The Variation of Serotonin Values in Dogs in Different Environmental Conditions
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Variant 1
3.2. Variant 2
3.3. Variant 3
4. Discussion
4.1. Variant 1
4.2. Variant 2
4.3. Variant 3
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Savolainen, P.; Zhang, Y.P.; Luo, J.; Lundeberg, J.; Leitner, T. Genetic evidence for an East Asian origin of domestic dogs. Science 2002, 298, 1610–1613. [Google Scholar] [CrossRef]
- Mariti, C.; Ricci, E.; Carlone, B.; Moore, J.L.; Sighieri, C.; Gazzano, A. Dog attachment to man: A comparison between pet and working dog. J. Vet. Behav. Clin. Appl. Res. 2012, 8, 135–145. [Google Scholar] [CrossRef]
- Sachs, B.D.; Ni, J.R.; Caron, M.G. Brain 5-HT deficiency increases stress vulnerability and impairs antidepressant responses following psychosocial stress. Proc. Natl. Acad. Sci. USA 2015, 112, 2557–2562. [Google Scholar] [CrossRef] [PubMed]
- Patronek, G.J.; Glickman, L.T.; Beck, A.M.; McCabe, G.P.; Ecker, C. Risk factors for relinquishment of dogs to an animal shelter. J. Am. Vet. Med. Assoc. 1996, 209, 572–581. [Google Scholar] [PubMed]
- Leon, M.; Rosado, B.; Garcia, S. Assessment of serotonin in serum, plasma and platelets of aggressive dogs. J. Vet. Behav. Clin. Appl. Res. 2012, 7, 348–352. [Google Scholar] [CrossRef]
- Amat, M.; Le Brech, S.; Camps, T.; Torrente, C.; Mariotti, V.M.; José, L.; Ruiz, J.L.; Manteca, X. Differences in serotonin levels between aggressive English cocker spaniels and aggressive dogs of other breeds. J. Vet. Behav. Clin. Appl. Res. 2013, 8, 19–25. [Google Scholar] [CrossRef]
- Overall, K.L. Manual of Clinical Behavioural Medicine for Dogs and Cats; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Bollen, K.S.; Horowitz, J. Behavioral evaluation and demographic information in the assessment of aggressiveness in shelter dogs. Appl. Anim. Behav. Sci. 2008, 112, 120–135. [Google Scholar] [CrossRef]
