Cranial Spinal Spreading of Canine Brain Gliomas after Hypofractionated Volumetric-Modulated Arc Radiotherapy and Concomitant Temozolomide Chemotherapy: A Four-Case Report
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
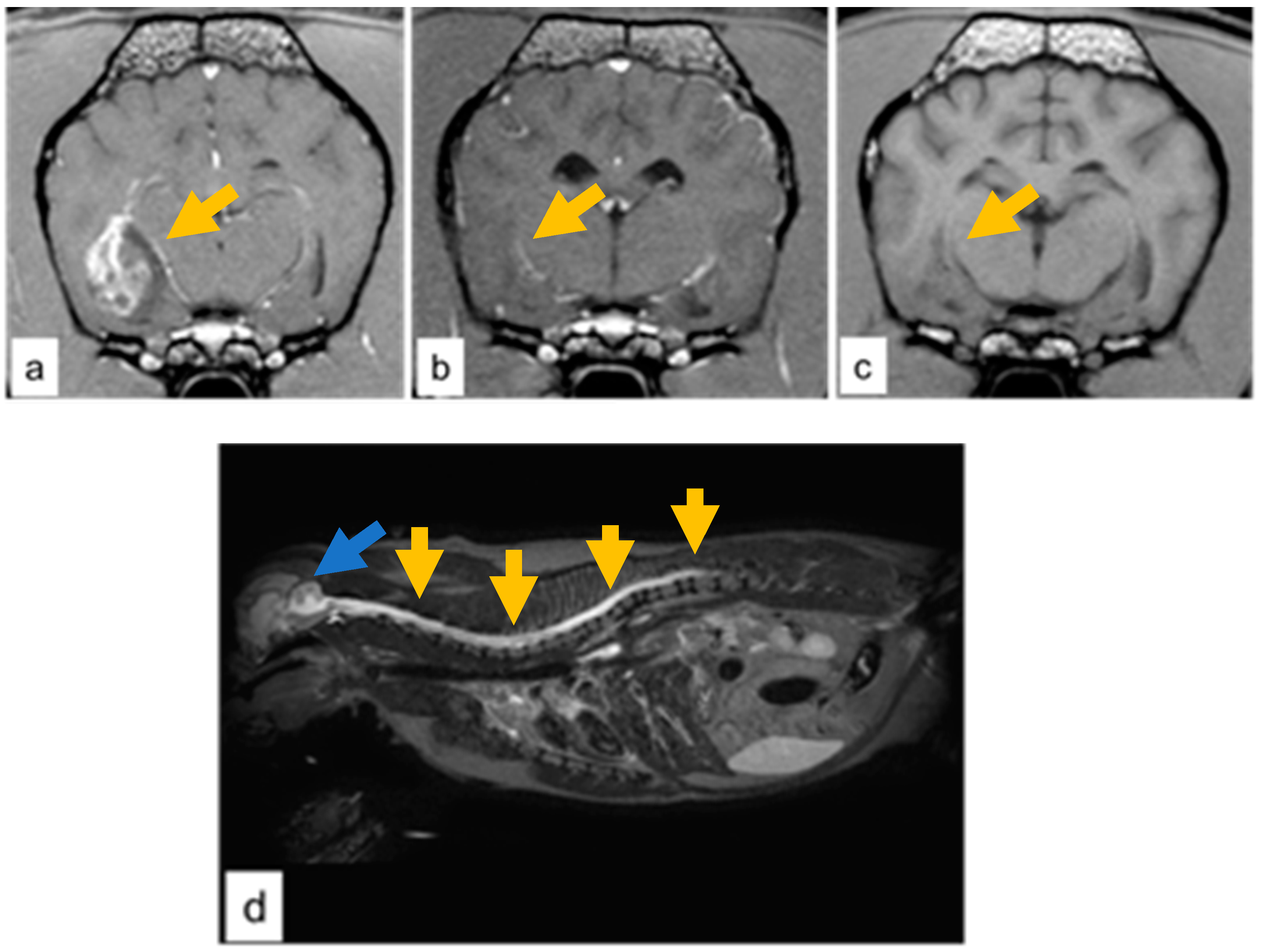
3.1. Case Descriptions
3.2. Post-Mortem Examination
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Song, R.B.; Vite, C.H.; Bradley, C.W.; Cross, J.R. Postmortem Evaluation of 435 Cases of Intracranial Neoplasia in Dogs and Relationship of Neoplasm with Breed, Age, and Body Weight. J. Vet. Intern. Med. 2013, 27, 1143–1152. [Google Scholar] [CrossRef] [PubMed]
- MacLellan, J.D.; Arnold, S.A.; Dave, A.C.; Hunt, M.A.; Pluhar, G.E. Association of magnetic resonance imaging-based preoperative tumour volume with postsurgical survival time in dogs with primary intracranial glioma. J. Am. Vet. Med. Assoc. 2018, 252, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.D.; Miller, C.R.; Rossmeisl, J.H. Canine Primary Intracranial Cancer: A Clinicopathologic and Comparative Review of Glioma, Meningioma, and Choroid Plexus Tumours. Front. Oncol. 2019, 9, 1151. [Google Scholar] [CrossRef]
- Kube, A.S.; Bruyette, D.S.; Hanson, S.M. Astrocytomas in Young Dogs. J. Am. Anim. Hosp. Assoc. 2003, 39, 288–293. [Google Scholar] [CrossRef] [PubMed]
- Snyder, J.M.; Shofer, F.S.; Van Winkle, T.J.; Massicotte, C. Canine intracranial primary neoplasia: 173 cases (1986–2003). J. Vet. Intern. Med. 2006, 20, 669–675. [Google Scholar] [CrossRef] [PubMed]
- Bannasch, D.; Young, A.; Myers, J.; Truvé, K.; Dickinson, P.; Gregg, J.; Davis, R.; Bongcam-Rudloff, E.; Webster, M.T.; Lindblad-Toh, K.; et al. Localization of canine brachycephaly using an across breed mapping approach. PLoS ONE 2010, 3, e9632. [Google Scholar] [CrossRef]
- Fox, M.W. Developmental abnormalities of the canine skull. Can. J. Comp. Med. Vet. Sci. 1963, 27, 219–222. [Google Scholar]
- Sokołowski, W.; Czubaj, N.; Skibniewski, M.; Barszcz, K.; Kupczyńska, M.; Kinda, W.; Kiełbowicz, Z. Rostral cranial fossa as a site for cerebrospinal fluid drainage—Volumetric studies in dog breeds of different size and morphotype. BMC Vet. Res. 2018, 14, 162. [Google Scholar] [CrossRef]
- Hussein, A.K.; Sullivan, M.; Penderis, J. Effect of brachycephalic, mesaticephalic, and dolichocephalic head conformations on olfactory bulb angle and orientation in dogs as determined by use of in vivo magnetic resonance imaging. Am. J. Vet. Res. 2012, 73, 946–951. [Google Scholar] [CrossRef]
- Truvé, K.; Dickinson, P.; Xiong, A.; York, D.; Jayashankar, K.; Pielberg, G.; Koltookian, M.; Murén, E.; Fuxelius, H.-H.; Weishaupt, H.; et al. Utilizing the Dog Genome in the Search for Novel Candidate Genes Involved in Glioma Development—Genome Wide Association Mapping followed by Targeted Massive Parallel Sequencing Identifies a Strongly Associated Locus. PLoS Genet. 2016, 12, e1006000. [Google Scholar] [CrossRef]
