Lipopolysaccharide Promotes the Proliferation and Differentiation of Goose Embryonic Myoblasts by Promoting Cytokine Expression and Appropriate Apoptosis Processes
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Animals and Embryonic Myoblast Isolation
2.3. Cell Treatments
2.4. Detection of Myoblast Proliferation Ability
2.5. Detection of Myoblast Differentiation Ability
2.6. Detection of Myoblast Apoptosis Processes
2.7. Western Blotting
2.8. Quantitative RT-qPCR
2.9. Statistical Analysis
3. Results
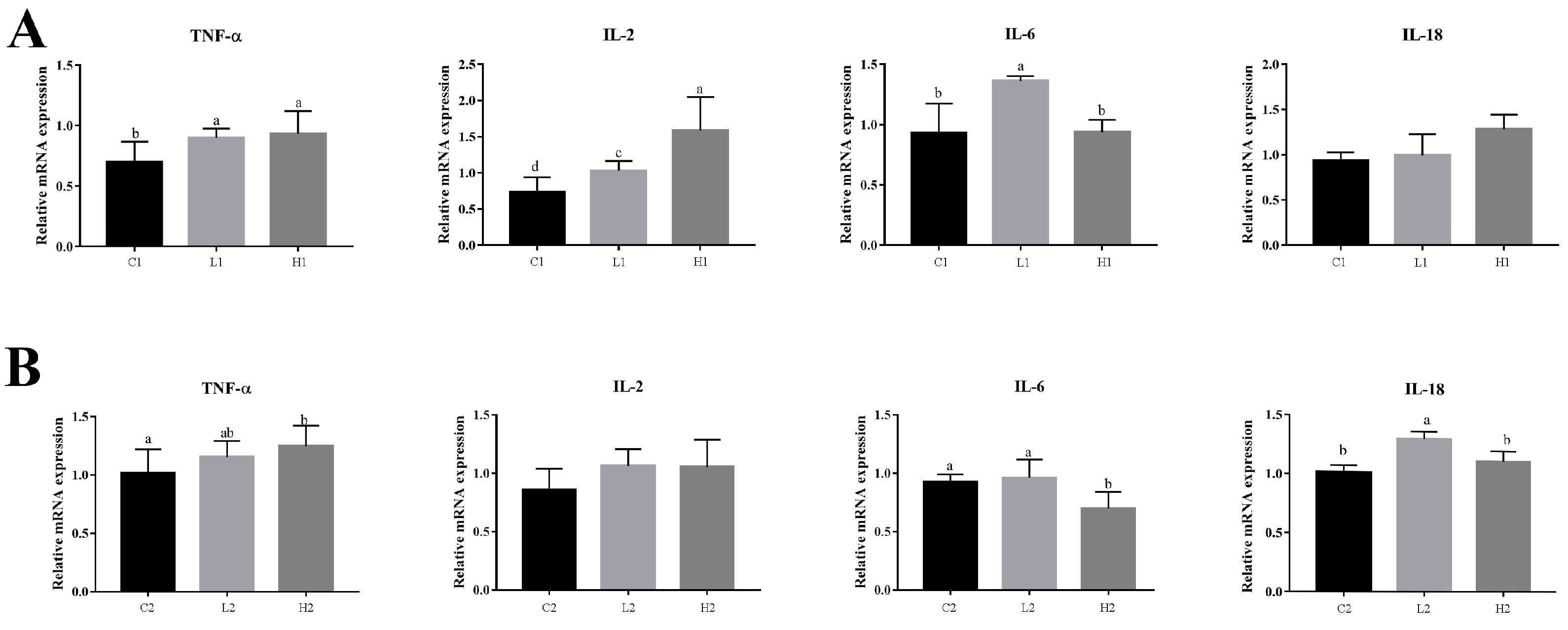
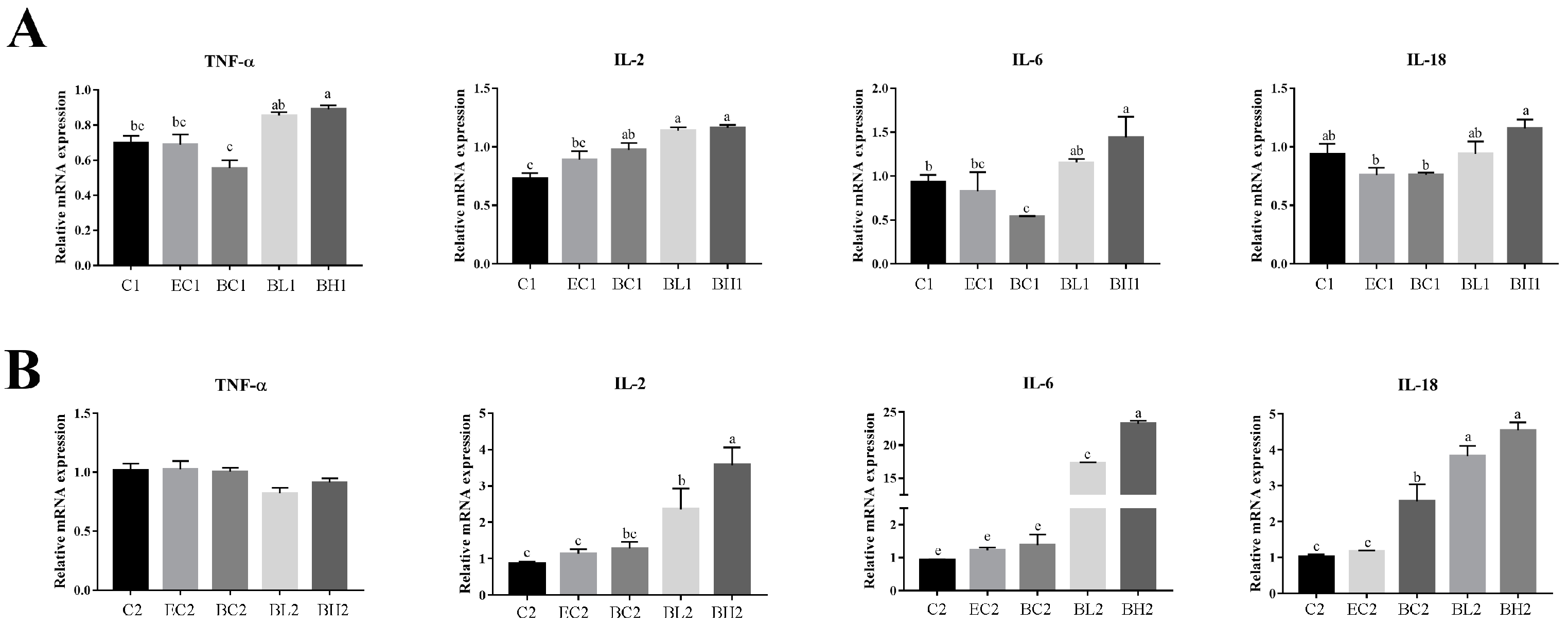
3.1. Exogenous LPS Promoted the Expression of Inflammatory Factors in Goose Embryonic Myoblasts
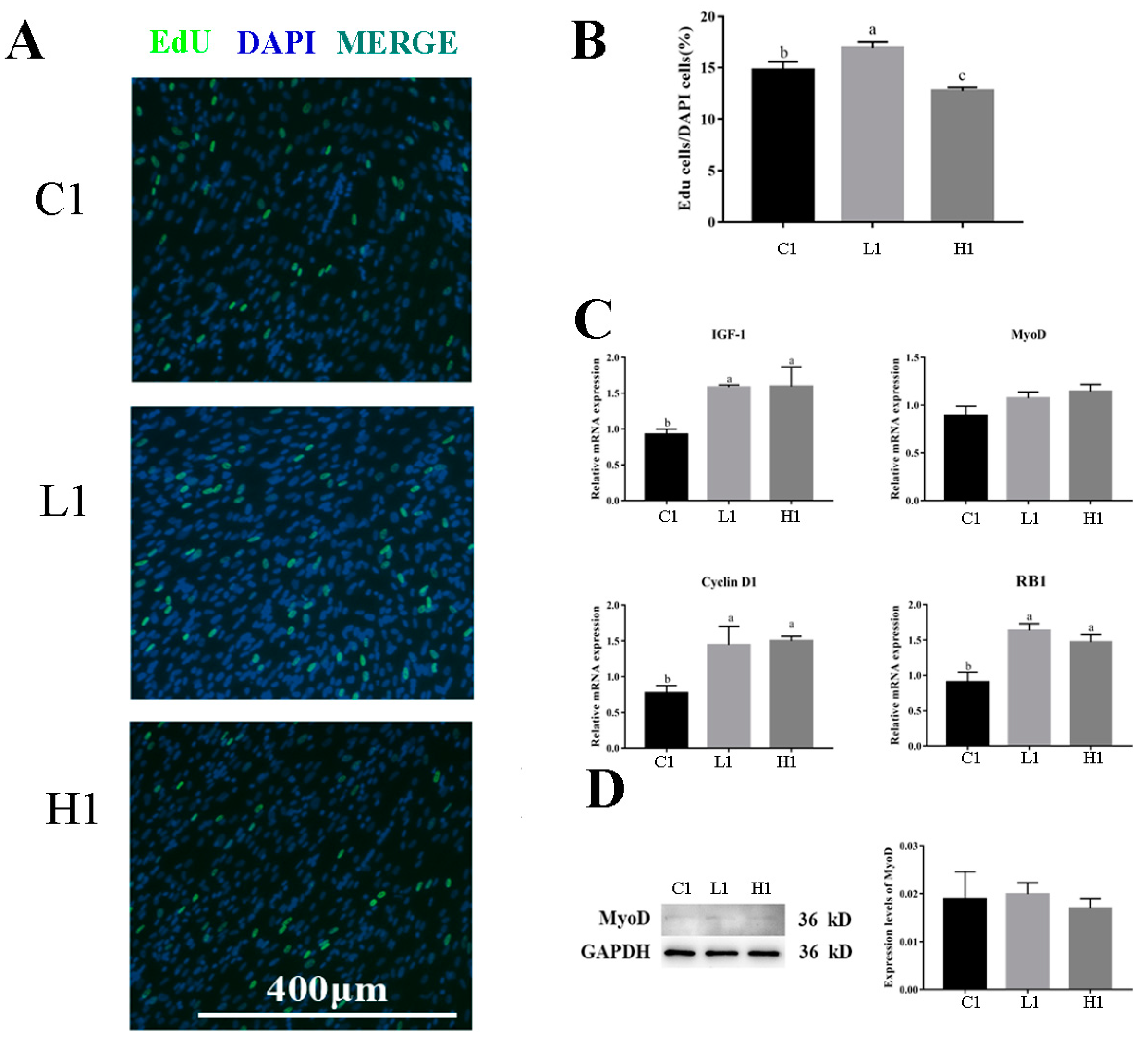
3.2. Exogenous LPS Significantly Promoted Proliferation Ability of Goose Embryonic Myoblasts
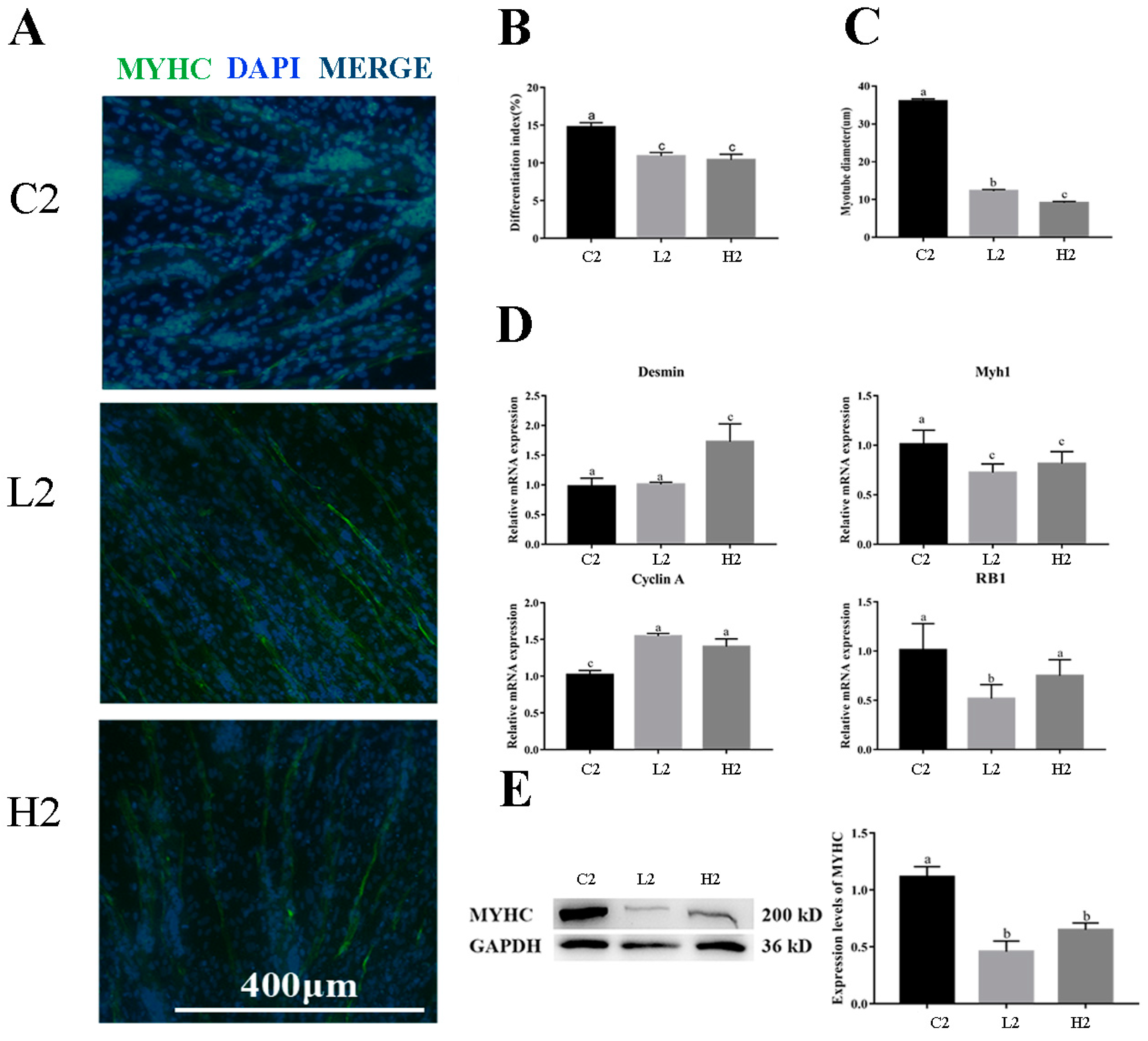
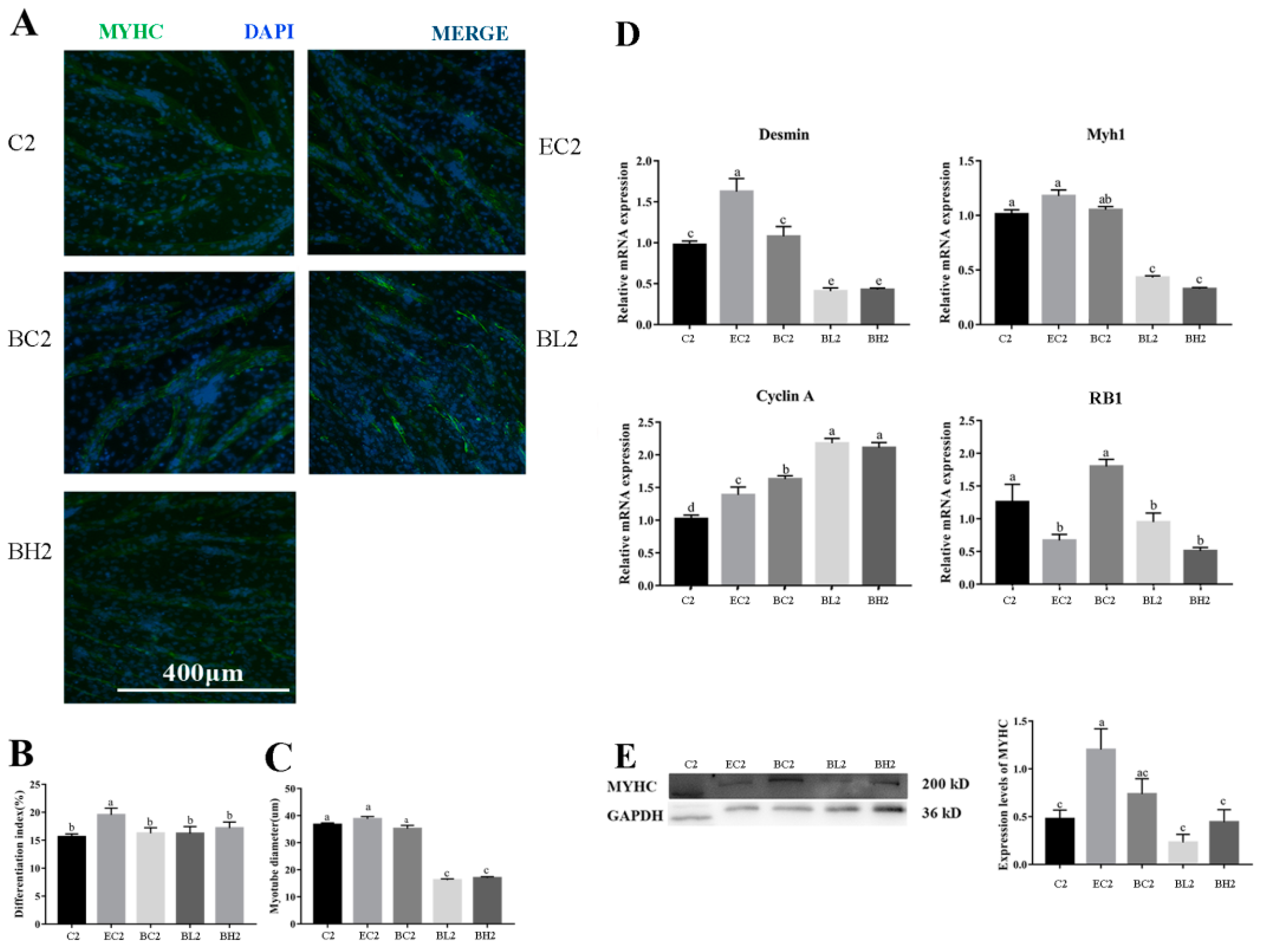
3.3. Exogenous LPS Significantly Reduced Differentiation Ability of Goose Embryonic Myoblasts
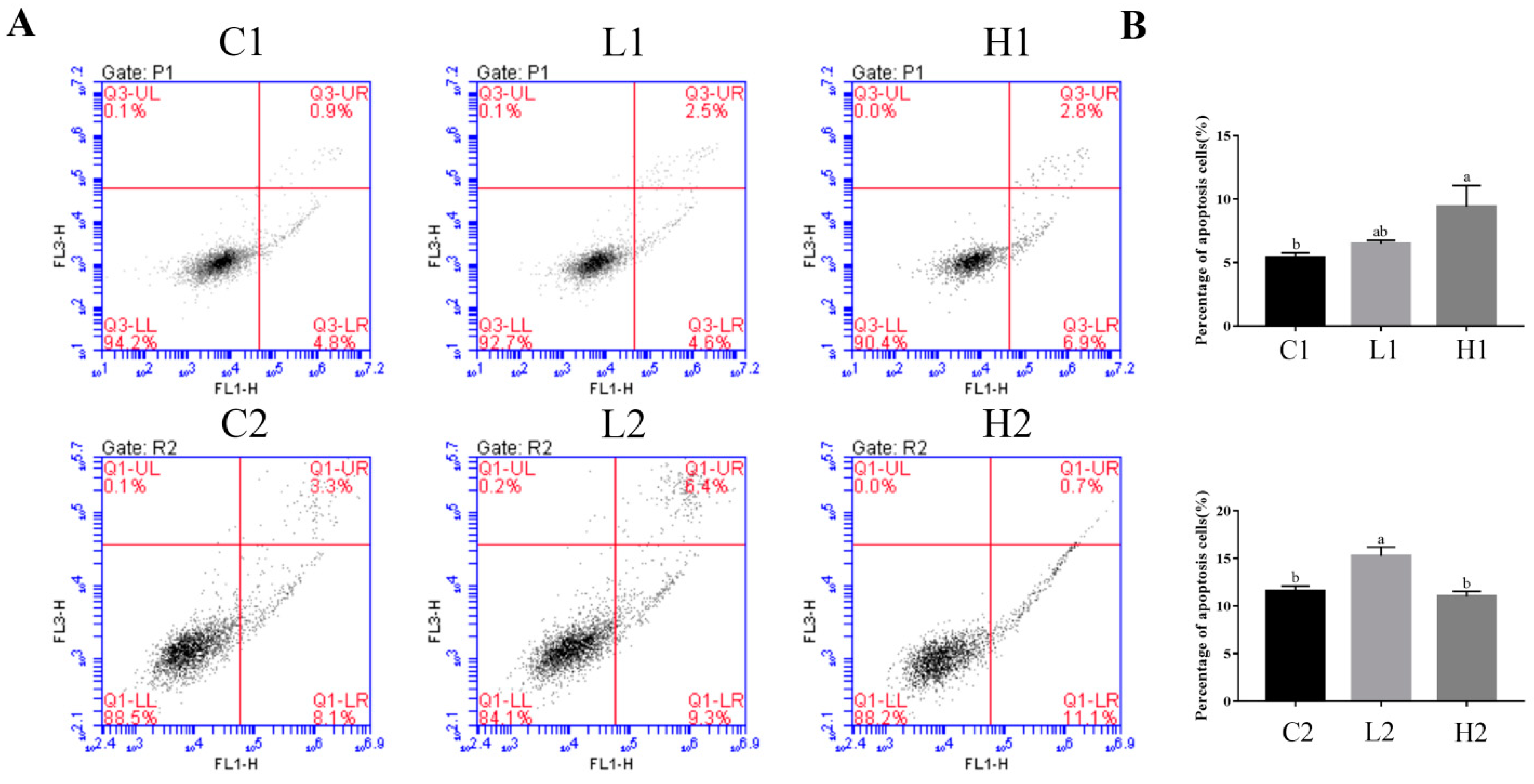
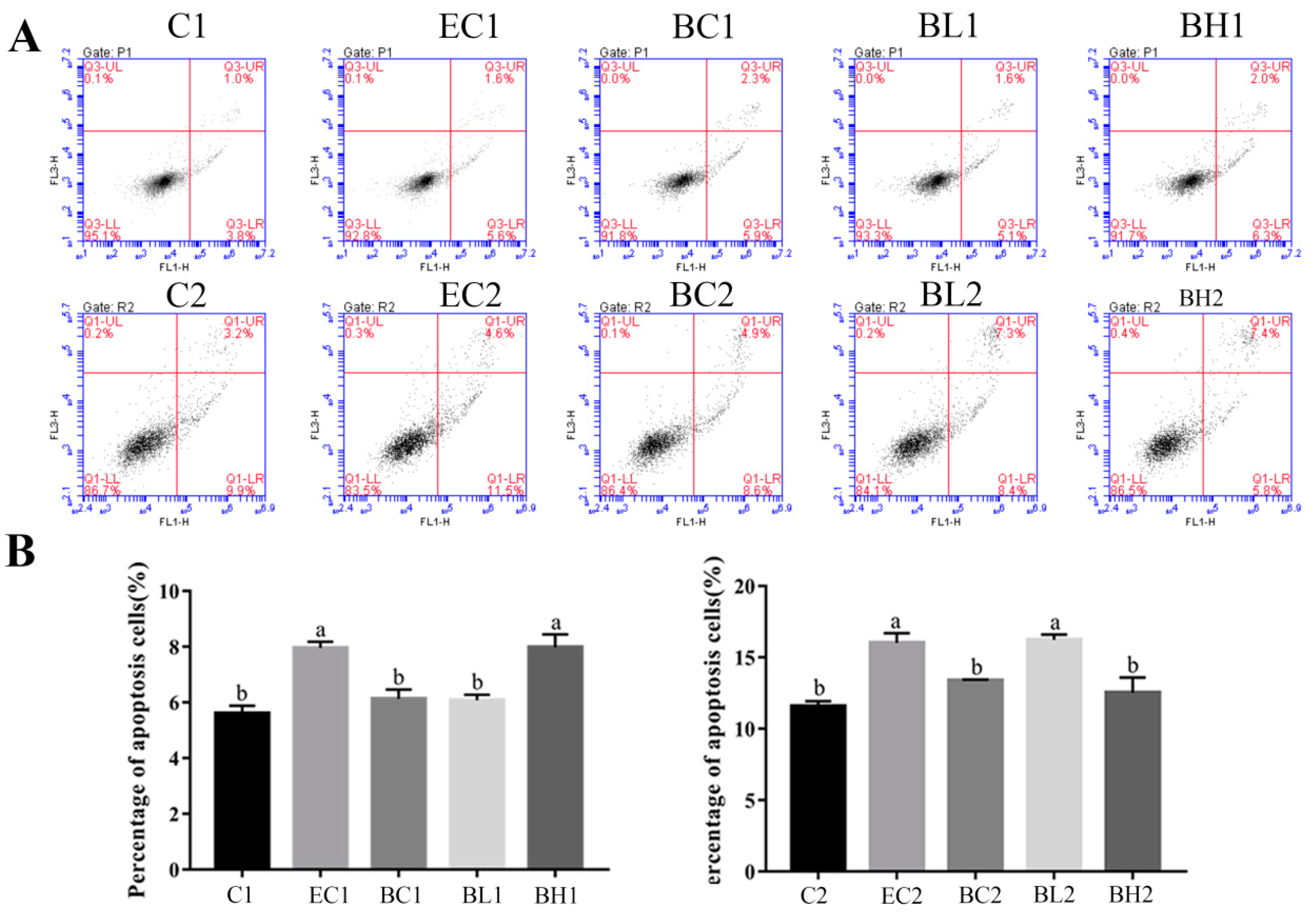
3.4. Exogenous LPS Significantly Promoted Apoptosis Processes in Both Proliferation and Differentiation Phases of Goose Embryonic Myoblasts
3.5. Effect of Embelin or Belnacasan and LPS Treatment on Expression of Inflammatory Cytokines in Goose Embryonic Myoblasts
3.6. Effect of Embelin or Belnacasan and LPS Treatment on Proliferation Ability of Goose Embryonic Myoblasts
3.7. Effect of Embelin or Belnacasan and LPS Treatment on Differentiation Ability of Goose Embryonic Myoblasts
3.8. Effect of Embelin or Belnacasan and LPS Treatment on Apoptosis Processes of Goose Embryonic Myoblasts
