Immune Activated Cellular Therapy for Drug Resistant Infections: Rationale, Mechanisms, and Implications for Veterinary Medicine
Abstract
Simple Summary
Abstract
1. Introduction
2. Principles of Cellular Therapy to Treat Bacterial Infection
2.1. Mechanisms of MSC Antimicrobial and Immunomodulatory Action
2.2. Cellular Activation Techniques
2.3. Route of Administration, Dosing, and Number of Injections
2.4. Combination of MSC with Antibiotics for Enhanced Bacterial Killing
3. Evidence for Antimicrobial Activity in Animal Models
3.1. Rodent Models of Infection
3.2. Naturally Occurring Canine Model of Chronic Infection
3.3. Induced Equine Model of Septic Arthritis
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- CDC. Antibiotic Resistance Threats in the United States. 2013. Available online: https://www.cdc.gov/drugresistance/threat-report-2013/pdf/ar-threats-2013-508.pdf (accessed on 4 April 2022).
- World Health Organization (WHO). Antimicrobial Resistance. 2018. Available online: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance (accessed on 4 April 2022).
- Ventola, C.L. The Antibiotic Resistance Crisis: Part 1: Causes and threats. Pharm. Ther. 2015, 40, 277–283. [Google Scholar]
- Aslam, B.; Wang, W.; Arshad, M.I.; Khurshid, M.; Muzammil, S.; Nisar, M.A.; Alvi, R.F.; Aslam, M.A.; Qamar, M.U.; Salamat, M.K.F.; et al. Antibiotic resistance: A rundown of a global crisis. Infect. Drug Resist. 2018, 11, 1645–1658. [Google Scholar] [CrossRef] [PubMed]
- Bin Zaman, S.; Hussain, M.A.; Nye, R.; Mehta, V.; Mamun, K.T.; Hossain, N. A Review on Antibiotic Resistance: Alarm Bells are Ringing. Cureus 2017, 9, e1403. [Google Scholar] [CrossRef] [PubMed]
- Cumper, C. The Antibiotic Resistance Crisis. 2018. Available online: http://eureka.criver.com/the-antibiotic-resistance-crisis/ (accessed on 8 April 2022).
- NRDC. Next FDA Commissioner Must Address Antibiotic Resistance Crisis. 2019. Available online: https://www.nrdc.org/media/2019/190305 (accessed on 8 April 2022).
- NIHMedline Plus. The End Antibiotics? 2018. Available online: https://medlineplus.gov/magazine/issues/winter18/articles/winter18pg8-11.html (accessed on 8 April 2022).
- Palma, E.; Tilocca, B.; Roncada, P. Antimicrobial Resistance in Veterinary Medicine: An Overview. Int. J. Mol. Sci. 2020, 21, 1914. [Google Scholar] [CrossRef]
- Sutton, M.T.; Fletcher, D.; Ghosh, S.K.; Weinberg, A.; van Heeckeren, R.; Kaur, S.; Sadeghi, Z.; Hijaz, A.; Reese, J.; Lazarus, H.M.; et al. Antimicrobial Properties of Mesenchymal Stem Cells: Therapeutic Potential for Cystic Fibrosis Infection, and Treatment. Stem Cells Int. 2016, 2016, 5303048. [Google Scholar] [CrossRef]
- Gupta, N.; Krasnodembskaya, A.; Kapetanaki, M.; Mouded, M.; Tan, X.; Serikov, V.; Matthay, M.A. Mesenchymal stem cells enhance survival and bacterial clearance in murine Escherichia coli pneumonia. Thorax 2012, 67, 533–539. [Google Scholar] [CrossRef]
- Krasnodembskaya, A.; Samarani, G.; Song, Y.; Zhuo, H.; Su, X.; Lee, J.-W.; Gupta, N.; Petrini, M.; Matthay, M.A. Human mesenchymal stem cells reduce mortality and bacteremia in gram-negative sepsis in mice in part by enhancing the phagocytic activity of blood monocytes. Am. J. Physiol. Lung Cell. Mol. Physiol. 2012, 302, L1003–L1013. [Google Scholar] [CrossRef]
- Alcayaga-Miranda, F.; Cuenca, J.; Khoury, M. Antimicrobial Activity of Mesenchymal Stem Cells: Current Status and New Perspectives of Antimicrobial Peptide-Based Therapies. Front. Immunol. 2017, 8, 339. [Google Scholar] [CrossRef]
- Yuan, Y.; Lin, S.; Guo, N.; Zhao, C.; Shen, S.; Bu, X.; Ye, H. Marrow mesenchymal stromal cells reduce methicillin-resistant Staphylococcus aureus infection in rat models. Cytotherapy 2014, 16, 56–63. [Google Scholar] [CrossRef]
- Criman, E.T.; Kurata, W.E.; Matsumoto, K.W.; Aubin, H.T.; Campbell, C.E.; Pierce, L.M. Bone Marrow–Derived Mesenchymal Stem Cells Enhance Bacterial Clearance and Preserve Bioprosthetic Integrity in a Model of Mesh Infection. Plast. Reconstr. Surg. Glob. Open 2016, 4, e751. [Google Scholar] [CrossRef]
- Johnson, V.; Webb, T.; Norman, A.; Coy, J.; Kurihara, J.; Regan, D.; Dow, S. Activated Mesenchymal Stem Cells Interact with Antibiotics and Host Innate Immune Responses to Control Chronic Bacterial Infections. Sci. Rep. 2017, 7, 9575. [Google Scholar] [CrossRef] [PubMed]
- Asami, T.; Ishii, M.; Namkoong, H.; Yagi, K.; Tasaka, S.; Asakura, T.; Suzuki, S.; Kamo, T.; Okamori, S.; Kamata, H. Anti-inflammatory roles of mesenchymal stromal cells during acute Streptococcus pneumoniae pulmonary infection in mice. Cytotherapy 2018, 20, 302–313. [Google Scholar] [CrossRef] [PubMed]
- Wood, C.R.; Al Dhahri, D.; Al-Delfi, I.; Pickles, N.A.; Sammons, R.L.; Worthington, T.; Wright, K.T.; Johnson, W.E.B. Human adipose tissue-derived mesenchymal stem/stromal cells adhere to and inhibit the growth of Staphylococcus aureus and Pseudomonas aeruginosa. J. Med. Microbiol. 2018, 67, 1789–1795. [Google Scholar] [CrossRef] [PubMed]
- Chow, L.; Johnson, V.; Coy, J.; Regan, D.; Dow, S. Mechanisms of Immune Suppression Utilized by Canine Adipose and Bone Marrow-Derived Mesenchymal Stem Cells. Stem Cells Dev. 2017, 26, 374–389. [Google Scholar] [CrossRef]
- Bujňáková, D.; Čuvalová, A.; Čížek, M.; Humenik, F.; Salzet, M.; Čížková, D. Canine Bone Marrow Mesenchymal Stem Cell Conditioned Media Affect Bacterial Growth, Biofilm-Associated Staphylococcus aureus and AHL-Dependent Quorum Sensing. Microorganisms 2020, 8, 1478. [Google Scholar] [CrossRef]
- Bahroudi, M.; Bakhshi, B.; Soudi, S.; Najar-Peerayeh, S. Antibacterial and antibiofilm activity of bone marrow-derived human mesenchymal stem cells secretome against Vibrio cholerae. Microb. Pathog. 2020, 139, 103867. [Google Scholar] [CrossRef]
