Equine Anthelmintic Resistance: Horse Owner and Yard Manager Perception of the Barriers Affecting Strategic Control Measures in England
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Data Collection
2.3. Data Analysis
2.4. Thematic Analysis
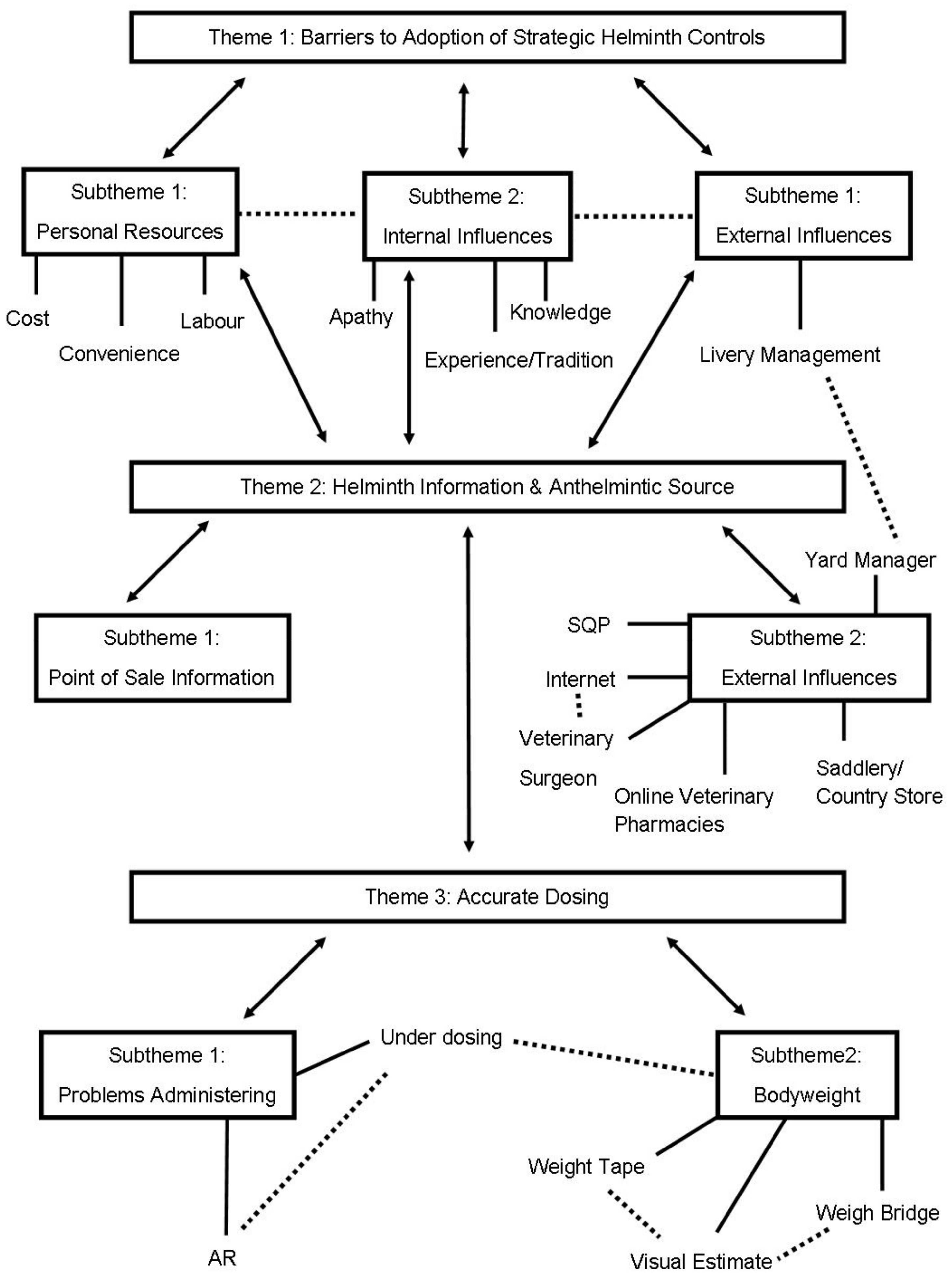
3. Results
3.1. Theme 1: Barriers to Adoption of Strategic Controls
3.1.1. Subtheme 1: Personal Resources
It would be so expensive to strategically worm properly. It’s going to waste more of my money.[HO1]
I guess the problem is the cost of the worm count is similar to that of the cost of a wormer and if you do a worm count and you still have to worm then you’re doubling the cost.[YM2]
Probably not cost if I really believed in it or if I was really concerned about worm resistance.[HO2]
I suppose I thought it might be a similar cost, so it’s easier to just stick a syringe in their mouth I guess.[HO3]
Manageability, the fact that I’d have to go to multiple different people to get the right things, knowing when and what to test for, I can’t get that information easily from one place that is reliable.[HO1]
The fact that, although I can’t pull the information to mind immediately, the fact that I know it’s not a comprehensive solution. Often, you still have to worm because you’ve got no idea about the different worm burdens that they might be suffering from that can’t be counted on a worm egg count.[HO2]
I just haven’t got round to it. Because it’s a fairly new business and it’s grown quite quickly and its one of the things that should have come to the forefront but, we’ve like poo picked constantly and worming, I think that has sufficed to a level, but it it’s an area I’d like to move to.[YM1]
As a large yard with a large field, poo picking is difficult, so that’s why we harrow.[HO4]
3.1.2. Subtheme 2: Internal Influences
I don’t know why really, I just haven’t. I haven’t thought about it or looked into it.[HO3]
Habit primarily, and it being the norm.[HO5]