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hou, S.-S.; Liu, L.-Z. Current situation of waterfowl industry in 2019, future development trend and suggestions. Chin. J. Anim. Sci. 2020, 56, 135–140. (In Chinese) [Google Scholar]
- Guha, M.; Mackman, N. LPS induction of gene expression in human monocytes. Cell. Signal. 2001, 13, 85–94. [Google Scholar] [CrossRef]
- Zhang, X.; Yu, S.; Ci, X.; An, N.; Fan, J.; Cui, J.; Deng, X. Effects of florfenicol on early cytokine responses and survival in murine endotoxemia. Int. Immunopharmacol. 2008, 8, 982–988. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Yeh, W.; Ohashi, P.S. LPS/TLR4 signal transduction pathway. Cytokine 2008, 42, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.I.; Ernst, R.K.; Bader, M.W. LPS, TLR4 and infectious disease diversity. Nat. Rev. Microbiol. 2005, 3, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Liu, L.; Wang, C.; Chen, F.; Sun, A.; Shi, Z. Raising on Water Stocking Density Reduces Geese Reproductive Performances via Water Bacteria and Lipopolysaccharide Contaminations in “Geese-Fish” Production System. Agric. Sci. China 2011, 10, 1459–1466. [Google Scholar] [CrossRef]
- Frost, R.A.; Nystrom, G.J.; Lang, C.H. Lipopolysaccharide regulates proinflammatory cytokine expression in mouse myoblasts and skeletal muscle. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2002, 283, R698–R709. [Google Scholar] [CrossRef]
- Jiang, H.; Stewart, C.A.; Leu, R.W. Tumor-derived factor synergizes with IFN-gamma and LPS, IL-2 or TNF-alpha to promote macrophage synthesis of TNF-alpha and TNF receptors for autocrine induction of nitric oxide synthase and enhanced nitric oxide-mediated tumor cytotoxicity. Immunobiology 1995, 192, 321–342. [Google Scholar] [CrossRef]