- Marx, C.; Gardner, S.; Harman, R.M.; Van de Walle, G.R. The mesenchymal stromal cell secretome impairs methicillin-resistant Staphylococcus aureus biofilms via cysteine protease activity in the equine model. Stem Cells Transl. Med. 2020, 9, 746–757. [Google Scholar] [CrossRef]
- Marx, C.; Gardner, S.; Harman, R.M.; Wagner, B.; Van de Walle, G.R. Mesenchymal Stromal Cell-Secreted CCL2 Promotes Antibacterial Defense Mechanisms Through Increased Antimicrobial Peptide Expression in Keratinocytes. Stem Cells Transl. Med. 2021, 10, 1666–1679. [Google Scholar] [CrossRef]
- Pezzanite, L.M.; Chow, L.; Johnson, V.; Griffenhagen, G.M.; Goodrich, L.; Dow, S. Toll-like receptor activation of equine mesenchymal stromal cells to enhance antibacterial activity and immunomodulatory cytokine secretion. Vet. Surg. 2021, 50, 858–871. [Google Scholar] [CrossRef]
- Pezzanite, L.M.; Chow, L.; Phillips, J.; Griffenhagen, G.M.; Moore, A.R.; Schaer, T.P.; Engiles, J.B.; Werpy, N.; Gilbertie, J.; Schnabel, L.V.; et al. TLR-activated mesenchymal stromal cell therapy and antibiotics to treat multi-drug resistant Staphyloccocal septic arthritis in an equine model. Ann. Trans. Med. 2022, in press. [Google Scholar]
- Johnson, V.; Chow, L.; Harrison, J.; Soontararak, S.; Dow, S. Activated Mesenchymal Stromal Cell Therapy for Treatment of Multi-Drug Resistant Bacterial Infections in Dogs. Front. Vet. Sci. 2022, 9, 925701. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Xu, F.; Zheng, X.; Yang, S.; Ren, Z.; Yang, J. Human Umbilical Cord Mesenchymal Stem Cells Prevent Bacterial Biofilm Formation. BioMed Res. Int. 2022, 2022, 1530525. [Google Scholar] [CrossRef] [PubMed]
- Liotta, F.; Angeli, R.; Cosmi, L.; Filì, L.; Manuelli, C.; Frosali, F.; Mazzinghi, B.; Maggi, L.; Pasini, A.; Lisi, V.; et al. Toll-Like Receptors 3 and 4 Are Expressed by Human Bone Marrow-Derived Mesenchymal Stem Cells and Can Inhibit Their T-Cell Modulatory Activity by Impairing Notch Signaling. Stem Cells 2008, 26, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Opitz, C.A.; Litzenburger, U.M.; Lutz, C.; Lanz, T.V.; Tritschler, I.; Köppel, A.; Tolosa, E.; Hoberg, M.; Anderl, J.; Aicher, W.K.; et al. Toll-Like Receptor Engagement Enhances the Immunosuppressive Properties of Human Bone Marrow-Derived Mesenchymal Stem Cells by Inducing Indoleamine-2,3-dioxygenase-1 via Interferon-β and Protein Kinase R. Stem Cells 2009, 27, 909–919. [Google Scholar] [CrossRef]
- Romieu-Mourez, R.; Francois, M.; Boivin, M.-N.; Bouchentouf, M.; Spaner, D.E.; Galipeau, J. Cytokine Modulation of TLR Expression and Activation in Mesenchymal Stromal Cells Leads to a Proinflammatory Phenotype. J. Immunol. 2009, 182, 7963–7973. [Google Scholar] [CrossRef]
- DelaRosa, O.; Lombardo, E. Modulation of Adult Mesenchymal Stem Cells Activity by Toll-Like Receptors: Implications on Therapeutic Potential. Mediat. Inflamm. 2010, 2010, 865601. [Google Scholar] [CrossRef]
- Cassatella, M.A.; Mosna, F.; Micheletti, A.; Lisi, V.; Tamassia, N.; Cont, C.; Calzetti, F.; Pelletier, M.; Pizzolo, G.; Krampera, M. Toll-Like Receptor-3-Activated Human Mesenchymal Stromal Cells Significantly Prolong the Survival and Function of Neutrophils. Stem Cells 2011, 29, 1001–1011. [Google Scholar] [CrossRef]
- Lei, J.; Wang, Z.; Hui, D.; Yu, W.; Zhou, D.; Xia, W.; Chen, C.; Zhang, Q.; Wang, Z.; Zhang, Q.; et al. Ligation of TLR2 and TLR4 on murine bone marrow-derived mesenchymal stem cells triggers differential effects on their immunosuppressive activity. Cell. Immunol. 2011, 271, 147–156. [Google Scholar] [CrossRef]
- Giuliani, M.; Bennaceur-Griscelli, A.; Nanbakhsh, A.; Oudrhiri, N.; Chouaib, S.; Azzarone, B.; Durrbach, A.; Lataillade, J.-J. TLR Ligands Stimulation Protects MSC from NK Killing. Stem Cells 2014, 32, 290–300. [Google Scholar] [CrossRef]
- Wang, L.; Zhu, C.-Y.; Ma, D.-X.; Gu, Z.-Y.; Xu, C.-C.; Wang, F.-Y.; Chen, J.-G.; Liu, C.-J.; Guan, L.-X.; Gao, R.; et al. Efficacy and safety of mesenchymal stromal cells for the prophylaxis of chronic graft-versus-host disease after allogeneic hematopoietic stem cell transplantation: A meta-analysis of randomized controlled trials. Ann. Hematol. 2018, 97, 1941–1950. [Google Scholar] [CrossRef]
- Gorskaya, Y.F.; Tukhvatulin, A.I.; Nesterenko, V.G. NNLR2 and TLR3, TLR4, TLR5 ligands, injected in vivo, improve after 1 h the efficiency of cloning and proliferative activity of bone marrow multipotent stromal cells and reduce the content of osteogenic multipotent stromal cells in CBA mice. Microbiol. Immunol. 2017, 163, 356–360. [Google Scholar] [CrossRef]
- Rashedi, I.; Gómez-Aristizábal, A.; Wang, X.-H.; Viswanathan, S.; Keating, A. TLR3 or TLR4 Activation Enhances Mesenchymal Stromal Cell-Mediated Treg Induction via Notch Signaling. Stem Cells 2017, 35, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Petri, R.M.; Hackel, A.; Hahnel, K.; Dumitru, C.A.; Bruderek, K.; Flohe, S.B.; Paschen, A.; Lang, S.; Brandau, S. Activated Tissue-Resident Mesenchymal Stromal Cells Regulate Natural Killer Cell Immune and Tissue-Regenerative Function. Stem Cell Rep. 2017, 9, 985–998. [Google Scholar] [CrossRef] [PubMed]
- Cassano, J.M.; Schnabel, L.V.; Goodale, M.B.; Fortier, L.A. The immunomodulatory function of equine MSCs is enhanced by priming through an inflammatory microenvironment or TLR3 ligand. Vet. Immunol. Immunopathol. 2018, 195, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Cassano, J.M.; Schnabel, L.V.; Goodale, M.B.; Fortier, L.A. Inflammatory licensed equine MSCs are chrondroprotective and exhibit enhanced immunomodulation in an inflammatory environment. Stem Cell Res. Ther. 2018, 9, 82. [Google Scholar] [CrossRef]
- Cortés-Araya, Y.; Amilon, K.; Rink, B.E.; Black, G.; Lisowski, Z.; Donadeu, F.X.; Esteves, C.L. Comparison of Antibacterial and Immunological Properties of Mesenchymal Stem/Stromal Cells from Equine Bone Marrow, Endometrium, and Adipose Tissue. Stem Cells Dev. 2018, 27, 1518–1525. [Google Scholar] [CrossRef]