You know, it’s how we’ve always done it. And so it’s a bit of changing the yard owner’s mindset to actually let us [perform FWECs].[YM3]
I’m not very good with worming.[HO1]
I didn’t have a clue what worm egg counting was, until a good few years ago when it started coming around.[HO4]
I think the other biggest thing is that people just don’t understand it, how to worm strategically. I think not enough people know about it effectively.[HO1]
The knowledge is really not there for novice owners.[HO4]
I think the companies selling the faecal worm egg counting kits and things, they’re selling the products that they can sell, but they can’t do a blood test. So they aren’t marketing that oh actually there’s this vital bit of information needed here.[HO1]
I’ve dealt with a few vets and you have your routine vaccinations and everything else but unless somethings wrong you don’t really get pushed to talk about worming.[HO4]
3.1.3. Subtheme 3: External Influences
I think for us, because we have, do have quite a lot of traffic. We have young horses arriving that have come from Ireland or dealers, basically places that I don’t know their worming history and I’m pretty sure that a lot of them that come to us have never ever been wormed. So I’m a little leaned towards getting a wormer in them in the first place.[YM2]
I think if you’re on a yard, for example similar to mine and you’re on full livery, your horse’s management is out of your hands. If your horse, for example like mine, over winter they don’t poo pick, so I know that’s a barrier for me because if they’re not poo picking the fields I know he’s more likely to have a high worm burden an I am more likely to be required to worm.” “how my horse is managed, i.e., on full livery, I can’t control that they don’t poo pick the fields, the shared pastures on a daily basis in winter. You know, and the fields are rotated quite frequently, as in different horses on there.