- Rossmeisl, J.H., Jr.; Jones, J.C.; Zimmerman, K.; Robertson, J.L. Survival time following hospital discharge in dogs with palliatively treated primary brain tumours. J. Am. Vet. Med. Assoc. 2013, 242, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Barker, A.; Harcourt-Brown, T.; Jeffery, N. Systematic Review of Brain Tumour Treatment in Dogs. J. Vet. Intern. Med. 2015, 29, 1456–1463. [Google Scholar] [CrossRef] [PubMed]
- Heading, K.L.; Brockley, L.K.; Bennett, P.F. CCNU (lomustine) toxicity in dogs: A retrospective study (2002-07). Aust. Vet. J. 2011, 89, 109–116. [Google Scholar] [CrossRef]
- Batzorf, U.; Selch, M.T. Brain tumours. In Cancer Treatment, 2nd ed.; Haskell, C.M., Ed.; Saunders, W.B.: Philadelphia, PA, USA, 1985; p. 660. [Google Scholar]
- Evans, S.M.; Dayrell-Hart, B.; Powlis, W.; Christy, G.; Van Winkle, T. Radiation Therapy of Canine Brain Masses. J. Vet. Intern. Med. 1993, 7, 216–219. [Google Scholar] [CrossRef] [PubMed]
- Debreuque, M.; De Fornel, P.; David, I.; Delisle, F.; Ducerveau, M.N.; Devauchelle, P.; Thibaud, J.L. Definitive-intent uniform megavoltage fractioned radiotherapy protocol for presumed canine intracranial gliomas: Retrospective analysis of survival and prognostic factors in 38 cases (2013–2019). BMC Vet. Res. 2020, 16, 412. [Google Scholar] [CrossRef] [PubMed]
- Choucair, A.K.; Levin, V.A.; Gutin, P.H.; Davis, R.L.; Silver, P.; Edwards, M.S.; Wilson, C.B. Development of multiple lesions during radiation therapy and chemotherapy in patients with gliomas. J. Neurosurg. 1986, 65, 654–658. [Google Scholar] [CrossRef] [PubMed]
- Chamberlain, M.C.; Tredway, T.L. Adult primary intradural spinal cord tumours: A review. Curr. Neurol. Neurosci. Rep. 2011, 11, 320–328. [Google Scholar] [CrossRef]
- Tinchon, A.; Oberndorfer, S.; Marosi, C.; Rudà, R.; Sax, C.; Calabek, B.; Grisold, W. Malignant spinal cord compression in cerebral glioblastoma multiforme: A multicenter case series and review of the literature. J. Neurooncol. 2012, 110, 221–226. [Google Scholar] [CrossRef]
- Mariniello, G.; Peca, C.; Del Basso De Caro, M.; Carotenuto, B.; Formicola, F.; Elefante, A.; Maiuri, F. Brain gliomas presenting with symptoms of spinal cord metastasis. Neuroradiol. J. 2015, 28, 478–482. [Google Scholar] [CrossRef]
- Ng, H.K.; Sun, D.T.; Poon, W.S. Anaplastic oligodendroglioma with drop metastasis to the spinal cord. Clin. Neurol. Neurosurg. 2002, 104, 383–386. [Google Scholar] [CrossRef]
- Chen-Zhao, X.; Aznar-García, L. Diagnosis and management of spinal metastasis of primary brain tumours. AME Case Rep. 2018, 2, 26. [Google Scholar] [CrossRef] [PubMed]
- Munshey, A.; Moore, J.; Maclean, C.; Longano, A.; Goldschlager, T. Cranial Pilocytic Astrocytoma With Spinal Drop Metastasis in an Adult: Case Report and Literature Review. World Neurosurg. 2017, 98, 883.e7–883.e12. [Google Scholar] [CrossRef] [PubMed]