- Abdi, J.; Rashedi, I.; Keating, A. Concise review: TLR pathway-miRNA interplay in mesenchymal stromal cells: Regulatory roles and therapeutic directions. Cells. 2018, 36, 1655–1662. [Google Scholar] [CrossRef]
- Olsen, A.; Johnson, V.; Webb, T.; Santangelo, K.S.; Dow, S.; Duerr, F.M. Evaluation of Intravenously Delivered Allogeneic Mesenchymal Stem Cells for Treatment of Elbow Osteoarthritis in Dogs: A Pilot Study. Vet. Comp. Orthop. Traumatol. 2019, 32, 173–181. [Google Scholar] [CrossRef]
- Kurte, M.; Vega-Letter, A.M.; Luz-Crawford, P.; Djouad, F.; Noël, D.; Khoury, M.; Carrión, F. Time-dependent LPS exposure commands MSC immunoplasticity through TLR4 activation leading to opposite therapeutic outcome in EAF. Stem Cell Res. Ther. 2020, 11, 416. [Google Scholar] [CrossRef]
- Aqdas, M.; Singh, S.; Amir, M.; Maurya, S.K.; Pahari, S.; Agrewala, J.N. Cumulative Signaling Through NOD-2 and TLR-4 Eliminates the Mycobacterium Tuberculosis Concealed Inside the Mesenchymal Stem Cells. Front. Cell. Infect. Microbiol. 2021, 11, 669168. [Google Scholar] [CrossRef]
- Aziz, S.G.-G.; Alipour, S.; Ranjbarvan, P.; Azari, A.; Babaei, G.; Golchin, A. Critical roles of TLRs on the polarization of mesenchymal stem cells for cell therapy of viral infections: A notice for COVID-19 treatment. Comp. Clin. Pathol. 2021, 30, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Krasnodembskaya, A.; Song, Y.; Fang, X.; Gupta, N.; Serikov, V.; Lee, J.-W.; Matthay, M.A. Antibacterial Effect of Human Mesenchymal Stem Cells Is Mediated in Part from Secretion of the Antimicrobial Peptide LL-37. Stem Cells 2010, 28, 2229–2238. [Google Scholar] [CrossRef] [PubMed]
- Sung, D.K.; Chang, Y.S.; Sung, S.I.; Yoo, H.S.; Ahn, S.Y.; Park, W.S. Antibacterial effect of mesenchymal stem cells against Escherichia coli is mediated by secretion of beta- defensin- 2 via toll- like receptor 4 signalling. Cell. Microbiol. 2016, 18, 424–436. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.-K.; Kim, C.; Seo, C.H.; Park, Y. The therapeutic applications of antimicrobial peptides (AMPs): A patent review. J. Microbiol. 2017, 55, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Fabisiak, A.; Murawska, N.; Fichna, J. LL-37: Cathelicidin-related antimicrobial peptide with pleiotropic activity. Pharmacol. Rep. 2016, 68, 802–808. [Google Scholar] [CrossRef]
- Doss, M.; White, M.R.; Tecle, T.; Hartshorn, K.L. Human defensins and LL-37 in mucosal immunity. J. Leukoc. Biol. 2010, 87, 79–92. [Google Scholar] [CrossRef]
- Chakraborty, K.; Ghosh, S.; Koley, H.; Mukhopadhyay, A.K.; Ramamurthy, T.; Saha, D.R.; Mukhopadhyay, D.; Roychowdhury, S.; Hamabata, T.; Takeda, Y.; et al. Bacterial exotoxins downregulate cathelicidin (hCAP-18/LL-37) and human beta-defensin 1 (HBD-1) expression in the intestinal epithelial cells. Cell Microbiol. 2008, 10, 2520–2537. [Google Scholar] [CrossRef]
- Armitage, A.E.; Eddowes, L.A.; Gileadi, U.; Cole, S.; Spottiswoode, N.; Selvakumar, T.A.; Ho, L.-P.; Townsend, A.R.M.; Drakesmith, H. Hepcidin regulation by innate immune and infectious stimuli. Blood 2011, 118, 4129–4139. [Google Scholar] [CrossRef]
- Michels, K.; Nemeth, E.; Ganz, T.; Mehrad, B. Hepcidin and Host Defense against Infectious Diseases. PLoS Pathog. 2015, 11, e1004998. [Google Scholar] [CrossRef]
- Kaundal, U.; Bagai, U.; Rakha, A. Immunomodulatory plasticity of mesenchymal stem cells: A potential key to successful solid organ transplantation. J. Transl. Med. 2018, 16, 31. [Google Scholar] [CrossRef]
- Harman, R.M.; Yang, S.; He, M.K.; Van de Walle, G.R. Antimicrobial peptides secreted by equine mesenchymal stromal cells inhibit the growth of bacteria commonly found in skin wounds. Stem Cell Res. Ther. 2017, 8, 157. [Google Scholar] [CrossRef] [PubMed]
- Russell, K.A.; Garbin, L.C.; Wong, J.M.; Koch, T.G. Mesenchymal Stromal Cells as Potential Antimicrobial for Veterinary Use—A Comprehensive Review. Front. Microbiol. 2020, 11, 606404. [Google Scholar] [CrossRef] [PubMed]
- Kyurkchiev, D.; Bochev, I.; Ivanova-Todorova, E.; Mourdjeva, M.; Oreshkova, T.; Belemezova, K.; Kyurkchiev, S. Secretion of immunoregulatory cytokines by mesenchymal stem cells. World J. Stem Cells 2014, 6, 552–570. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Rey, E.; Gonzalez, M.A.; Varela, N.; O’Valle, F.; Hernandez-Cortes, P.; Rico, L.; Büscher, D.; Delgado, M. Human adipose-derived mesenchymal stem cells reduce inflammatory and T cell responses and induce regulatory T cells in vitro in rheumatoid arthritis. Ann. Rheum. Dis. 2010, 69, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Davies Lindsay, C.; Heldring, N.; Kadri, N.; Le Blanc, K. Mesenchymal stromal cell secretion of programmed death-1 ligands regulates t cell mediated immunosuppression. Stem Cells 2016, 35, 766–776. [Google Scholar] [CrossRef]
- Kadle, R.L.; Abdou, S.A.; Villarreal-Ponce, A.; Soares, M.A.; Sultan, D.L.; David, J.A.; Massie, J.; Rifkin, W.J.; Rabbani, P.; Ceradini, D.J. Microenvironmental cues enhance mesenchymal stem cell-mediated immunomodulation and regulatory T-cell expansion. PLoS ONE 2018, 13, e0193178. [Google Scholar] [CrossRef]
- Zhou, K.; Guo, S.; Tong, S.; Sun, Q.; Li, F.; Zhang, X.; Qiao, Y.; Liang, G. Immunosuppression of human adipose-derived stem cells on t-cell subsets via the reduction of nf-kappab activation mediated by pd-l1/pd-1 and gal-9/tim-3 pathways. Stem Cells Dev. 2018, 27, 1191–1202. [Google Scholar] [CrossRef]
- Rozenberg, A.; Rezk, A.; Boivin, M.-N.; Darlington, P.J.; Nyirenda, M.; Li, R.; Jalili, F.; Winer, R.; Artsy, E.A.; Uccelli, A.; et al. Human Mesenchymal Stem Cells Impact Th17 and Th1 Responses Through a Prostaglandin E2 and Myeloid-Dependent Mechanism. Stem Cells Transl. Med. 2016, 5, 1506–1514. [Google Scholar] [CrossRef]
- Takeda, K.; Webb, T.L.; Ning, F.; Shiraishi, Y.; Regan, D.P.; Chow, L.; Smith, M.J.; Ashino, S.; Guth, A.M.; Hopkins, S.; et al. Mesenchymal Stem Cells Recruit CCR2+ Monocytes to Suppress Allergic Airway Inflammation. J. Immunol. 2018, 200, 1261–1269. [Google Scholar] [CrossRef]