So we have a barn of 20 horses and every single one of those 20 horses could go in the same paddock as mine, and they are all exposed to the same worms and eggs I suppose, so you know that’s a barrier for me, because what’s the point of me testing when the likelihood is that my horse is going to have a high burden because the paddocks aren’t poo picked, it’s going to waste more of my money.[HO1]
What I say at my yard is that we run a worm programme, they have to join. And if they don’t join it I want to know why, and if they don’t worm I want to know why. If they basically have a worm programme in place from a previous yard where they wormed and didn’t do egg counts, I persuade them to do the egg counts and to be honest, everyone comes over onto the egg counts. I have found a few barriers, people are a bit old fashioned aren’t they and they only like what they know, and they’re the type of people to just worm once a year and think that’s okay, but then actually when you do a worm count they’ve got a horribly high redworm count, or roundworm count and they’re like horrified.[YM6]
3.2. Theme 2: Helminth Information -and Anthelmintic Source
3.2.1. Subtheme 1: Point of Sale Information
Depends who serves you. I’ve had information given in the past and sometimes I’ve not, I’ve just been handed it.[HO4]
They don’t really give me a lot of information about to me I suppose because I think they must know that I know what I’m on about. To some people they might, but they don’t really tell me a lot about the products because I sort of know what I’m aiming at.[YM6]
The dose, what wormer I should use, when I should give and she does tell me why as well, why I need to give it.[HO1]
Just tells me which worms it kills. Pretty much it’s just sort of standard stuff isn’t it. It tells you up to 700 kg or and then just in theory what it kills.[YM1]
They require you to give information about the horses, what size and age.[YM4]
3.2.2. Subtheme 2: External Influences
If I was after general information, I’d probably just Google. If I thought they were severely affected by a worm burden I’d bring it up with a vet.[HO2]
I’d google stuff like that usually and ask the vet if he was here at the time.[YM6]
The vets, I always get veterinary advice on it.[YM4]
I guess it would be a collaborative effort, in conjunction with myself and the vets.[YM2]
To be honest through I just speak to an SQP quite regularly to understand what I should do with him as I’m not very good with worming.[HO1]
I used to kind of shop around a bit, but the prices are very much similar really, it’s only a couple of pounds and you’re not buying them every week, it only every few months isn’t it so, but now I’ve tended to, I’ve found someone who gives more valid advice, and quite reliable and seems to know their stuff, I tend to stick with the one pharmacy that I get mine from.[HO1]
Just get them from [Online veterinary pharmacy]. To be honest I just google cheap [wormers]. Depends which ones [the vets are] saying and I just google it and get from wherever is cheapest.[YM1]
I go the local suppliers, a suppliers for farmers, a local one.[HO3]
3.3. Theme 3: Accurate Dosing
I make sure he hasn’t got any food in his mouth first, so I pull any hay out of his mouth.[HO1]
I like to keep their head up until they have swallowed it.[YM4]
3.3.1. Subtheme 1: Problems Administering
I’m under five foot and I can worm any horse because I’ve got a technique.[YM3]
Well, it depends which one, there is a variety of different strategies. [One of the horses] won’t let you touch her nose with anything, so it has to be quite strategic. The rest of them, you can pretty much do headcollar-less. And sometimes try and give them a treat afterwards if I’m organised.[HO2]
Multiple problems, especially with horses that aren’t keen with their mouths.[HO4]
If it’s naughty I’d go to more efforts to control it, if we have any that are really naughty, we’d put it in their feed.[YM2]
Yeah I’m pretty alright to be honest, I mean a few of them aren’t overly keen but its done before they know if you’re quick. So yeah I’m quite happy the way we do worm if we have to.[YM1]
Er, either just wrestle them. Or try and put it in their feed maybe. But it’s a tricky one, especially if you’ve got a big horse that doesn’t want it being done. To be fair it’s not just the big ones, I’ve had a little tiny pony every year we have to worm put its legs over my shoulder, rearing up. It was a nightmare, I dread that time when I have to worm that pony.[HO4]