- Carlsen, J.G.; Tietze, A.; Lassen, Y.A.; Rosendal, F. Paraplegia due to drop metastases from anaplastic oligodendroglioma. Br. J. Neurosurg. 2012, 26, 94–95. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.H.; Yoon, H.I.; Yi, S.; Park, J.W.; Cho, J.; Shin, D.A.; Ha, Y.; Kim, D.S.; Kim, S.H.; Lee, S.K.; et al. Treatment outcomes of radiotherapy for primary spinal cord glioma. Behandlungsergebnisse der Strahlentherapie beim primären Rückenmarksgliom. Strahlenther. Onkol. 2019, 195, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Elefante, A.; Peca, C.; Del Basso De Caro, M.L.; Russo, C.; Formicola, F.; Mariniello, G.; Brunetti, A.; Maiuri, F. Symptomatic spinal cord metastasis from cerebral oligodendroglioma. Neurol. Sci. 2012, 33, 609–613. [Google Scholar] [CrossRef][Green Version]
- Kim, J.G.; Park, C.O.; Hyun, D.K.; Ha, Y.S. Spinal epidural metastasis of cerebral oligodendroglioma. Yonsei Med. J. 2003, 44, 340–346. [Google Scholar] [CrossRef]
- Rohrer Bley, C.; Staudinger, C.; Bley, T.; Marconato, L.; Sabattini, S.; Beckmann, K. Canine presumed glial brain tumours treated with radiotherapy: Is there an inferior outcome in tumours contacting the subventricular zone? Vet. Comp. Oncol. 2022, 20, 29–37. [Google Scholar] [CrossRef]
- Vigeral, M.; Bentley, R.T.; Rancilio, N.J.; Miller, M.A.; Heng, H.G. Imaging diagnosis—Antemortem detection of oligodendroglioma “cerebrospinal fluid drop metastases” in a dog by serial magnetic resonance imaging. Vet. Radiol. Ultrasound 2018, 59, 32–37. [Google Scholar] [CrossRef]
- Bentley, R.T.; Yanke, A.B.; Miller, M.A.; Heng, H.G.; Cohen-Gadol, A.; Rossmeisl, J.H. Cerebrospinal Fluid Drop Metastases of Canine Glioma: Magnetic Resonance Imaging Classification. Front. Vet. Sci. 2021, 8, 650320. [Google Scholar] [CrossRef]
- Bordignon, K.C.; Neto, M.C.; Ramina, R.; de Meneses, M.S.; Zazula, A.D.; de Almeida, L.G. Patterns of neuroaxis dissemination of gliomas: Suggestion of a classification based on magnetic resonance imaging findings. Surg. Neurol. 2006, 65, 472–477. [Google Scholar] [CrossRef]
- Teasdale, G.; Jennett, B. Assessment and prognosis of coma after head injury. Acta Neurochir. 1976, 34, 45–55. [Google Scholar] [CrossRef]
- Dolera, M.; Malfassi, L.; Bianchi, C.; Carrara, N.; Finesso, S.; Marcarini, S.; Mazza, G.; Pavesi, S.; Sala, M.; Urso, G. Frameless stereotactic radiotherapy alone and combined with temozolomide for presumed canine gliomas. Vet. Comp. Oncol. 2018, 16, 90–101. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N.; Ohgaki, H.; Wiestler, O.D.; Cavenee, W.K.; Burger, P.C.; Jouvet, A.; Scheithauer, B.W.; Kleihues, P. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007, 114, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Benedict, S.H.; Yenice, K.M.; Followill, D.; Galvin, J.M.; Hinson, W.; Kavanagh, B.; Keall, P.; Lovelock, M.; Meeks, S.; Papiez, L.; et al. Stereotactic body radiation therapy: The report of AAPM Task Group 101. Med. Phys. 2010, 37, 4078–4101. [Google Scholar] [CrossRef] [PubMed]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- Ladue, T.; Klein, M.K.; Veterinary Radiation Therapy Oncology Group. Toxicity criteria of the veterinary radiation therapy oncology group. Vet. Radiol. Ultrasound 2001, 42, 475–476. [Google Scholar] [CrossRef]