- Luo, G.; Hershko, D.D.; Robb, B.W.; Wray, C.J.; Hasselgren, P.O. IL-1β stimulates IL-6 production in cultured skeletal muscle cells through activation of MAP kinase signaling pathway and NF-κB. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003, 284, R1249–R1254. [Google Scholar] [CrossRef]
- Horstmann, J.; Marzi, I.; Relja, B. Adrenergic stimulation alters the expression of inflammasome components and interleukins in primary human monocytes. Exp. Ther. Med. 2016, 11, 297–302. [Google Scholar] [CrossRef]
- Cao, Y.-Y.; Wang, Z.; Yu, T.; Zhang, Y.; Wang, Z.-H.; Lu, Z.-M.; Lu, W.-H.; Yu, J.-B. Sepsis induces muscle atrophy by inhibiting proliferation and promoting apoptosis via PLK1-AKT signalling. J. Cell. Mol. Med. 2021, 25, 9724–9739. [Google Scholar] [CrossRef] [PubMed]
- Fang, W.-Y.; Tseng, Y.-T.; Lee, T.-Y.; Fu, Y.-C.; Chang, W.-H.; Lo, W.-W.; Lin, C.-L.; Lo, Y.-C. Triptolide prevents LPS-induced skeletal muscle atrophy via inhibiting NF-κB/TNF-α and regulating protein synthesis/degradation pathway. Br. J. Pharmacol. 2021, 178, 2998–3016. [Google Scholar] [CrossRef] [PubMed]
- Ono, Y.; Sakamoto, K. Lipopolysaccharide inhibits myogenic differentiation of C2C12 myoblasts through the Toll-like receptor 4-nuclear factor-κB signaling pathway and myoblast-derived tumor necrosis factor-α. PLoS ONE 2017, 12, e182040. [Google Scholar]
- Shang, K.; Zhang, J.; Amna, T.; Yang, J.; Cheng, X.; Zhang, C.; Hwang, I. Attenuation of cellular toxicity by calpain inhibitor induced by bacterial endotoxin: A mechanistic study using muscle precursor cells as a model system. Mol. Biol. Rep. 2015, 42, 1281–1288. [Google Scholar] [CrossRef]
- Yang, X.; Liu, L.; Jiang, D.; Wang, C.; Sun, A.; Shi, Z. Improving Geese Production Performance in “Goose-Fish” Production System by Competitive Reduction of Pathogenic Bacteria in Pond Water. J. Integr. Agric. 2012, 11, 993–1001. [Google Scholar] [CrossRef]