You can’t wrestle with her, she’s too strong. We’ve got to touch it on her cheek, and scrub it, and then slide it down her face. And then like don’t stretch your hand out to push the wormer in yet because it’s just going to squirt all over the place because she’ll make you jump when she drags you along. And then just keep taking it away and back, so the whole thing takes about 10 minutes. I like push it on her cheek, take it away, push it a bit further down, take it away, and then in the end, insert it in her mouth a bit and shoot as fast I can and hold her head in the air really quickly afterwards.[HO2]
I’ve got a couple that won’t have a syringe and I have to give granules. But you can’t with tapeworm, I struggle to tapeworm as there isn’t a granule for tapeworm.[YM6]
What did someone say to me if they’re like 500 kg and you only give them enough for 400 kg then you needn’t have bothered giving them any at all as it’s not enough. You’ve got to give them the right amount for what they weigh, otherwise there’s no point in giving half of it. You’ve got to like give them the full amount that’s needed for the weight of the horse, I don’t know why but you’re supposed to do that.[HO3]
If you don’t administer enough wormer for the weight of your horse, then some worms can survive, hence leading to long term resistance.[HO1]
I guess I don’t worry about overdosing, I do worry about underdosing. You’d rather get a bit more in because obviously there’ll be a bit on the face and a bit spat out or whatever.[HO2]
I just happily over worm them all. As my vet has told me that it’s not a problem to over worm it’s a problem to under worm.[YM2]
The only ones I don’t give a full wormer to are my little ponies, and I didn’t used to give a full wormer to my younger horses when they were smaller, so I guess I do adjust the dose to some extent but in terms of a 400 kg horse versus a 550 kg horse, I’d just give them both a full dose of wormer. My theory on that would be that they probably spit a bit out so they’d be better having the whole lot, rightly or wrongly.[YM2]
Yeah, it’s an important factor so we’re not giving a pony a full syringe but also for wastage, if we can get two ponies out of one syringe it’s going to cost a lot less.[YM3]
3.3.2. Subtheme 2: Bodyweight
I quite often frequent to the vets unfortunately for other instances, and every time I’m there I make sure he goes on the weighbridge. When my saddle fitter comes out he always uses a weigh tape every time so that’s another way. And often visually as well, because I have a bit of a baseline as to what he is, I can tell if he’s put weight on or if he’s lost weight.[HO1]
We weight tape on a fairly regularly basis so we have a good idea. All our horses go on a weigh bridge twice a year.[YM4]
Not very often, only if we see any sort of problem. Obviously this time of year [late spring/summer] we’ve got so much grass at the minute so it’s important to keep an eye on the weight.[YM5]
The liveries use weight tapes, but I think they’re a bit of an average, I don’t think they give you a very accurate result.[YM6]
I know what he weighs, I use my weigh tape.[HO3]
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A. Semi-Structured Interview Guide
- How many horses do currently own/have previously owned?
- How are your horses kept/What livery services does your yard offer?
- Would you class yourself as a leisure rider or competition rider/are your livery clients leisure riders or competition riders?
- How long have you been a part of the equine industry/how long have you worked in the equine industry?
- Do you have any relevant equine/animal qualifications (BHS/UKCC etc)?
- Can you tell me how you manage your horses’ worm burdens?
- What are the details of your programme?
- Who helped you develop your programme?
- Where do you source your [de]wormers?
- How often do you obtain [de]wormers from that source?
- What information is given at the point of sale?
- [In addition to those already stated] Do you know who can give you [de]worming advice and sell [de]worming products?
- What do you know about horse worms and what is the consequence of high worm burdens?
- Where do you find or look for information on worms?
- Are you aware of equine [de]wormer resistance?
- What do you think the problem is?
- What do you think the consequence(s) of the problem is?
- How do you worm your horse, can you talk me through it?
- Do you have any problems administering [de]wormers?
- Do you consider weight an important factor in [de]worming?
- How do you determine your horses’ weights?
- Are there any barriers preventing you from implementing a “testing before treating” style programme?