- Bamberger, M.; Houpt, K.A. Signalment factors, comorbidity, and trends in behavior diagnoses in dogs: 1644 cases (1991–2001). J. Am. Vet. Med. Assoc. 2006, 229, 1591–1601. [Google Scholar] [CrossRef]
- Fatjó, J.; Amat, M.; Mariotti, V.; Ruiz de la Torre, J.L.; Manteca, X. Analysis of 1040 cases of canine aggression in a referral practice in Spain. J. Vet. Behav. 2007, 2, 158–165. [Google Scholar] [CrossRef]
- Mikkelsen, J.; Lund, J.D. Euthanasia of dogs due to behavioural problems: An epidemiological study of euthanasia of dogs in Denmark, with a special focus on problems of aggression. Eur. J. Comp. Anim. Pract. 1999, 10, 143–150. [Google Scholar]
- Nelson, R.J. Biology of Aggression; Oxford University Press: Oxford, UK, 2006; pp. 250–265. [Google Scholar]
- Ruddick, J.; Evans, A.; Nutt, D.; Lightman, S.; Rook, G.; Lowry, C. Tryptophan metabolism in the central nervous system: Medical implications. Expert Rev. Mol. Med. 2006, 8, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Cakiroglu, D.; Meral, Y.; Sanca, A.A.; Cifti, G. Relationship between the serum concentrations of serotonin and lipids and aggression in dogs. Vet. Record 2007, 161, 59–61. [Google Scholar] [CrossRef] [PubMed]
- Berger, M.; Gray, J.; Roth, B. The expanded biology of serotonin. Annu. Rev. Med. 2009, 60, 355–366. [Google Scholar] [CrossRef] [PubMed]
- Lentz, S. Serotonin and 5-hydroxyindolacetic acid in cerebrospinal fluid, serum and plama in dominat-aggresive and normal dogs. In Proceedings of the American College of Veterinary Internal Medicine Meeting, Washington, DC, USA; 2000; pp. 101–105. [Google Scholar]