- Parker, M.H.; Seale, P.; Rudnicki, M.A. Looking back to the embryo: Defining transcriptional networks in adult myogenesis. Nat. Rev. Genet. 2003, 4, 497–507. [Google Scholar] [CrossRef]
- Bentzinger Florian, C.; Wang, Y.X.; Rudnicki, M.A. Building Muscle: Molecular Regulation of Myogenesis. Cold Spring Harb Perspect. Biol. 2012, 4, a008342. [Google Scholar] [CrossRef]
- Chal, J.; Pourquié, O. Making muscle: Skeletal myogenesis in vivo and in vitro. Development 2017, 144, 2104–2122. [Google Scholar] [CrossRef]
- Hochreiter-Hufford, A.E.; Chang, S.L.; Kinchen, J.M.; Sokolowski, J.D.; Arandjelovic, S.; Call, J.A.; Klibanov, A.L.; Yan, Z.; Mandell, J.W.; Ravichandran, K.S. Phosphatidylserine receptor BAI1 and apoptotic cells as new promoters of myoblast fusion. Nature 2013, 497, 263–267. [Google Scholar] [CrossRef]
- Krauss, R.S.; Joseph, G.A.; Goel, A.J. Keep Your Friends Close: Cell–Cell Contact and Skeletal Myogenesis. Cold Spring Harb. Perspect. Biol. 2017, 9, a29298. [Google Scholar] [CrossRef]
- Krauss, R.S. Close encounters: Regulation of vertebrate skeletal myogenesis by cell-cell contact. J. Cell Sci. 2005, 118, 2355–2362. [Google Scholar] [CrossRef] [PubMed]
- Nie, Y.; Cai, S.; Yuan, R.; Ding, S.; Zhang, X.; Chen, L.; Chen, Y.; Mo, D. Zfp422 promotes skeletal muscle differentiation by regulating EphA7 to induce appropriate myoblast apoptosis. Cell Death Differ. 2020, 27, 1644–1659. [Google Scholar] [CrossRef]
- Cooper, R.N.; Tajbakhsh, S.; Mouly, V.; Cossu, G.; Buckingham, M.; Butler-Browne, G.S. In vivo satellite cell activation via Myf5 and MyoD in regenerating mouse skeletal muscle. J. Cell Sci. 1999, 112, 2895. [Google Scholar] [CrossRef] [PubMed]