References
- Köhler, P. The biochemical basis of anthelmintic action and resistance. Int. J. Parasitol. 2001, 31, 336–345. [Google Scholar] [CrossRef]
- Lester, H.E.; Spanton, J.; Stratfor, C.H.; Bartley, D.J.; Morgan, E.R.; Hodgkinson, J.E.; Coumbe, K.; Mair, T.; Swan, B.; Lemon, G.; et al. Anthelmintic efficacy against cyathostomins in horses in South England. Vet. Parasitol. 2013, 197, 189–196. [Google Scholar] [CrossRef]
- Relf, V.E.; Morgan, E.R.; Hodgkinson, J.E.; Matthews, J.B. Helminth egg excretion with regard to age, gender and management practices on UK Thoroughbred studs. Parasitology 2013, 140, 641–652. [Google Scholar] [CrossRef]
- Andersen, U.V.; Howe, D.K.; Olsen, S.N.; Nielsen, M.K. Recent advances in diagnosing pathogenic equine gastrointestinal helminths: The challenge of prepatent detection. Vet. Parasitol. 2013, 192, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Giles, C.J.; Urquhard, K.A.; Longstaffe, J.A. Larval cyathostomiasis (immature trichonema-induced enteropathy): A report of 15 clinical cases. Equine Vet. J. 1985, 17, 196–201. [Google Scholar] [CrossRef]
- Cribb, N.C.; Cote, N.M.; Boure, L.P.; Peregrine, A.S. Acute small intestinal obstruction associated with Parascaris equorum infection in young horses: 25 cases (1985–2004). N. Z. Vet. J. 2006, 54, 338–343. [Google Scholar] [CrossRef]
- Ryu, S.H.; Jang, J.D.; Bak, U.B.; Lee, C.W.; Youn, H.J.; Lee, Y.L. Gastrointestinal impaction by Parascaris equorum in a Thoroughbred foal in Jeju, Korea. J. Vet. Sci. 2004, 5, 181–182. [Google Scholar] [CrossRef]
- Von Samson-Himmelstjerna, G. Anthelmintic resistance in equine parasites–detection, potential clinical relevance and implications for control. Vet. Parasitol. 2012, 185, 2–8. [Google Scholar] [CrossRef]
- Reinemeyer, C.R.; Nielsen, M.K. Parasitism and colic. Vet. Clin. Equine Pract. 2009, 25, 233–245. [Google Scholar] [CrossRef]
- Nielsen, M.K.; Reinemeyer, C.R.; Donecker, J.M.; Leathwick, D.M.; Marchiondo, A.A.; Kaplan, R.M. Anthelmintic resistance in equine parasites—Current evidence and knowledge gaps. Vet. Parasitol. 2014, 204, 55–63. [Google Scholar] [CrossRef]
- Matthews, J.B. Anthelmintic resistance in equine nematodes. Int. J. Parasitol. Drugs Drug Resist. 2014, 4, 310–315. [Google Scholar] [CrossRef]
- Rendle, D.; Austin, C.; Bowen, M.; Cameron, I.; Furtado, T.; Hodgkinson, J.; McGorum, B.; Matthews, J. Equine de-worming: A consensus on current best practice. UK-Vet. Equine 2019, 3, 1–14. [Google Scholar] [CrossRef]
- Fissiha, W.; Kinde, M.Z. Anthelmintic Resistance and Its Mechanism: A Review. Infect. Drug Resist. 2021, 14, 5403. [Google Scholar] [CrossRef] [PubMed]
- American Association of Equine Practitioners. AAEP Parasite Control Guidelines. Available online: https://aaep.org/sites/default/files/Documents/InternalParasiteGuidelinesFinal5.23.19.pdf (accessed on 23 July 2022).
- Shalaby, H.A. Anthelmintics resistance; how to overcome it? Iran. J. Parasitol. 2013, 8, 18. [Google Scholar] [PubMed]
- Rendle, D.; Mountford, D.; Roberts, C.; Owers, R.; Mair, T.; Bowen, M.; Matthews, J.; Richards, I.; Hodgkinson, J.; Furtado, T.; et al. Anthelmintic resistance in equids. Vet. Rec. 2021, 188, 230–231. [Google Scholar] [CrossRef] [PubMed]
- Easton, S.; Pinchbeck, G.L.; Tzelos, T.; Bartley, D.J.; Hotchkiss, E.; Hodgkinson, J.E.; Matthews, J.B. Investigating interactions between UK horse owners and prescribers of anthelmintics. Prev. Vet. Med. 2016, 135, 17–27. [Google Scholar] [CrossRef]
- Sustainable Control of Parasites. The SCOPS Principles. Available online: https://www.scops.org.uk/ (accessed on 18 July 2022).