- Ferrari, P.F.; Palanza, P.; Parmigiani, S.; de Almeida, R.M.M.; Miczek, K.A. Serotonin and aggressive behavior in rodents and nonhuman primates: Predispositions and plasticity. Eur. J. Pharmacol. 2005, 526, 259–273. [Google Scholar] [CrossRef]
- Howell, S.; Westergaard, G.; Hoos, B.; Chavanne, T.J.; Shoaf, S.E.; Clevelan, A. Serotonergic influences on life-history outcomes in free-ranging mal rhesus macaques. Am. J. Primatol. 2007, 69, 851–865. [Google Scholar] [CrossRef] [PubMed]
- Mehlman, P.T.; Higley, J.D.; Fauscher, I.; Lilly, A.A.; Taub, D.M.; Vickers, J. Low CSF 5-HIAA concentrations and severe aggression and impaired impulse control in nonhuman primates. Am. J. Psychiatry 1994, 151, 1485–1491. [Google Scholar]
- Stanley, B.; Molcho, A.; Stanley, M.; Winchel, R.; Gameroff, M.J.; Parsons, B.; Mann, J.J. Association of aggressive behavior with altered serotonergic function in patients who are not suicidal. Am. J. Psychiatry 2000, 157, 609–614. [Google Scholar] [CrossRef]
- Sih, A.; Bell, A.; Johnson, J.C. Behavioral syndromes: An ecological and evolutionary overview. Trends Ecol. Evol. 2004, 19, 372–378. [Google Scholar] [CrossRef]
- Alberghina, D.M.; Rizzo, G.; Piccione, C.; Giannetto, M.P. An exploratory study about the association between serum serotonin concentrations and canine-human social interactions in shelter dogs (Canis familiaris). J. Vet. Behav. Clin. Appl. Res. 2017, 18, 96–101. [Google Scholar] [CrossRef]
- Rosado, B.; Garcia-Belenguer, S.; Leon, M.; Chacon, G. Blood concentrations of serotonin, cortisol and dehydroepiandrosterone in aggressive dogs. Appl. Anim. Behav. Sci. 2010, 123, 124–130. [Google Scholar] [CrossRef]
- Alberghina, D.; Rizzo, M.; Piccione, G.; Giannetto, C.; Panzera, M. Serum serotonin (5-HT) in dogs (Canis familiaris): Preanalytical factors and analytical procedure for use of reference values in behavioral medicine. J. Vet. Behav. Clin. Appl. Res. 2019, 32, 72–75. [Google Scholar] [CrossRef]