- Tapscott, S.J. The circuitry of a master switch: Myod and the regulation of skeletal muscle gene transcription. Development 2005, 132, 2685–2695. [Google Scholar] [CrossRef] [PubMed]
- Folpe Andrew, L. MyoD1 and myogenin expression in human neoplasia: A review and update. Adv. Anat. Pathol. 2002, 9, 198. [Google Scholar] [CrossRef]
- Baldin, V.; Lukas, J.; Marcote, M.J.; Pagano, M.; Draetta, G. Cyclin D1 is a nuclear protein required for cell cycle progression in G1. Genes Dev. 1993, 7, 812–821. [Google Scholar] [CrossRef]
- Pagano, M.; Pepperkok, R.; Verde, F.; Ansorge, W.; Draetta, G. Cyclin A is required at two points in the human cell cycle. EMBO J. 1992, 11, 961–971. [Google Scholar] [CrossRef]
- Weinberg, R.A. The retinoblastoma protein and cell cycle control. Cell 1995, 81, 323–330. [Google Scholar] [CrossRef]
- Wang, J.; Huang, Y.; Li, X.; Shen, X.; Fu, X.; Jiang, D.; Ouyang, H.; Fan, D.; Huang, X.; Zhang, X. In vitro culture and identification of Magang goosemyoblasts and effect of temperature on cellproliferation and differentiation. J. Zhongkai Univ. Agric. Eng. 2020, 33, 21–27. (In Chinese) [Google Scholar]
- Ning, N.-Z.; Liu, X.; Chen, F.; Zhou, P.; Hu, L.; Huang, J.; Li, Z.; Huang, J.; Li, T.; Wang, H. Embelin Restores Carbapenem Efficacy against NDM-1-Positive Pathogens. Front. Microbiol. 2018, 9, 71. [Google Scholar] [CrossRef]
- Park, N.; Baek, H.S.; Chun, Y. Embelin-Induced Apoptosis of Human Prostate Cancer Cells Is Mediated through Modulation of Akt and β-Catenin Signaling. PLoS ONE 2015, 10, e134760. [Google Scholar] [CrossRef] [PubMed]