- Control of Worms Sustainably. What Is COWS? Available online: https://www.cattleparasites.org.uk/ (accessed on 18 July 2022).
- Charlton, K.; Robinson, P.A. A qualitative investigation of the attitudes and practices of farmers and veterinarians in Wales regarding anthelmintic resistance in cattle. Vet. Ital. 2019, 55, 327–337. [Google Scholar] [PubMed]
- Golding, S.E.; Ogden, J.; Higgins, H.M. Shared goals, different barriers: A qualitative study of UK veterinarians’ and farmers’ beliefs about antimicrobial resistance and stewardship. Front. Vet. Sci. 2019, 6, 132. [Google Scholar] [CrossRef]
- Velde, F.V.; Charlier, J.; Hudders, L.; Cauberghe, V.; Claerebout, E. Beliefs, intentions, and beyond: A qualitative study on the adoption of sustainable gastrointestinal nematode control practices in Flanders’ dairy industry. Prev. Vet. Med. 2018, 153, 15–23. [Google Scholar] [CrossRef]
- Tzelos, T.; Morgan, E.R.; Easton, S.; Hodgkinson, J.E.; Matthews, J.B. A survey of the level of horse owner uptake of evidence-based anthelmintic treatment protocols for equine helminth control in the UK. Vet. Parasitol. 2019, 274, 108926. [Google Scholar] [CrossRef] [PubMed]
- Allison, K.; Taylor, N.M.; Wilsmore, A.J.; Garforth, C. Equine anthelmintics: Survey of the patterns of use, beliefs and attitudes among horse owners in the UK. Vet. Rec. 2011, 168, 483. [Google Scholar] [CrossRef]
- Furtardo, T.; Perkins, E.; McGowan, C.; Pinchbeck, G. Equine Management in UK Livery Yards during the COVID-19 Pandemic—“As Long As the Horses Are Happy, We Can Work Out the Rest Later”. Animals 2021, 11, 1416. [Google Scholar] [CrossRef]
- Carroll, G.A.; Groarke, J.M. The importance of the social sciences in reducing tail biting prevalence in pigs. Animals 2019, 9, 591. [Google Scholar] [CrossRef]
- Jackson, C.; Eliasson, Â.L.; Barber, N.; Weinman, J. Applying COM-B to medication adherence: A suggested framework for research and interventions. Eur. Health Psychol. 2014, 16, 7–17. [Google Scholar]
- McDonald, J.L.; Farnworth, M.J.; Clements, J. Integrating trap-neuter-return campaigns into a social framework: Developing long-term positive behavior change toward unowned cats in urban areas. Front. Vet. Sci. 2018, 5, 258. [Google Scholar] [CrossRef] [PubMed]
- Thompson, L.M.; Diaz-Artiga, A.; Weinstein, J.R.; Handley, M.A. Designing a behavioral intervention using the COM-B model and the theoretical domains framework to promote gas stove use in rural Guatemala: A formative research study. BMC Public Health 2018, 18, 253. [Google Scholar] [CrossRef] [PubMed]
- Pathak, V.; Jena, B.; Kalra, S. Qualitative research. Perspect. Clin. Res. 2013, 4, 115389. [Google Scholar]
- Onwuegbuzie, A.J.; Leech, N.L. Sampling designs in qualitative research: Making the sampling process more public. Qual. Rep. 2007, 12, 238–254. [Google Scholar] [CrossRef]
- Kvale, S.; Brinkmann, S. Interviews: Learning the Craft of Qualitative Research Interviewing, 2nd ed.; Sage Publications Inc.: Newbury Park, CA, USA, 2009; pp. 97–109. [Google Scholar]
- Charmaz, K. Constructing Grounded Theory, 2nd ed.; Sage Publications Ltd.: London, UK, 2014; pp. 83–108. [Google Scholar]
- Braun, V.; Clarke, V. Using thematic analysis in psychology. Qual. Res. Psychol. 2006, 3, 77–101. [Google Scholar] [CrossRef]
- Novick, G. Is there a bias against telephone interviews in qualitative research? Res. Nurs. Health 2008, 31, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Sturges, J.E.; Hanrahan, K.J. Comparing telephone and face-to-face qualitative interviewing: A research note. Qual. Res. 2004, 4, 107–118. [Google Scholar] [CrossRef]
- Otter.ai. Available online: https://otter.ai/ (accessed on 4 June 2022).