- LeBlanc, A.K.; Atherton, M.; Bentley, R.T.; Boudreau, C.E.; Burton, J.H.; Curran, K.M.; Dow, S.; Giuffrida, M.A.; Kellihan, H.B.; Mason, N.J.; et al. Veterinary cooperative oncology group—Common terminology criteria for adverse events (VCOG-CTCAE) following chemotherapy or biological antineoplastic therapy in dogs and cats v1.1. Vet. Comp. Oncol. 2016, 14, 417–446. [Google Scholar] [CrossRef]
- Plattner, B.L.; Kent, M.; Summers, B.; Platt, S.R.; Freeman, A.C.; Blas-Machado, U.; Clemans, J.; Cheville, N.F.; Garcia-Tapia, D. Gliomatosis cerebri in two dogs. J. Am. Anim. Hosp. Assoc. 2012, 48, 359–365. [Google Scholar] [CrossRef]
- Giron, C.; Paquette, D.; Culang, D.; Doré, M.; Masseau, I. Diffuse meningeal oligodendrogliomatosis characterized by spinal intra-parenchymal nodules on magnetic resonance imaging in a dog. Can. Vet. J. 2020, 61, 1312–1318. [Google Scholar]
- Rissi, D.R.; Donovan, T.A.; Porter, B.F.; Frank, C.; Miller, A.D. Canine Gliomatosis Cerebri: Morphologic and immunohistochemical characterization is supportive of glial histogenesis. Vet. Pathol. 2021, 58, 293–304. [Google Scholar] [CrossRef]
- Porter, B.; de Lahunta, A.; Summers, B. Gliomatosis cerebri in six dogs. Vet. Pathol. 2003, 40, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Koestner, A.; Bilzer, T.; Schulman, F.Y.; Summers, B.A.; VanWinkle, T.J. Histological Classification of Tumours of the Nervous System of Domestic Animals; Armed Forces Institute of Pathology; American Registry of Pathology: Washington, DC, USA, 1999; p. 71. [Google Scholar]
- Ranjan, S.; Warren, K.E. Gliomatosis Cerebri: Current Understanding and Controversies. Front. Oncol. 2017, 7, 165. [Google Scholar] [CrossRef] [PubMed]
- Schweizer-Gorgas, D.; Henke, D.; Oevermann, A.; Lang, J.; Vandevelde, M.; Steffen, F. Magnetic resonance imaging features of canine gliomatosis cerebri. Vet. Radiol. Ultrasound 2018, 59, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Sebastian, P.R.; Fisher, M.; Smith, T.W.; Davidson, R.I. Intramedullary spinal cord metastasis. Surg. Neurol. 1981, 16, 336–339. [Google Scholar] [CrossRef]
- Pietschmann, S.; von Bueren, A.O.; Henke, G.; Kerber, M.J.; Kortmann, R.D.; Müller, K. An individual patient data meta-analysis on characteristics, treatments and outcomes of the glioblastoma/gliosarcoma patients with central nervous system metastases reported in literature until 2013. J. Neuro-Oncol. 2014, 120, 451–457. [Google Scholar] [CrossRef]
- Chan, M.; Hsiao, E. Incidental Finding of Cerebellar Medulloblastoma on 68Ga-DOTATATE PET/CT in a Patient with Appendiceal Carcinoid. Clin. Nucl. Med. 2016, 41, 886–887. [Google Scholar] [CrossRef]
- Costigan, D.A.; Winkelman, M.D. Intramedullary spinal cord metastasis. A clinic pathological study of 13 cases. J. Neurosurg. 1985, 62, 227–233. [Google Scholar] [CrossRef]