- Brandau, S.; Jakob, M.; Bruderek, K.; Bootz, F.; Giebel, B.; Radtke, S.; Mauel, K.; Jäger, M.; Flohe, S.; Lang, S. Mesenchymal Stem Cells Augment the Anti-Bacterial Activity of Neutrophil Granulocytes. PLoS ONE 2014, 9, e106903. [Google Scholar] [CrossRef]
- de Witte, S.F.H.; Luk, F.; Parraga, J.M.S.; Gargesha, M.; Merino, A.; Korevaar, S.S.; Shankar, A.S.; O'Flynn, L.; Elliman, S.J.; Roy, D.; et al. Immunomodulation by therapeutic mesenchymal stromal cells (MSC) is triggered through phagocytosis of MSC by monocytic cells. Stem Cells 2018, 36, 602–615. [Google Scholar] [CrossRef] [PubMed]
- Salami, F.; Tavassoli, A.; Mehrzad, J.; Parham, A. Immunomodulatory effects of mesenchymal stem cells on leukocytes with emphasis on neutrophils. Immunobiology 2018, 223, 786–791. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-N.; Lee, H.-J.; Jeon, M.-S.; Yi, T.; Song, S.U. Galectin-9 is involved in immunosuppression mediated by human bone marrow-derived clonal mesenchymal stem cells. Immune Netw. 2015, 15, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Yagi, H.; Soto-Gutierrez, A.; Parekkadan, B.; Kitagawa, Y.; Tompkins, R.G.; Kobayashi, N.; Yarmush, M.L. Mesenchymal Stem Cells: Mechanisms of Immunomodulation and Homing. Cell Transplant. 2010, 19, 667–679. [Google Scholar] [CrossRef]
- Chow, L.; Johnson, V.; Impastato, R.; Coy, J.; Strumpf, A.; Dow, S. Antibacterial activity of human mesenchymal stem cells mediated directly by constitutively secreted factors and indirectly by activation of innate immune effector cells. Stem Cells Transl. Med. 2020, 9, 235–249. [Google Scholar] [CrossRef]
- Bjarnsholt, T. The role of bacterial biofilms in chronic infections. APMIS 2013, 121, 1–58. [Google Scholar] [CrossRef]
- Bjarnsholt, T.; Ciofu, O.; Molin, S.; Givskov, M.; Høiby, N. Applying insights from biofilm biology to drug development—Can a new approach be developed? Nat. Rev. Drug Discov. 2013, 12, 791–808. [Google Scholar] [CrossRef]
- Walton, K.D.; Lord, A.; Kendall, L.V.; Dow, S.W. Comparison of 3 real-time, quantitative murine models of staphylococcal biofilm infection by using in vivo bioluminescent imaging. Comp. Med. 2014, 64, 25–33. [Google Scholar]
- Shirjang, S.; Mansoori, B.; Solali, S.; Hagh, M.F.; Shamsasenjan, K. Toll-like receptors as a key regulator of mesenchymal stem cell function: An up-to-date review. Cell. Immunol. 2017, 315, 1–10. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, Z.; Zhou, H.; Zhou, G. Characterization of mesenchymal stem cells under the stimulation of Toll-like receptor agonists. Dev. Growth Differ. 2014, 56, 233–244. [Google Scholar] [CrossRef]
- Saeedi, P.; Halabian, R.; Fooladi, A.A.I. Antimicrobial effects of mesenchymal stem cells primed by modified LPS on bacterial clearance in sepsis. J. Cell. Physiol. 2018, 234, 4970–4986. [Google Scholar] [CrossRef] [PubMed]
- Waterman, R.S.; Tomchuck, S.L.; Henkle, S.L.; Betancourt, A.M. A New Mesenchymal Stem Cell (MSC) Paradigm: Polarization into a Pro-Inflammatory MSC1 or an Immunosuppressive MSC2 Phenotype. PLoS ONE 2010, 5, e10088. [Google Scholar] [CrossRef] [PubMed]
- Ward, C.L.; Sanchez, C.J., Jr.; Pollot, B.E.; Romano, D.R.; Hardy, S.K.; Becerra, S.C.; Rathbone, C.R.; Wenke, J.C. Soluble factors from biofilms of wound pathogens modulate human bone marrow-derived stromal cell differentiation, migration, angiogenesis, and cytokine secretion. BMC Microbiol. 2015, 15, 75. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.-C.; Wang, L.-T.; Yao, C.-L.; Lai, H.-Y.; Chan, K.-Y.; Liu, B.-S.; Chong, P.; Lee, O.K.-S.; Chen, H.-W. Mesenchymal stem cells promote neutrophil activation by inducing IL-17 production in CD4+ CD45RO+ T cells. Immunobiology 2013, 218, 90–95. [Google Scholar] [CrossRef]
- Pezzanite, L.; Chow, L.; Griffenhagen, G.; Dow, S.; Goodrich, L. Impact of Three Different Serum Sources on Functional Properties of Equine Mesenchymal Stromal Cells. Front. Vet. Sci. 2021, 8, 634064. [Google Scholar] [CrossRef]
- Deng, J.; Li, D.; Huang, X.; Li, W.; Zhao, F.; Gu, C.; Shen, L.; Cao, S.; Ren, Z.; Zuo, Z.; et al. Interferon-y enhances the immunosuppressive ability of canine bone marrow-derived mesenchymal stem cells by activating the TLR3-dependent IDO/kynurenine pathway. Mol. Biol. Rep. 2022, 49, 8337–8347. [Google Scholar] [CrossRef] [PubMed]
- Ryu, D.-B.; Lim, J.-Y.; Lee, S.-E.; Park, G.; Min, C.-K. Induction of Indoleamine 2,3-dioxygenase by Pre-treatment with Poly(I:C) May Enhance the Efficacy of MSC Treatment in DSS-induced Colitis. Immune Netw. 2016, 16, 358–365. [Google Scholar] [CrossRef] [PubMed]
- Hirakawa, M.P.; Tjahjono, N.; Light, Y.K.; Celebi, A.N.; Celebi, N.N.; Chintalapudi, P.; Butler, K.S.; Branda, S.S.; Krishnakumar, R. Upregulation of CD14 in mesenchymal stromal cells accelerates lipopolysaccharide-induced response and enhances antibacterial properties. iScience 2022, 25, 103759. [Google Scholar] [CrossRef] [PubMed]
- Schallmoser, K.; Rohde, E.; Reinisch, A.; Bartmann, C.; Thaler, D.; Drexler, C.; Obenauf, A.; Lanzer, G.; Linkesch, W.; Strunk, D. Rapid Large-Scale Expansion of Functional Mesenchymal Stem Cells from Unmanipulated Bone Marrow Without Animal Serum. Tissue Eng. Part C Methods 2008, 14, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Pedrazza, L.; Cunha, A.A.; Luft, C.; Nunes, N.K.; Schimitz, F.; Gassen, R.B.; Breda, R.V.; Donadio, M.V.F.; de Souza Wyse, A.T.; Pitrez, P.M.C.; et al. Mesenchymal stem cells improves survival in LPS-induced acute lung injury acting through inhibition of NETs formation. J. Cell. Physiol. 2017, 232, 3552–3564. [Google Scholar] [CrossRef] [PubMed]
- Berglund, A.K.; Long, J.M.; Robertson, J.B.; Schnabel, L.V. TGF-ß2 reduces the cell-mediated immunogenicity of equine MHC-mismatched bone marrow-derived mesenchymal stem cells without altering immunomodulatory properties. Front. Cell Dev. Biol. 2021, 9, 628382. [Google Scholar] [CrossRef] [PubMed]