- Arhant, C.; Bubna-Littitz, H.; Bartels, A.; Futschik, A.; Troxler, J. Behaviour of smaller and larger dogs: Effects of training methods, inconsistency of owner behaviour and level of engagement in activities with the dog. Appl. Anim. Behav. Sci. 2010, 123, 131–142. [Google Scholar] [CrossRef]
- Takeuchi, Y.; Houpt, K.A. Behavior genetics. Clin. Tech. Small Anim. Pract. 2004, 19, 194–204. [Google Scholar] [CrossRef]
- Scott, J.P.; Fuller, J.L. The critical period. In Genetics and the Social Behavior of the Dog; The Classic Study; The University of Chicago Press: Chicago, IL, USA, 1965; pp. 117–182. [Google Scholar]
- Hart, B.L.; Hart, L.A. Selecting pet dogs on the basis of cluster analysis of breed behavior profiles and gender. J. Am. Vet. Med. Assoc. 1985, 186, 1181–1185. [Google Scholar] [PubMed]
- Svartberg, K. Breed-typical behavior in dogs—Fistorical remnants or recent constructs? Appl. Anim. Behav. Sci. 2006, 96, 293–313. [Google Scholar] [CrossRef]
- Duffy, D.L.; Hsu, H.; Serpell, J. Breed differences in canine aggression. Appl. Anim. Behav. Sci. 2008, 114, 441–460. [Google Scholar] [CrossRef]
- De Napoli, J.; Dodman, N. Effect of dietary protein content and tryptophan supplementation on dominance aggression, territorial aggression, and hyperactivity in dogs. J. Am. Vet. Med. Assoc. 2000, 217, 102–105. [Google Scholar]
- Gazzano, A.; Ogi, A.; Torracca, B.; Mariti, C.; Casini, L. Plasma Tryptophan/Large Neutral Amino Acids Ratio in Domestic Dogs Is Affected by a Single Meal with High Carbohydrates Level. Animals. 2018, 8, 63. [Google Scholar] [CrossRef] [PubMed]