- Liao, Z.; Li, S.; Liu, R.; Feng, X.; Shi, Y.; Wang, K.; Li, S.; Zhang, Y.; Wu, X.; Yang, C. Autophagic Degradation of Gasdermin D Protects against Nucleus Pulposus Cell Pyroptosis and Retards Intervertebral Disc Degeneration In Vivo. Oxid. Med. Cell. Longev. 2021, 2021, 5584447. [Google Scholar] [CrossRef] [PubMed]
- Chitra, M.; Sukumar, E.; Suja, V.; Devi, C.S. Antitumor, anti-inflammatory and analgesic property of embelin, a plant product. Comp. Stud. 1994, 40, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Gong, Z.; Zhou, J.; Zhao, S.; Tian, C.; Wang, P.; Xu, C.; Chen, Y.; Cai, W.; Wu, J. Chenodeoxycholic acid activates NLRP3 inflammasome and contributes to cholestatic liver fibrosis. Oncotarget 2016, 7, 83951–83963. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Gu, L.; Wu, K.; Wang, K.; Ru, J.; Yang, S.; Wang, Z.; Zhuge, Q.; Huang, L.; Huang, S. VX-765 enhances autophagy of human umbilical cord mesenchymal stem cells against stroke-induced apoptosis and inflammatory responses via AMPK/mTOR signaling pathway. CNS Neurosci. Ther. 2020, 26, 952–961. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, X.; Wang, Q.; Yang, X. Roles of the pyroptosis signaling pathway in a sepsis-associated encephalopathy cell model. J. Int. Med. Res. 2020, 48, 1220749319. [Google Scholar] [CrossRef] [PubMed]
- Ondee, S.; Sithisarn, P.; Mangmool, S.; Rojsanga, P. Chemical Standardization and Anti-Proliferative Activity of Ardisia elliptica Fruit against the HCT116 Human Colon Cancer Cell Line. Molecules 2020, 25, 1023. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Zhang, D.; Lee, H.; Wu, J.; Hu, K.; Jin, Y. Macrophage-derived apoptotic bodies promote the proliferation of the recipient cells via shuttling microRNA-221/222. J. Leukoc. Biol. 2017, 101, 1349–1359. [Google Scholar] [CrossRef]