- Rowland, A.L.; Miller, D.; Berglund, A.; Schnabel, L.V.; Levine, G.J.; Antczak, D.F.; Watts, A.E. Cross-matching of allogeneic mesenchymal stromal cells eliminates recipient immune targeting. Stem Cells Transl. Med. 2021, 10, 694–710. [Google Scholar] [CrossRef] [PubMed]
- Arifka, M.; Wilar, G.; Elamin, K.M.; Wathoni, N. Polymeric Hydrogels as Mesenchymal Stem Cell Secretome Delivery System in Biomedical Applications. Polymers 2022, 14, 1218. [Google Scholar] [CrossRef] [PubMed]
- Kudinov, V.A.; Artyushev, R.I.; Zurina, I.M.; Lapshin, R.D.; Snopova, L.B.; Mukhina, I.V.; Grinakovskaya, O.S.; Saburina, I.N. Anntimicrobial and regenerative effects of placental multipotent mesenchymal stromal cell secretome-based chitosan gel on infected burns in rats. Pharmceuticals 2021, 14, 1263. [Google Scholar] [CrossRef] [PubMed]
- Fisher, S.A.; Cutler, A.; Doree, C.; Brunskill, S.J.; Stanworth, S.J.; Navarrete, C.; Girdlestone, J. Mesenchymal stromal cells as treatment or prophylaxis for acute or chronic graft-versus-host disease in haematopoietic stem cell transplant (HSCT) recipients with a haematological condition. Cochrane Database Syst. Rev. 2019, 2019, CD009768. [Google Scholar] [CrossRef]
- Frisbie, D.D.; Cross, M.W.; McIlwraith, C.W. A comparative study of articular cartilage thickness in the stifle of animal species used in human pre-clinical studies compared to articular cartilage thickness in the human knee. Vet. Comp. Orthop. Traumatol. 2006, 19, 142–146. [Google Scholar] [CrossRef]
- McIlwraith, C.W.; Fortier, L.A.; Frisbie, D.D.; Nixon, A.J. Equine Models of Articular Cartilage Repair. Cartilage 2011, 2, 317–326. [Google Scholar] [CrossRef]
- McIlwraith, C.W.; Frisbie, D.D.; Kawcak, C.E. The horse as a model of naturally occurring osteoarthritis. Bone Jt. Res. 2012, 1, 297–309. [Google Scholar] [CrossRef]
- Chu, C.R.; Szczodry, M.; Bruno, S. Animal Models for Cartilage Regeneration and Repair. Tissue Eng. Part B Rev. 2010, 16, 105–115. [Google Scholar] [CrossRef]
- Reesink, H.; Watts, A.; Mohammed, H.; Jay, G.; Nixon, A. Lubricin/proteoglycan 4 increases in both experimental and naturally occurring equine osteoarthritis. Osteoarthr. Cartil. 2017, 25, 128–137. [Google Scholar] [CrossRef]
- Frisbie, D.D.; Kawcak, C.E.; Trotter, G.W.; Powers, B.E.; Walton, R.M.; McIlwraith, C.W. Effects of triamcinolone acetonide on an in vivo equine osteochondral fragment exercise model. Equine Vet. J. 1997, 29, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Jin, S.; Ding, C.; Wang, Y.; He, D.; Liu, Y. Mesenchymal Stem Cell-Derived Exosome Therapy of Microbial Diseases: From Bench to Bed. Front. Microbiol. 2022, 12, 804813. [Google Scholar] [CrossRef] [PubMed]
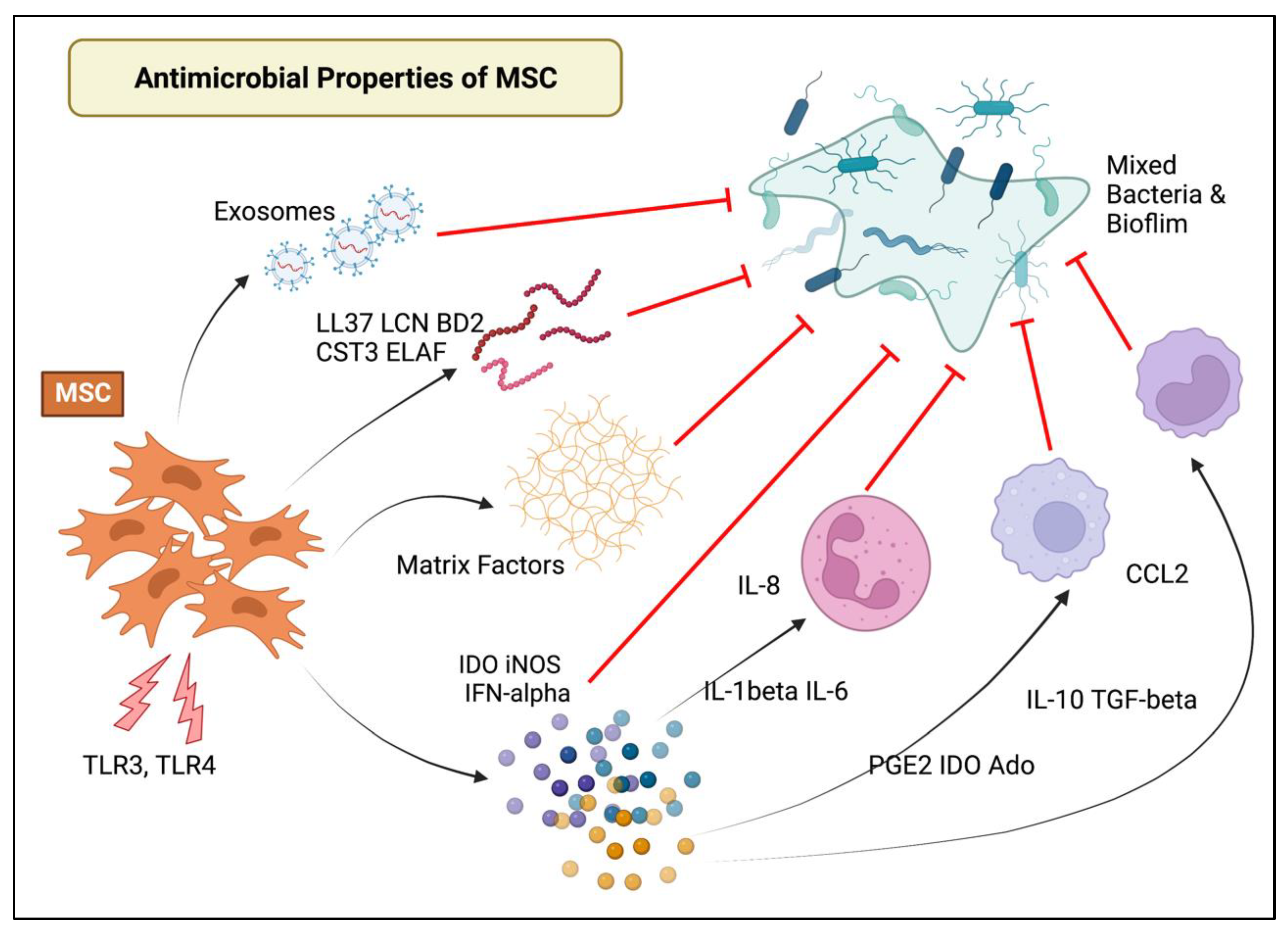
| Investigator | Reference | Species | Culture Conditions or Lesion | Cell Source | Cell Dose | Protocol | Route of Administration | Outcome Parameters | Main Findings |
|---|---|---|---|---|---|---|---|---|---|
| Yuan et al. (2014) | [14] | Rat | Subcutaneous infection MRSA | Bone marrow | 2 × 107, 2 × 106, | Dosed daily for 4 doses | Intravenous | Quantitative cultures | MSC reduced bacterial colonies. |
| or 2 × 105 cells/rat | Immunoassays cytokines | MSC reduced cytokine expression (IL1, IL6, IL10, CCL5). | |||||||
| Criman et al. (2016) | [15] | Rat | Subcutaneous E.coli | Bone marrow | 7.5 × 105 MSC/mesh | MSC seeded meshes | Seeded in meshes | Microbiologic mesh evaluation | Augmentation of bioprosthetic materials with MSC enhanced |
| inoculated meshes | vs non-seeded meshes | Histologic mesh evaluation | resistance to bacterial infection. | ||||||
| Johnson et al. (2017) | [16] | Murine | Staphylococcus aureus | Adipose | 1 × 106 cells/injection | TLR-3 poly I:C activated or not | Intravenous | IVIS luminescence imaging | Activated MSC co-administered with antibiotics was most |
| implant infection model | with or without antibiotics | to determine bacterial burden | effective to reduce bacterial bioburden. | ||||||
| Dosed every 3 days, 3 doses | Wound tissue histology | ||||||||
| Canine | Naturally occurring wounds | Adipose | 2 × 106 cells/kg | TLR-3 poly I:C activated + antibiotics | Intravenous | Quantitative cultures | Repeated MSC injection resulted in clearance of bacteria | ||
| Dosed every 2 weeks, 3 doses | Clinical signs | and wound healing. | |||||||
| Phone follow-up | |||||||||
| Asami et al. (2018) | [17] | Murine | Streptococcus pneumoniae | Bone marrow | 1 × 106 cells/injection | Once1 hour after bacterial inoculation | Intravenous | Bacteria bronchoalveolar lavage | MSC-CM modulates TNFα, IL-6, IL-10 after |
| pulmonary infection | Myeloperoxidase activity assay | stimulation with TLR2, TLR4, TLR9 ligands. | |||||||
| Bichinchoninic acid protein assay | MSC-CM suppresses CXCL1, CXCL2 production | ||||||||
| Histopathologic examination | after stimulation with TLR2 and TLR9 ligands. | ||||||||
| MSC IV decreased total cells, neutrophils, and | |||||||||
| myeloperoxidase activity during pulmonary infection. | |||||||||
| MSC IV decreased BALF cytokine levels TNFα, IL-6, | |||||||||
| IFN-γ, CCL2, GM-CSF during pulmonary infection. | |||||||||
| Wood et al. (2018) | [18] | Human | In vitro Staphylococcus aureus, | Adipose | N/A | In vitro | Scanning electron microscopy | MSC inhibited P. aeruginosa biofilm formation | |
| Pseudomonas co-culture | Colony forming units | due to bacterial adhesion, engulfment/phagocytosis | |||||||
| Biofilm assay | and secretion of antibacterial factors. | ||||||||
| Chow et al. (2019) | [19] | Human | Staphylococcus aureus | ||||||
| In vitro biofilm assay | Bone marrow | N/A | TLR and Nod-like receptor agonists | In vitro | Live/dead biofilms confocal microscopy | MSC secreted factors disrupted MRSA biofilm formation. | |||