- Takano, S.; Kamiyama, H.; Mashiko, R.; Osuka, S.; Ishikawa, E.; Matsumura, A. Metronomic treatment of malignant glioma xenografts with irinotecan (CPT-11) inhibits angiogenesis and tumour growth. J. Neurooncol. 2010, 99, 177–185. [Google Scholar] [CrossRef]
- Bergers, G.; Benjamin, L.E. Tumourigenesis and the angiogenic switch. Nat. Rev. Cancer 2003, 3, 401–410. [Google Scholar] [CrossRef]
- Louveau, A.; Harris, T.H.; Kipnis, J. Revisiting the Mechanisms of CNS Immune Privilege. Trends Immunol. 2015, 36, 569–577. [Google Scholar] [CrossRef]
- Noell, S.; Ritz, R.; Wolburg-Buchholz, K.; Wolburg, H.; Fallier-Becker, P. An allograft glioma model reveals the dependence of aquaporin-4 expression on the brain microenvironment. PLoS ONE 2012, 7, e36555. [Google Scholar] [CrossRef] [PubMed]
- De Bonis, P.; Anile, C.; Pompucci, A.; Fiorentino, A.; Balducci, M.; Chiesa, S.; Lauriola, L.; Maira, G.; Mangiola, A. The influence of surgery on recurrence pattern of glioblastoma. Clin. Neurol. Neurosurg. 2013, 115, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Milano, M.T.; Okunieff, P.; Donatello, R.S.; Mohile, N.A.; Sul, J.; Walter, K.A.; Korones, D.N. Patterns and timing of recurrence after temozolomide-based chemoradiation for glioblastoma. Int. J. Radiat. Oncol. Biol. Phys. 2010, 78, 1147–1155. [Google Scholar] [CrossRef] [PubMed]
- Milano, M.T.; Johnson, M.D.; Sul, J.; Mohile, N.A.; Korones, D.N.; Okunieff, P.; Walter, K.A. Primary spinal cord glioma: A Surveillance, Epidemiology, and End Results database study. J. Neuro-Oncol. 2010, 98, 83–92. [Google Scholar] [CrossRef]
- Lawton, C.D.; Nagasawa, D.T.; Yang, I.; Fessler, R.G.; Smith, Z.A. Leptomeningeal spinal metastases from glioblastoma multiforme: Treatment and management of an uncommon manifestation of disease. J. Neurosurg. Spine 2012, 17, 438–448. [Google Scholar] [CrossRef] [PubMed]
- Beier, D.; Röhrl, S.; Pillai, D.R.; Schwarz, S.; Kunz-Schughart, L.A.; Leukel, P.; Proescholdt, M.; Brawanski, A.; Bogdahn, U.; Trampe-Kieslich, A.; et al. Temozolomide preferentially depletes cancer stem cells in glioblastoma. Cancer Res. 2008, 68, 5706–5715. [Google Scholar] [CrossRef]
- Seystahl, K.; Wick, W.; Weller, M. Therapeutic options in recurrent glioblastoma: An update. Crit. Rev. Oncol. Hematol. 2016, 99, 389–408. [Google Scholar] [CrossRef]
- Hegi, M.E.; Diserens, A.C.; Gorlia, T.; Hamou, M.F.; de Tribolet, N.; Weller, M.; Kros, J.M.; Hainfellner, J.A.; Mason, W.; Mariani, L.; et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N. Engl. J. Med. 2005, 352, 997–1003. [Google Scholar] [CrossRef]
- Weller, M.; Tabatabai, G.; Kästner, B.; Felsberg, J.; Steinbach, J.P.; Wick, A.; Schnell, O.; Hau, P.; Herrlinger, U.; Sabel, M.C.; et al. MGMT promoter methylation is a strong prognostic biomarker for benefit from dose-intensified temozolomide rechallenge in progressive glioblastoma: The DIRECTOR trial. Clin. Cancer Res. 2015, 21, 2057–2064. [Google Scholar] [CrossRef]