- Sechi, S.; Di Cerbo, A.; Canello, S.; Guidetti, G.; Chiavolelli, F.; Fiore, F.; Cocco, R. Effects in dogs with behavioural disorders of a commercial nutraceutical diet on stress and neuroendocrine parameters. Vet. Rec. 2017, 180, 18. [Google Scholar] [CrossRef]
- Riggio, G.; Mariti, C.; Sergi, V.; Diverio, S.; Gazzano, A. Serotonin and Tryptophan Serum Concentrations in Shelter Dogs Showing Different Behavioural Responses to a Potentially Stressful Procedur. Vet. Sci. 2021, 8, 1. [Google Scholar]
- Overall, K.L. Clinical Behavioral Medicine for Small Animals, 1st ed.; Mosby: Mosby, MO, USA, 1997; pp. 88–137. [Google Scholar]
- Sacks, J.J.; Sinclair, L.; Gilchrist, J.; Golab, G.C.; Lockwood, R. Breed of dogs involved in fatal human attacks in The United States between 1979 and 1998. J. Am. Vet. Assoc. 2000, 217, 836–840. [Google Scholar] [CrossRef] [PubMed]
- Reisner, I. An overview of aggression. In Manual of Canine and Feline Behavioural Medicine; British Small Animal Veterinary Association: Waterwells, UK, 2002; pp. 181–194. [Google Scholar]
- Houpt, K.A. Genetics of Canine Behavior. Acta Vet. Brno 2007, 76, 431–444. [Google Scholar] [CrossRef]
- Karpiński, M.; Ognik, K.; Garbiec, A.; Czyżowski, P.; Krauze, M. Effect of Stroking on Serotonin, Noradrenaline, and Cortisol Levels in the Blood of Right- and Left-Pawed Dogs. Animals 2021, 11, 331. [Google Scholar] [CrossRef]
- Mengoli, M.; Oliva, J.L.; Tiago, M.; Chabaud, C.; Arroub, S.; Lafont-Lecuelle, C.; Cozzi, A.; Pageat, P.; Bienboire-Frosini, C. Neurohormonal Profiles of Assistance Dogs Compared to Pet Dogs: What Is the Impact of Different Lifestyles? Animals 2021, 11, 2594. [Google Scholar] [CrossRef] [PubMed]
- Blackwell, E.J.; Twells, C.; Seawright, A.; Casey, R.A. The relationship between training methods and the occurrence of behaviour problems, as reported by owners, in a population of domestic dogs. J. Vet. Behav. Clin. Appl. Res. 2008, 3, 207–217. [Google Scholar] [CrossRef]