- Leech, N.L.; Onwuegbuzie, A.J. Beyond constant comparison qualitative data analysis: Using NVivo. Sch. Psychol. Q. 2011, 26, 70–84. [Google Scholar] [CrossRef]
- Department for Environment Food & Rural Affairs. Code of Practice for the Welfare of Horses, Ponies, Donkeys and Their Hybrids. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/700200/horses-welfare-codes-of-practice-april2018.pdf (accessed on 24 July 2022).
- Hughes, P.L.; Dowling, A.F.; Callinan, A.P.L. Resistance to macrocyclic lactone anthelmintics and associated risk factors on sheep farms in the lower North Island of New Zealand. N. Z. Vet. J. 2007, 55, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Whitaker, T.C.; Goupil, X.; Roy, O.; Marciat, D.; McGahie, D. Evaluation and comparison under field conditions of the stress response induced in horses when administered endoparasiticides in tablet or paste formulations. Int. J. Appl. Res. 2011, 9, 6. [Google Scholar]
- Veterinary Medicines Directorate. VMD Hosts Second Equine Anthelmintic Resistance Stakeholder Meeting. Available online: https://www.gov.uk/government/news/vmd-hosts-second-equine-anthelmintic-resistance-stakeholder-meeting (accessed on 18 June 2022).
- Clough, H.; Roshier, M.; England, G.; Burford, J.; Freeman, S. Qualitative study of the influence of horse-owner relationship during some key events within a horse’s lifetime. Vet. Rec. 2021, 188, e79. [Google Scholar] [CrossRef]
- Murray, B. ‘It’s a False Economy’: Owners Urged Not to Scrimp on Horse Care as Cost of Living Crisis Continues. Available online: https://www.horseandhound.co.uk/news/owners-urged-not-to-scrimp-on-horse-care-as-cost-of-living-crisis-continues-793940 (accessed on 7 July 2022).
- Best, C.M.; Pyatt, A.Z.; Roden, J.; Behnke, M.; Phillips, K. Sheep farmers’ attitudes towards lameness control: Qualitative exploration of factors affecting adoption of the lameness Five-Point Plan. PLoS ONE 2021, 16, e0246798. [Google Scholar] [CrossRef]
- Fisher, M.; Holden, S.T.; Thierfelder, C.; Katengeza, S.P. Awareness and adoption of conservation agriculture in Malawi: What difference can farmer-to-farmer extension make? J. Sustain. Agric. 2018, 16, 310–325. [Google Scholar] [CrossRef]
- Mohammed, F.; Nuhu, H.; Gwary, M.; Mustapha, S. Review of Experiences and Effectiveness of Farmer-to-Farmer Extension Approach in Developing Countries. Fudma Jaat 2018, 4, 55–60. [Google Scholar]
- Hii, C.; Dhand, N.K.; Toribio, J.A.L.; Taylor, M.R.; Wiethoelter, A.; Schembri, N.; Sawford, K.; Kung, N.; Moloney, B.; Wright, T.; et al. Information delivery and the veterinarian-horse owner relationship in the context of Hendra virus in Australia. Prev. Vet. Med 2020, 179, 104988. [Google Scholar] [CrossRef]
- Wagner, E.L.; Tyler, P.J. A Comparison of Weight Estimation Methods in Adult Horses. J. Equine Vet. Sci. 2011, 31, 706–710. [Google Scholar] [CrossRef]
- Hitchens, P.L.; Hultgren, J.; Frössling, J.; Emanuelson, U.; Keeling, L.J. Prevalence and risk factors for overweight horses at premises in Sweden assessed using official animal welfare control data. Acta Vet. Scand. 2016, 58, 31–35. [Google Scholar] [CrossRef]