| Mouse mesh implant model | 1 × 106 cells/injection | TLR-3 poly I:C activated with antibiotics | Intravenous | bacterial density via IVIS live imaging | Activated MSC treatment decreases bacterial bioburden | ||||
| dosed every 3 days for 4 doses | in mouse chronic biofilm infection model. | ||||||||
| Bujnakova et al. (2020) | [20] | Canine | In vitro biofilm | Bone marrow | N/A | In vitro coculture S. aureus, E.coli biofilms | In vitro | Disc diffusion test | MSC-CM inhibited biofilm formation and quorum sensing. |
| Staphylococcus aureus | Spectrophotometric crystal violet assay | ||||||||
| Escherichia coli | Bioluminescence assay | ||||||||
| Bahroudi et al. (2020) | [21] | Human | In vitro Vibrio cholerae | Bone marrow | N/A | MSC secretome coculture | In vitro | Plate crystal violet assay | MSC secretome prevented biofilm formation |
| co-culture with MSC secretome | V. cholerae 1:8 to 1:128 | of Vibrio cholerae in a dose-dependent manner. | |||||||
| Marx et al. (2020) | [22] | Equine | In vitro Pseudomonas, | Peripheral blood | N/A | In vitro co-culture with Pseudomonas | In vitro | Protease array | MSC secretome inhibits biofilm formation and mature |
| Staphylococcus biofilms | and Staphylococcus biofilms | Confocal microscopy biofilm composition | biofilms of Pseudomonas and Staphylococcus spp. | ||||||
| Western blot analysis | MSC secrete cysteine proteases that destabilize MRSA | ||||||||
| biofilms increasing efficacy of antibiotics. | |||||||||
| Marx et al. (2021) | [23] | Equine | Ex vivo equine skin | Peripheral blood | N/A | In vitro co-culture MSC-CM | In vitro explant | Immunofluorescence activity | MSC decreased MRSA viability in mature biofilms. |
| biofilm explant model | with MRSA and MSSA | Biofilm live/dead staining | Equine MSCs secrete CCL2 that increased antimicrobial | ||||||
| peptide secretion by equine keratinocytes. | |||||||||
| Pezzanite et al. (2021) | [24] | Equine | In vitro MRSA biofilm assays | Bone marrow | N/A | TLR-3, TLR-4 NOD activated MSC | In vitro biofilms | Bactericidal activity | MSC stimulation TLR3 poly I:C suppressed biofilm formation |
| Neutrophil bacterial phagocytosis | enhanced neutrophil phagocytosis | ||||||||
| Cytokine analysis | increased MCP-1 secretion, | ||||||||
| Antimicrobial peptide secretion | enhanced antimicrobial peptide production. | ||||||||
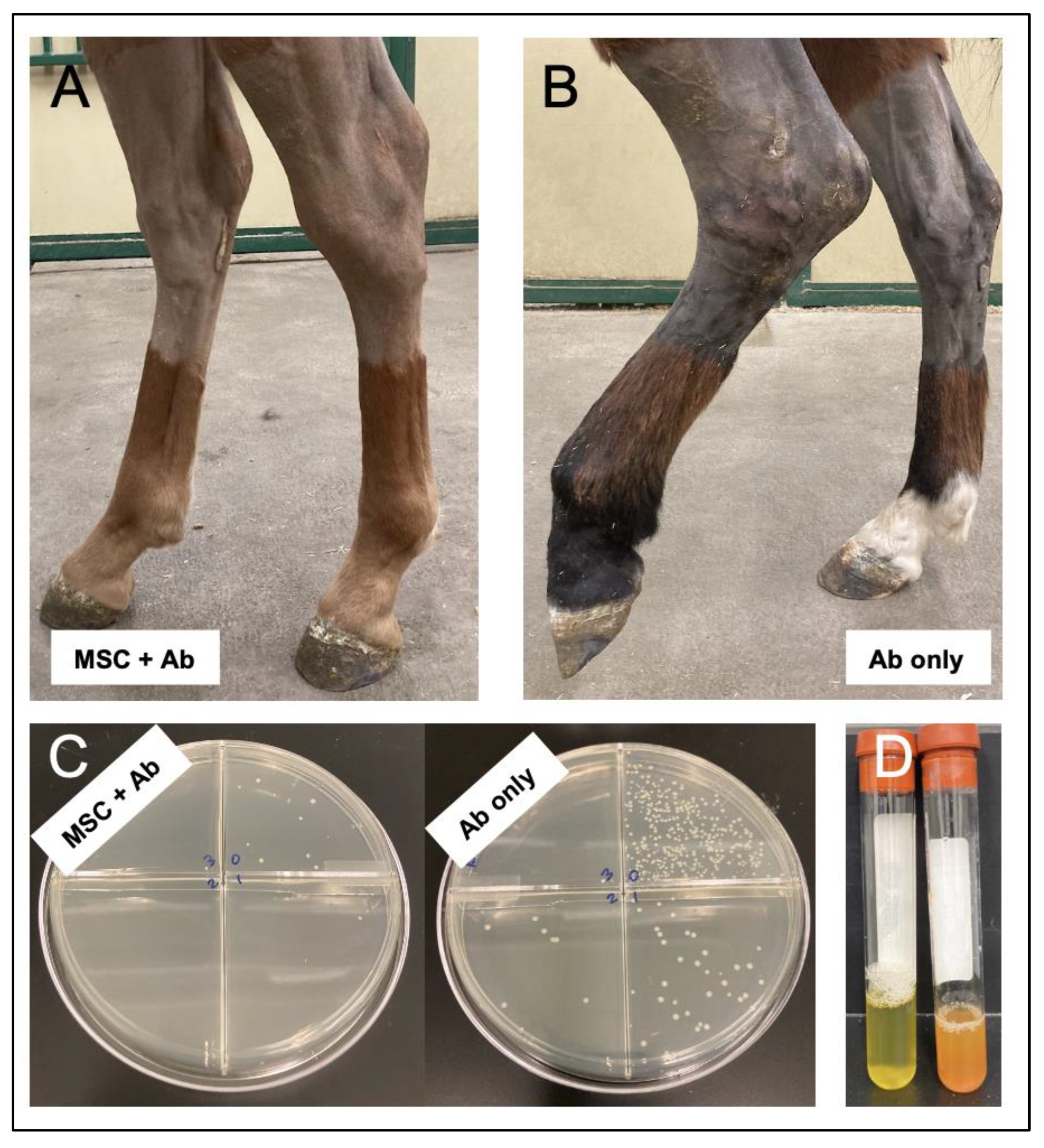
| Pezzanite et al. (2022) | [25] | Equine | In vivo MRSA septic arthritis | Bone marrow | 20 × 106 cells/joint | TLR-3 poly I:C activated MSC | Intra-articular | Clinical pain scoring | Activated MSC therapy resulted in improved pain scores, |
| Quantitative bacterial cultures | ultrasound and MRI scoring, quantittative bacterial counts, | ||||||||
| Complete blood counts | systemic neutrophil and serum amyloid A, | ||||||||
| Dosed every 3 days for 3 doses | Cytokines synovial fluid, plasma | synovial fluid lactate and serum amyloid A | |||||||
| Imaging (radiographs, ultrasound, MRI) | synovial fluid IL-6 and IL-18. | ||||||||
| Macroscopic joint scoring | |||||||||
| Histologic changes | |||||||||
| Johnson et al. (2022) | [26] | Canine | Naturally occurring chronic | Adipose | 2 × 106 cells/kg | TLR-3 poly I:C activated with antibiotics | Intravenous | Quantitative cultures | Repeated delivery of activated allogeneic MSC resulted |
| multidrug resistant infections | Dosed every 2 weeks for 3 doses | Clinical signs | in infection clearance and wound healing. | ||||||
| Phone follow-up | |||||||||
| Yang et al. (2022) | [27] | Human | Pseudomonas aeruginosa | Umbilical cord | N/A | In vitro co-culture, 8 MSC concentrations | In vitro biofilms | Titration MSC concentration | Antibacterial peptides from MSC affected biofim formation |
| inoculated tracheal tubes | Anti-biofilm experiment | by downregulating polysaccharide biosynthesis | |||||||
| Bacterial motility assay | protein which correlated to MSC concentration. | ||||||||
| DNA microarray experiment |
| Investigator | Reference | Species | Culture Conditions or Lesion | Cell Source | Cell Dose | Protocol | Route | Outcome Parameters | Main Findings |
|---|---|---|---|---|---|---|---|---|---|
| Liotta et al. (2008) | [28] | Human | In vitro TLR activation | Bone marrow | N/A | TLR-3 poly I:C or TLR-4 LPS activation | In vitro | Flow cytometric evaluation | BM-MSCs expressed high levels TLR3 and 4 which induce nuclear factor k- activity, IL6, IL8, CXCL10 |
| T-cell co-culture | MSC differentiation assays | Ligation TLR3 and TLR4 on MSCs inhibited ability of MSC to suppress T-cell proliferation without | |||||||
| T-cell proliferation assays | influencing immunophenotype or differentiation potential | ||||||||
| ELISA cytokines/chemokines analysis | TLR-triggering was related to impaired Notch receptor signaling in T cells | ||||||||
| IDO activity measures | TLR3 and TLR4 expression on MSCs provide effective mechanisms to block immunosuppressive activities | ||||||||
| Confocal microscopy | and restore efficient T-cell response to infection such as viruses or Gram-negative bacteria | ||||||||
| Quantitative analysis NFK- translocation | |||||||||
| RNA extraction and rtPCR | |||||||||
| Opitz et al. (2009) | [29] | Human | In vitro co-culture | Bone marrow | N/A | MSC T-cells in mixed leukocyte reactions | In vitro | Karyotype analysis of MSC | TLR ligation activates innate and adaptive immune response pathways to protect against pathogens |
| MSC with T-cells | TLR-3 poly I:C or TLR-4 LPS activation | Flow cytometric analysis MSC | TLR expressed on human bm-MSC enhanced immunosuppressive phenotype of MSC | ||||||
| Mixed leukocyte reactions | Immnunosuppression mediated by TLR was dependent on production of IDO1 | ||||||||
| Quantitative rt-PCR | Induction of IDO1 by TLR involved autocrine interferon signaling loop which depended on protein kinase R | ||||||||
