Crushed Loose Mineral Salt for Horses a | 91 | 0.35 | 0.06 | 0.03 |
| Himalayan Pink Salt b | 50–70 | 0.18–0.31 | 0.13–0.25 | 0.21–0.35 |
| Dead Sea Salt c | 30 | 7 | 25 | 2 |
| Ocean salt | 97 | |||
| Perform’N Win d | 33 | 0.024 | 0.03 | 18 |
| Equine Sweat e | 48 | 0.04 | 0.05 | 14 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lindinger, M.I. Oral Electrolyte and Water Supplementation in Horses. Vet. Sci. 2022, 9, 626. https://doi.org/10.3390/vetsci9110626
Lindinger MI. Oral Electrolyte and Water Supplementation in Horses. Veterinary Sciences. 2022; 9(11):626. https://doi.org/10.3390/vetsci9110626
Chicago/Turabian StyleLindinger, Michael Ivan. 2022. "Oral Electrolyte and Water Supplementation in Horses" Veterinary Sciences 9, no. 11: 626. https://doi.org/10.3390/vetsci9110626
APA StyleLindinger, M. I. (2022). Oral Electrolyte and Water Supplementation in Horses. Veterinary Sciences, 9(11), 626. https://doi.org/10.3390/vetsci9110626