- Lit, L.; Schweitzer, J.B.; Iosif, A.M.; Oberbauer, A.M. Owner reports of attention, activity, and impulsivity in dogs: A replication study. Behav. Brain Funct. 2010, 6, 1–10. [Google Scholar] [CrossRef]
- Howell, T.; King, T.; Bennett, P. Puppy parties and beyond: The role of early age socialization practices on adult dog behaviour. Vet. Med. Res. Rep. 2015, 6, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Aldridge, J.E.; Meyer, A.; Seidler, F.J.; Slotkin, T.A. Developmental exposure to terbutaline and chlorpyrifos: Pharmacotherapy of preterm labor and an environmental neurotoxicant converge on serotonergic systems in neonatal rat brain regions. Toxicol. Appl. Pharmacol. 2005, 203, 132–144. [Google Scholar] [CrossRef] [PubMed]
- Chourbaji, S.; Hortnagl, H.; Molteni, R.; Riva, M.A.; Gass, P.; Hellweg, R. The impact of environmental enrichment on sex-specific neurochemical circuitries—Effects on brain-derived neurotrophic factor and the serotonergic system. Neuroscience 2012, 220, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Rayment, D.J.; Petersb, R.A.; Marstona, L.C.; De Groef, B. Relationships between serum serotonin, plasma cortisol, and behavioral factors in a mixed-breed, -sex, and -age group of pet dogs. J. Vet. Behav. 2020, 38, 96–102. [Google Scholar] [CrossRef]
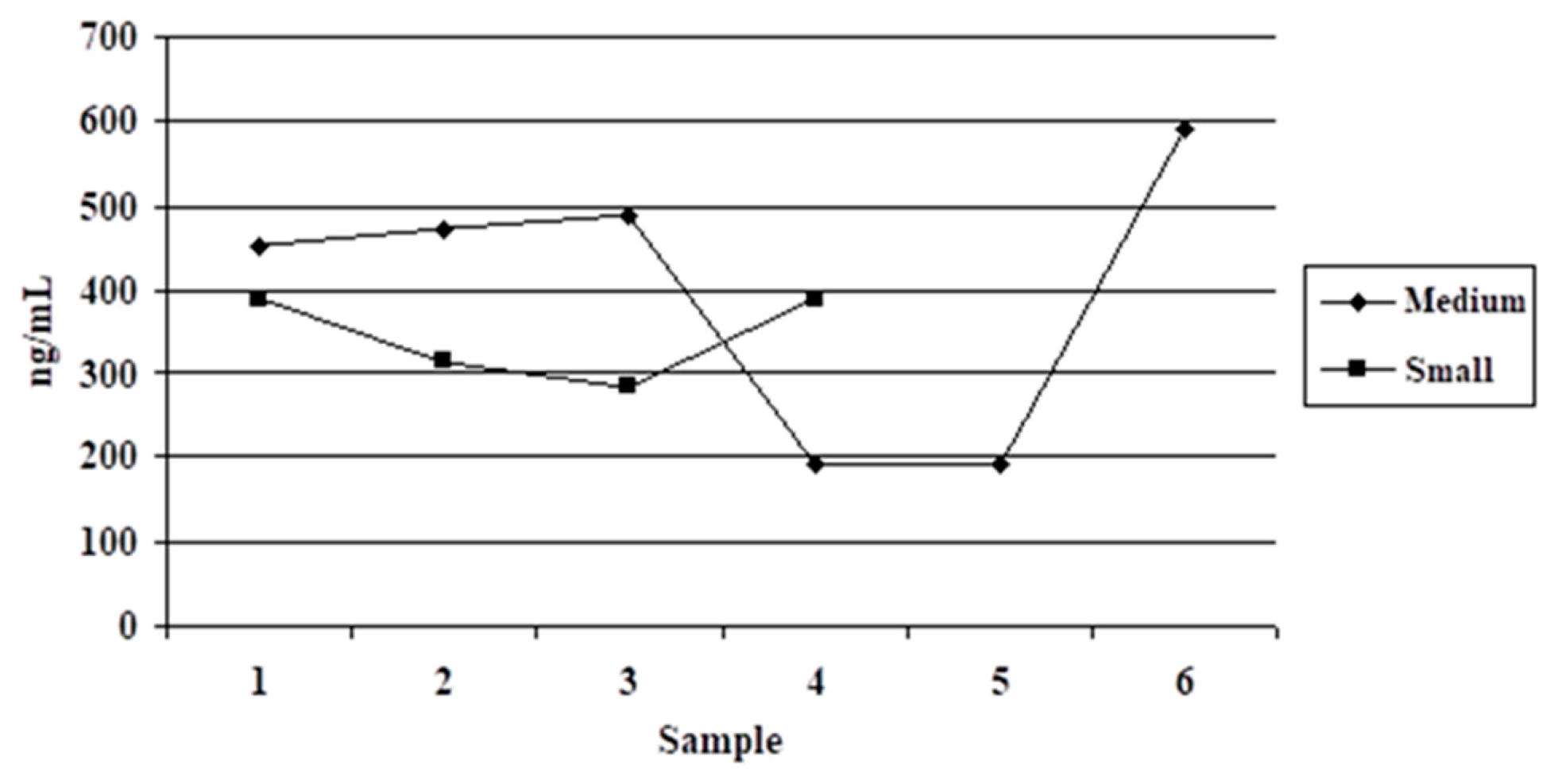
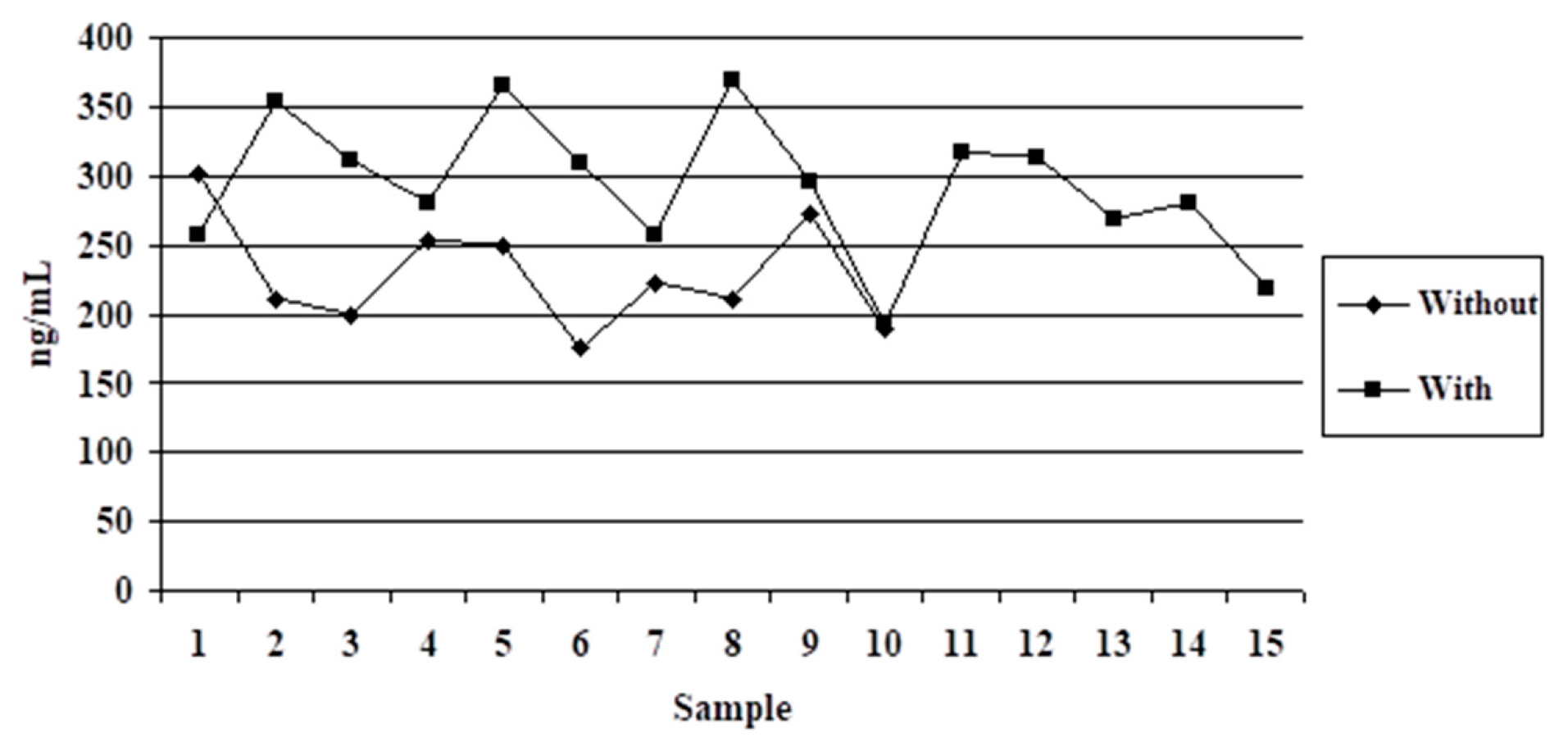
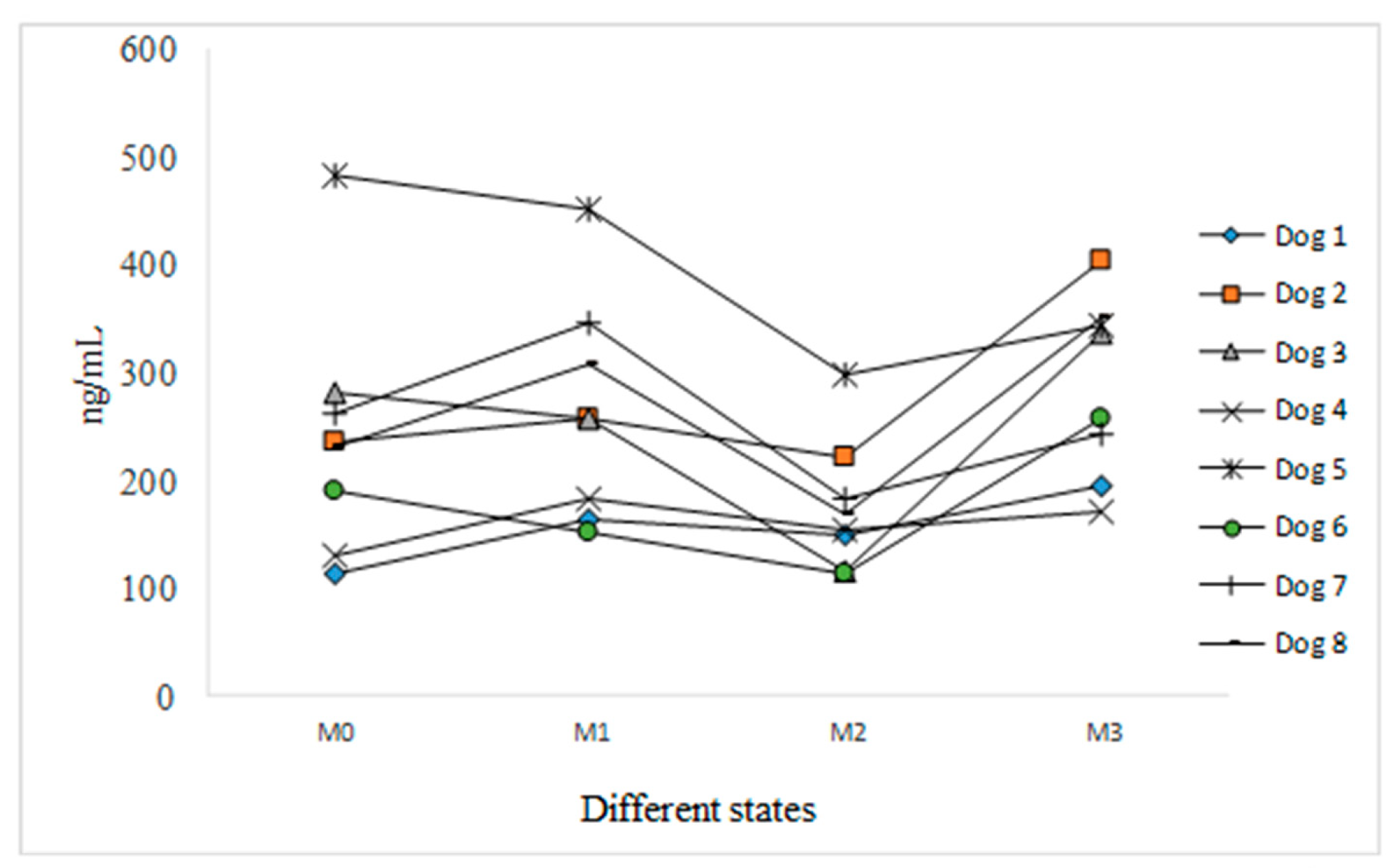
| Medium Size (10–20 kg) Group | Small Size (4–10 kg) Group | Difference | ||||
|---|---|---|---|---|---|---|
| n | X ± SD | CV% | n | X ± SD | CV% | (ng/mL) |
| 6 | 397.62 ± 167.72 | 42.18 | 4 | 343.73 ± 52.45 | 15.26 | 53.89 ns |
| Without Owner | With Owner | Difference | ||||
|---|---|---|---|---|---|---|