| Liquid chromatography | |||||||||
| Western blot analysis, siRNA | |||||||||
| ELISA cell culture supernatants | |||||||||
| Romieu-mourez et al. (2009) | [30] | Human | In vitro activation | Bone marrow | N/A | TLR-3 poly I:C or TLR-4 LPS activation | In vitro | Flow cytometric analysis | Human MSC and macrophages expressed TLR3 and TLR4 at comparable levels |
| cytokines, TLR agonists | real-time RT-PCR | TLR-mediated activation of MSC resulted in production inflammatory mediators IL-1, IL-6, IL-8/CXCL8, CCL5 | |||||||
| Immunoblot analysis | IFN priming combined with TLR activation increases immune responses induced by Ag-presenting MSC | ||||||||
| Growth response to TNF-α, IFN-α, IFN-γ | TLR activation resulted in inflammatory site attracting innate immune cells | ||||||||
| Immune effector infiltration analysis | |||||||||
| Neutrophil chemotaxis assay | |||||||||
| Cassatella et al. (2011) | [32] | Human | In vitro activated | Bone marrow | N/A | TLR-3 poly I:C or TLR-4 LPS activation | In vitro | Cytofluorometric analysis | TLR-3 MSC activation enhanced anti-apoptosis of neutrophils more than TLR-4 |
| MSC neutrophil coculture | ELISA immunoassays | TLR-3 and TLR-4 activation enhanced respiratory burst ability and CD11b expression by PMN | |||||||
| Respiratory burst cytochrome C reduction | TLR-3 activation effects mediated by IL-6, IFN- and GM-CSF | ||||||||
| TLR-4 activation effects mediated by GM-CSF | |||||||||
| Lei et al. (2011) | [33] | Murine | In vitro TLR activation | Bone marrow | N/A | TLR-2 or TLR-4 activation | In vitro | MSC migration | TLR2 ligation (but not TLR4) inhibited MSC migration, MSC mediated immunosuppression on allo-MLR, |
| Allogeneic mixed lymphocyte reaction | and reduced MSC mediated expansion of Treg cells | ||||||||
| Induction Treg cell | TLR2 activation induced lower CXCL10 mRNA and protein expressions | ||||||||
| TLR2 and TLR4 had different effects on immunomodulatory capacity of MSC | |||||||||
| Giuliani et al. (2014) | [34] | Human | In vitro MSC NK cell coculture | Bone marrow | N/A | TLR-3 or TLR-4 activation | In vitro | Flow cytometry CD107 degranulation | TLR primed MSC are more resistant than unprimed MSC to IL-2 activated NK-induced killing |
| Embryonic | NK cell MSC coculture | ELISA culture supernatants | TLR-primed MSC modulated naturall killer group 2D ligands MHC class I chain A, ULBP3, DNAM-1 ligands | ||||||
| Chromium release assay | MSC adapt their immunobehavior in inflammatory context, decreasing susceptibility to NK killing | ||||||||
| TLR3 but not TL4 primed MSC enhance suppressive functionns against NK cells | |||||||||
| Johnson et al. (2017) | [16] | Murine | Staphylococcus aureus | Adipose | 1 × 106 cells/ | TLR-3 poly I:C activation +/- antibiotics | Intravenous | Bacterial burden IVIS imaging | Activated MSC co-administered with antibiotics was most effective to reduce bacterial bioburden |
| implant infection model | /injection | dosed every 3 days for 3 doses | Wound tissue histology | ||||||
| Canine | Naturally occurring wounds | Adipose | 2 × 106 cells/kg | TLR-3 poly I:C activated with antibiotics | Intravenous | Quantitative cultures | Clearance of bacteria and wound healing following repeated IV injection | ||
| dosed every 2 weeks for 3 doses | Clinical signs, Phone follow-up | ||||||||
| Gorskaya et al. (2017) | [36] | Murine | Intraperitoneal injection | Bone marrow | NLR/TLR ligands | NLR2 and TLR (LPS, flagellin, CpG, poly I:C) | Intraperitoneal | Efficiency bone marrow MSC colony formation | NLR, TLR and S. typhimurium antigenic complex increase efficiency of MSC cloning and content by 1 hr |
| NLR, TLR, S. typhimurium | 10 µg/mouse | and S. typhimurium antigenic complex | |||||||
| Rashedi et al. (2017) | [37] | Human | In vitro activation TLR ligands | Bone marrow | N/A | TLR-3, TLR-4 effect on MSC Treg induction | In vitro | MSC, CD4+ lymphocyte co-culture assays | TLR3/4 activation MSC enhanced Treg generation in CD4+ lymphocyte/MSC cultures |
| Gene and protein expression analysis | TLR3/4 activation augmented Treg induction via Notch pathway | ||||||||
| Flow cytometric analysis | |||||||||
| Quantification cytokines culture medium | |||||||||
| Petri et al. (2017) | [38] | Human | In vitro coculture TLR-3 | Nasal mucosa | N/A | TLR-3 activated MSC effect on NK cells | In vitro | ELISA immunoassays | Early time points TLR3-activated MSC secrete type I interferon to enhance NK cell effector function |
| TLR-3 activated | Flow cytometric analysis | Later time points NK cell function limited by TGF- and IL-6 | |||||||
| MSCs and NK cells | Surface/intracellular staining | Feedback regulatory NK cells to MSCs promote survival, proliferation, pro-angiogenic properties | |||||||
| Cytotoxicity assays | |||||||||
| Degranulation assays | |||||||||
| NK cell proliferation assays | |||||||||
| MSC invasion and proliferation assays | |||||||||
| Cassano et al. (2018) | [39] | Equine | In vitro co-culture TLR ligands | Bone marrow | N/A | TLR-3 or TLR-4 activation | In vitro | T-cell proliferation via flow cytometry | TLR3/4 priming increased MSC expression IL6, CCL2, CXCL10 |
| MSC co-culture inflammatory macrophages | Macrophage RNA gene expression | TLR3/4 priming or exposure to inflammatory macrophages enhanced immunomodulatory function | |||||||
| Suppression T-cell proliiferation assay | demonstrated by decreased T-cell proliferation | ||||||||
| Cortes-Araya et al. (2018) | [41] | Equine | In vitro comparison MSC tissue sources | Endometrium | N/A | TLR-4 primed MSC versus unprimed | In vitro | Antimicrobial peptide immunocytochemistry | Lipocalin-2 was expressed at higher levels in EM-MSC than AD or BMD |
| In vitro activation with TLR4 ligand | Adipose | Cytokine secretion via ELISA | TLR-4 stimulated lipocalin-2 production by all three cell types | ||||||