- Zhang, K.; Wu, S.; Li, Z.; Zhou, J. MicroRNA-211/BDNF axis regulates LPS-induced proliferation of normal human astrocyte through PI3K/AKT pathway. Biosci. Rep. 2017, 37, BSR20170755. [Google Scholar] [CrossRef]
- Zhang, M.; Hao, Y.; Wei, W.; Wang, F.; Mao, D. Effects of LPS on the accumulation of lipid droplets, proliferation, and steroidogenesis in goat luteinized granulosa cells. J. Biochem. Mol. Toxicol. 2019, 33, e22329. [Google Scholar] [CrossRef]
- Ahn, K.S.; Sethi, G.; Aggarwal, B.B. Embelin, an inhibitor of X chromosome-linked inhibitor-of-apoptosis protein, blocks nuclear factor-kappaB (NF-kappaB) signaling pathway leading to suppression of NF-kappaB-regulated antiapoptotic and metastatic gene products. Mol. Pharmacol. 2007, 71, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Nikolovska-Coleska, Z.; Xu, L.; Hu, Z.; Tomita, Y.; Li, P.; Roller, P.P.; Wang, R.; Fang, X.; Guo, R.; Zhang, M.; et al. Discovery of embelin as a cell-permeable, small-molecular weight inhibitor of XIAP through structure-based computational screening of a traditional herbal medicine three-dimensional structure database. J. Med. Chem. 2004, 47, 2430–2440. [Google Scholar] [CrossRef] [PubMed]
- Baker, L.A.; Martin, N.R.W.; Kimber, M.C.; Pritchard, G.J.; Lindley, M.R.; Lewis, M.P. Resolvin E1 (Rv E1) attenuates LPS induced inflammation and subsequent atrophy in C2C12 myotubes. J. Cell. Biochem. 2018, 119, 6094–6103. [Google Scholar] [CrossRef] [PubMed]

| Gene Name | Forward (5′-3′) | Reverse (3′-5′) |
|---|---|---|
| IL-18 | AAGTGAGGCTCAACATTGCG | CGGTAGAAGATGAAGCGGGT |
| IL-2 | ACCGAGAGCTGACCAACTTT | ATCACCCACACTAAGAGCAT |
| IL-6 | TTCGACGAGGAGAAATGCTT | CCTTATCGTCGTTGCCAGAT |
| TNF-α | ATGAACCCTCCTCCGTACAC | AGAGGCCACCACATGATAGC |
| MyoD | AAGGCGTGCAAGAGGAAGAC | TGGTTGGGGTTGGTGGA |