| n | X ± SD | CV % | n | X ± SD | CV % | (ng/mL) |
| 10 | 229.04 ± 40.28 | 17.59 | 15 | 292.89 ± 50.29 | 17.17 | 63.85 ** |
| States | Results | |||
|---|---|---|---|---|
| n | X ± SD | CV % | % | |
| M0 | 8 | 240.41 ± 114.92 | 47.80 | 100 |
| M1 (happy) | 8 | 264.32 ± 102.77 | 38.88 | 109.95 |
| M2 (aggressive) | 8 | 174.71 ± 60.91 | 34.86 | 72.67 |
| M3 (calmed by owner) | 8 | 287.49 ± 83.15 | 28.92 | 119.59 |
| Different States | M0 | M1 | M2 | M3 |
|---|---|---|---|---|
| M0 | - | +23.92 ns | −65.69 ns | +47.08 ns |
| M1 | +23.92 ns | - | −89.61 * | +23.17 ns |
| M2 | −65.69 ns | −89.61 * | - | +112.78 ** |
| M3 | +47.08 ns | +23.17 ns | +112.78 ** | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bochiș, T.A.; Imre, K.; Marc, S.; Vaduva, C.; Florea, T.; Dégi, J.; Voia, O.S.; Pop, C.; Ţibru, I. The Variation of Serotonin Values in Dogs in Different Environmental Conditions. Vet. Sci. 2022, 9, 523. https://doi.org/10.3390/vetsci9100523
Bochiș TA, Imre K, Marc S, Vaduva C, Florea T, Dégi J, Voia OS, Pop C, Ţibru I. The Variation of Serotonin Values in Dogs in Different Environmental Conditions. Veterinary Sciences. 2022; 9(10):523. https://doi.org/10.3390/vetsci9100523
Chicago/Turabian StyleBochiș, Timea Andrea, Kálmán Imre, Simona Marc, Cristina Vaduva, Tiana Florea, János Dégi, Octavian Sorin Voia, Călin Pop, and Ioan Ţibru. 2022. "The Variation of Serotonin Values in Dogs in Different Environmental Conditions" Veterinary Sciences 9, no. 10: 523. https://doi.org/10.3390/vetsci9100523
APA StyleBochiș, T. A., Imre, K., Marc, S., Vaduva, C., Florea, T., Dégi, J., Voia, O. S., Pop, C., & Ţibru, I. (2022). The Variation of Serotonin Values in Dogs in Different Environmental Conditions. Veterinary Sciences, 9(10), 523. https://doi.org/10.3390/vetsci9100523