| Bone marrow | Gene expression analyses | TLR-4 induced expression IL-6, IL-8, MCP-1, chemokine ligand-5, TLR4 by all three cell types | |||||||
| Asami et al. (2018) | [17] | Murine | In vitro activation with TLR ligands | Bone marrow | 1 × 106 cells | 1 injection 1 hour after bacterial inoculation | Intravenous | Bacteria bronchoalveolar lavage | MSC-CM modulates TNFα, IL-6, IL-10 after |
| Streptococcus pneumoniae | /injection | Myeloperoxidase activity assay | stimulation with TLR2, TLR4, TLR9 ligands. | ||||||
| pulmonary infection | Bichinchoninic acid protein assay | MSC-CM suppresses CXCL1, CXCL2 production | |||||||
| Histopathologic examination | after stimulation with TLR2 and TLR9 ligands. | ||||||||
| MSC IV decreased total cells, neutrophils, and | |||||||||
| myeloperoxidase activity during pulmonary infection. | |||||||||
| MSC IV decreased BALF cytokine levels TNFα, IL-6, | |||||||||
| IFN-γ, CCL2, GM-CSF during pulmonary infection. | |||||||||
| Chow et al. (2019) | [19] | Human | In vitro Staphylococcus aureus biofilm assay | Bone marrow | N/A | Comparison TLR, NLR receptor agonists | In vitro | Live/dead biofilms via confocal microscopy | MSC secreted factors disrupted MRSA biofilm formation |
| Mice with mesh implant biofilm animal model | 1 × 106 cells | TLR-3 poly I:C activated with antibiotics | Intravenous | bacterial density by IVIS live imaging | Activated MSC treatment decreases bacterial bioburden in mouse chronic biofilm infection model | ||||
| /injection | Dosed every 3 days for 4 doses | ||||||||
| Kurte et al. (2020) | [44] | Murine | In vitro splenocyte and MSC and Tcell | Bone marrow | N/A | In vitro | Quantitative real-time PCR | Time dependent LPS activation regulate IL6 and iNOS expression in MSCs. | |
| and MSC co-cultures | Subcutaneous | Flow cytometry | Immunosuppressive activity of MSCs on T cell proliferation depends on time dependent LPS activation. | ||||||
| Murine autoimmune encephalomyelitis (EAE) | Immunosuppression assay | Long exposure to LPS enhances MSC therapeutic potential in EAE. | |||||||
| Treg, Th17, Th1 differentiation assay | TLR4 expression involved in immunosuppressive capacity of MSCs in vitro. | ||||||||
| Thelper analysis in treated mouse lymph nodes | TLR4 inhibition disrupts capacity of MSCs to inhibit Th1 and Th17 cells in vitro. | ||||||||
| TLR4 deficiency reduces therapeutic effect of MSCs in EAE. | |||||||||
| Aqdas et al. (2021) | [45] | Murine | In vitro co-culture MSC with | Bone marrow | N/A | TLR-4 or NOD-2 activated MSC | In vitro | Cytokine secretion ELISA (IL-6, IL-10, IL-12, TNF-α) | TLR4/NOD-2 augmented pro-inflammatory cytokine secretion. |
| Mycobacterium tuberculosis (Mtb) | RT-qPCR (IL-6, IL-12, IL-10, iNOS, TNF-α, TGF-) | TLR4/NOD-2 co-localized Mtb in lysosomes. | |||||||
| Phenotypic charactization of MSC markers | TLR4-NOD-2 induced autophagy. | ||||||||
| Evaluation MSC differentiation | TLR4-NOD-2 enhanced NF-κ activity via p38 MAPK. | ||||||||
| Bacterial load determination post-infection | TLR4-NOD-2 reduced intracellular Mtb survival. | ||||||||
| Bacterial tracking into autolysosomes | Triggering TLR4-NOD-2 pathway may be future immunotherapy. | ||||||||
| Pezzanite et al. (2021) | [24] | Equine | In vitro MRSA biofilm assays | Bone marrow | N/A | TLR-3, TLR-4 and NOD activated MSC | In vitro | Bactericidal activity | MSC stimulation with TLR3 poly I:C suppressed biofilm formation, enhanced neutrophil phagocytosis, |
| Neutrophil bacterial phagocytosis | increased MCP-1 secretion and enhanced antimicrobial peptide cathetlicidin production | ||||||||
| Cytokine analysis | |||||||||
| Antimicrobial peptide secretion | |||||||||
| Johnson et al. (2022) | [26] | Canine | Naturally occurring chronic | Adipose | 2 × 106 cells/kg | TLR-3 poly I:C activated with antibiotics | Intravenous | Quantitative cultures | Repeated delivery of activated allogeneic MSC resulted in infection clearance and wound healing |
| multidrug resistant infections | dosed every 2 weeks for 3 doses | Clinical signs, Phone follow-up | |||||||
| Pezzanite et al. (2022) | [25] | Equine | MRSA inoculated septic arthritis | Bone marrow | 20 × 106 cells/joint | TLR-3 poly I:C activated MSC | Intra-articular | Clinical pain scoring | Activated MSC therapy resulted in improved pain scores, ultrasound and MRI scoring, quantitative |
| dosed every 3 days for 3 doses | Quantitative bacterial cultures | bacterial counts, systemic neutrophil and serum amyloid A, and synovial fluid lactate, serum | |||||||
| Complete blood counts | |||||||||
| Cytokine analyses (blood, synovial fluid) | |||||||||
| Imaging (radiographs, ultrasound, MRI) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pezzanite, L.M.; Chow, L.; Strumpf, A.; Johnson, V.; Dow, S.W. Immune Activated Cellular Therapy for Drug Resistant Infections: Rationale, Mechanisms, and Implications for Veterinary Medicine. Vet. Sci. 2022, 9, 610. https://doi.org/10.3390/vetsci9110610
Pezzanite LM, Chow L, Strumpf A, Johnson V, Dow SW. Immune Activated Cellular Therapy for Drug Resistant Infections: Rationale, Mechanisms, and Implications for Veterinary Medicine. Veterinary Sciences. 2022; 9(11):610. https://doi.org/10.3390/vetsci9110610
Chicago/Turabian StylePezzanite, Lynn M., Lyndah Chow, Alyssa Strumpf, Valerie Johnson, and Steven W. Dow. 2022. "Immune Activated Cellular Therapy for Drug Resistant Infections: Rationale, Mechanisms, and Implications for Veterinary Medicine" Veterinary Sciences 9, no. 11: 610. https://doi.org/10.3390/vetsci9110610
APA StylePezzanite, L. M., Chow, L., Strumpf, A., Johnson, V., & Dow, S. W. (2022). Immune Activated Cellular Therapy for Drug Resistant Infections: Rationale, Mechanisms, and Implications for Veterinary Medicine. Veterinary Sciences, 9(11), 610. https://doi.org/10.3390/vetsci9110610