| IGF-1 | AGGTCGTCCATCGTAGTCCTTGCACTTTTAAGAAGCAATGGA | ACAGCGTCGTTATCGTTCCTGCAAACACAGGCCAAGGTAG |
| Rb1 | CCCAGTAGTGAGATTTCTGCT | TGAGCATAGCAGGTGGTGAC |
| Cyclin D1 | GCTGCGAAGTGGAAACCATC | CCTCCTTCTGCACACATTTGAA |
| Cyclin A | GGCAGCTCCAACAATCAACC | TGCACAGAGATTCAGGCCAA |
| Myh1 | CTCCTCACGCTTTGGTAAAT | GCTCTGGCTTCTTGTTGGAC |
| Desmin | TGAAGGACGAGATGGCCCG | CATCACGGTCTTCTTGGTGTG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Fu, M.; Xu, D.; Cao, N.; Li, W.; Tian, Y.; Zhang, X.; Huang, Y. Lipopolysaccharide Promotes the Proliferation and Differentiation of Goose Embryonic Myoblasts by Promoting Cytokine Expression and Appropriate Apoptosis Processes. Vet. Sci. 2022, 9, 615. https://doi.org/10.3390/vetsci9110615
Wang J, Fu M, Xu D, Cao N, Li W, Tian Y, Zhang X, Huang Y. Lipopolysaccharide Promotes the Proliferation and Differentiation of Goose Embryonic Myoblasts by Promoting Cytokine Expression and Appropriate Apoptosis Processes. Veterinary Sciences. 2022; 9(11):615. https://doi.org/10.3390/vetsci9110615
Chicago/Turabian StyleWang, Jinhui, Mengsi Fu, Danning Xu, Nan Cao, Wanyan Li, Yunbo Tian, Xumeng Zhang, and Yunmao Huang. 2022. "Lipopolysaccharide Promotes the Proliferation and Differentiation of Goose Embryonic Myoblasts by Promoting Cytokine Expression and Appropriate Apoptosis Processes" Veterinary Sciences 9, no. 11: 615. https://doi.org/10.3390/vetsci9110615
APA StyleWang, J., Fu, M., Xu, D., Cao, N., Li, W., Tian, Y., Zhang, X., & Huang, Y. (2022). Lipopolysaccharide Promotes the Proliferation and Differentiation of Goose Embryonic Myoblasts by Promoting Cytokine Expression and Appropriate Apoptosis Processes. Veterinary Sciences, 9(11), 615. https://doi.org/10.3390/vetsci9110615