- Morrison, P.; Harris, P.; Maltin, C.; Grove-White, D.; Argo, C.; Barfoot, C. Perceptions of obesity in a UK leisure-based population of horse owners. Acta Vet. Scand. 2015, 57 (Suppl. S1), O6. [Google Scholar] [CrossRef]
- Stephenson, H.M.; Green, M.J.; Freeman, S.L. Prevalence of obesity in a population of horses in the UK. Vet. Rec. 2011, 168, 131. [Google Scholar] [CrossRef]
- Wyse, C.A.; McNie, K.A.; Tannahill, V.J.; Tannahil, V.J.; Murray, J.K.; Love, S. Prevalence of obesity in riding horses in Scotland. Vet. Rec. 2008, 162, 590–591. [Google Scholar] [CrossRef]
- Giles, S.L.; Nicol, C.J.; Harris, P.A.; Rands, S.A. Dominance rank is associated with body condition in outdoor-living domestic horses (Equus caballus). Appl. Anim. Behav. 2015, 166, 71–79. [Google Scholar] [CrossRef]
| Benzimidazoles | Macrocyclic Lactones | Tetrahydropyrimidines |
|---|---|---|
| Widespread cyathostomins resistance | Early indication of cyathostomins resistance | Reports of cyathostomins resistance |
| Early indication of P. equorum resistance | Widespread P. equorum resistance—particularly Ivermectin | Early indication of P. equorum resistance |
| Early indication of Oxyuris equi |
| Unique Code | Participant | Length of Time in the Equine Industry | Number of Horses | Livery Management |
|---|---|---|---|---|
| HO1 | Horse Owner | 25 years | 1 | Full livery |
| HO2 | Horse Owner | >25 years | 5 | DIY |
| HO3 | Horse Owner | >22 years | 2 | DIY |
| HO4 | Horse Owner | >10 years | 1 | DIY |
| HO5 | Horse Owner | 49 years | 2 | Full Livery |
| YM1 | Yard Manager | >30 years | 35 | Retirement Livery |
| YM2 | Yard Manager | >25 years | 24 | Full, Competition, Schooling, Rehabilitation, and Sales Livery |
| YM3 | Yard Manager | >26 years | 90 | Full, Part, Assisted, and DIY Livery in addition to Riding School Horses |
| YM4 | Yard Manager | >35 years | 12 | Full Livery |
| YM5 | Yard Manager | >24 years | 58 | Full, Part, DIY, and Grass Livery |
| YM6 | Yard Manager | >31 years | 65 | Full, Assisted, DIY, Holiday, and Schooling Livery |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
McTigue, F.E.; Mansbridge, S.C.; Pyatt, A.Z. Equine Anthelmintic Resistance: Horse Owner and Yard Manager Perception of the Barriers Affecting Strategic Control Measures in England. Vet. Sci. 2022, 9, 560. https://doi.org/10.3390/vetsci9100560
McTigue FE, Mansbridge SC, Pyatt AZ. Equine Anthelmintic Resistance: Horse Owner and Yard Manager Perception of the Barriers Affecting Strategic Control Measures in England. Veterinary Sciences. 2022; 9(10):560. https://doi.org/10.3390/vetsci9100560
Chicago/Turabian StyleMcTigue, Faye E., Stephen C. Mansbridge, and Alison Z. Pyatt. 2022. "Equine Anthelmintic Resistance: Horse Owner and Yard Manager Perception of the Barriers Affecting Strategic Control Measures in England" Veterinary Sciences 9, no. 10: 560. https://doi.org/10.3390/vetsci9100560
APA StyleMcTigue, F. E., Mansbridge, S. C., & Pyatt, A. Z. (2022). Equine Anthelmintic Resistance: Horse Owner and Yard Manager Perception of the Barriers Affecting Strategic Control Measures in England. Veterinary Sciences, 9(10), 560. https://doi.org/10.3390/vetsci9100560