- Koch, M.W.; Sánchez, M.D.; Long, S. Multifocal oligodendroglioma in three dogs. J. Am. Anim. Hosp. Assoc. 2011, 47, e77–e85. [Google Scholar] [CrossRef]
- Nakamoto, Y.; Fukunaga, D.; Uchida, K.; Mori, T.; Kishimoto, T.; Ozawa, T. Anaplastic oligodendroglioma with leptomeningeal dissemination in a French Bulldog. J. Vet. Med. Sci. 2018, 80, 1590–1595. [Google Scholar] [CrossRef] [PubMed]
- van Leeuwen, C.M.; Oei, A.L.; Crezee, J.; Bel, A.; Franken, N.A.P.; Stalpers, L.J.A.; Kok, H.P. The alfa and beta of tumours: A review of parameters of the linear-quadratic model, derived from clinical radiotherapy studies. Radiat. Oncol. 2018, 13, 96. [Google Scholar] [CrossRef] [PubMed]

| Dog Number | Signalment | Diagnosis | Signs | Neurological Examination | Best Response | First Event | Time to First Event |
|---|---|---|---|---|---|---|---|
| 1 | Boxer, female, 8 years old | Grade III glioma | Generalized seizures | Normal | a | Spinal cord lesion | 6 months |
| 2 | French bulldog, male, 8 years old | Grade III glioma | Generalized seizures | Normal | a | Spinal cord lesion | 4 months |
| 3 | French bulldog, male, 7 years old | Grade IV glioma | Generalized seizures | Proprioceptive deficits | b | Gliotic scar at primary tumor site | 6 months |
| 4 | Labrador, female, 8 years old | Grade IV glioma | Generalized seizures | Proprioceptive deficits | b | Spinal cord lesion | 9 months |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Urso, G.; Boncu, A.G.; Carrara, N.; Zaman, D.-T.; Malfassi, L.; Marcarini, S.; Minoli, L.; Pavesi, S.; Sala, M.; Scanziani, E.; et al. Cranial Spinal Spreading of Canine Brain Gliomas after Hypofractionated Volumetric-Modulated Arc Radiotherapy and Concomitant Temozolomide Chemotherapy: A Four-Case Report. Vet. Sci. 2022, 9, 541. https://doi.org/10.3390/vetsci9100541
Urso G, Boncu AG, Carrara N, Zaman D-T, Malfassi L, Marcarini S, Minoli L, Pavesi S, Sala M, Scanziani E, et al. Cranial Spinal Spreading of Canine Brain Gliomas after Hypofractionated Volumetric-Modulated Arc Radiotherapy and Concomitant Temozolomide Chemotherapy: A Four-Case Report. Veterinary Sciences. 2022; 9(10):541. https://doi.org/10.3390/vetsci9100541
Chicago/Turabian StyleUrso, Gaetano, Alexandra Gabriela Boncu, Nancy Carrara, Dragos-Teodor Zaman, Luca Malfassi, Silvia Marcarini, Lucia Minoli, Simone Pavesi, Massimo Sala, Eugenio Scanziani, and et al. 2022. "Cranial Spinal Spreading of Canine Brain Gliomas after Hypofractionated Volumetric-Modulated Arc Radiotherapy and Concomitant Temozolomide Chemotherapy: A Four-Case Report" Veterinary Sciences 9, no. 10: 541. https://doi.org/10.3390/vetsci9100541
APA StyleUrso, G., Boncu, A. G., Carrara, N., Zaman, D.-T., Malfassi, L., Marcarini, S., Minoli, L., Pavesi, S., Sala, M., Scanziani, E., & Dolera, M. (2022). Cranial Spinal Spreading of Canine Brain Gliomas after Hypofractionated Volumetric-Modulated Arc Radiotherapy and Concomitant Temozolomide Chemotherapy: A Four-Case Report. Veterinary Sciences, 9(10), 541. https://doi.org/10.3390/vetsci9100541






