Serological Evidence of Human Infection with Coxiella burnetii after Occupational Exposure to Aborting Cattle
Abstract
:1. Introduction
2. Materials and Methods
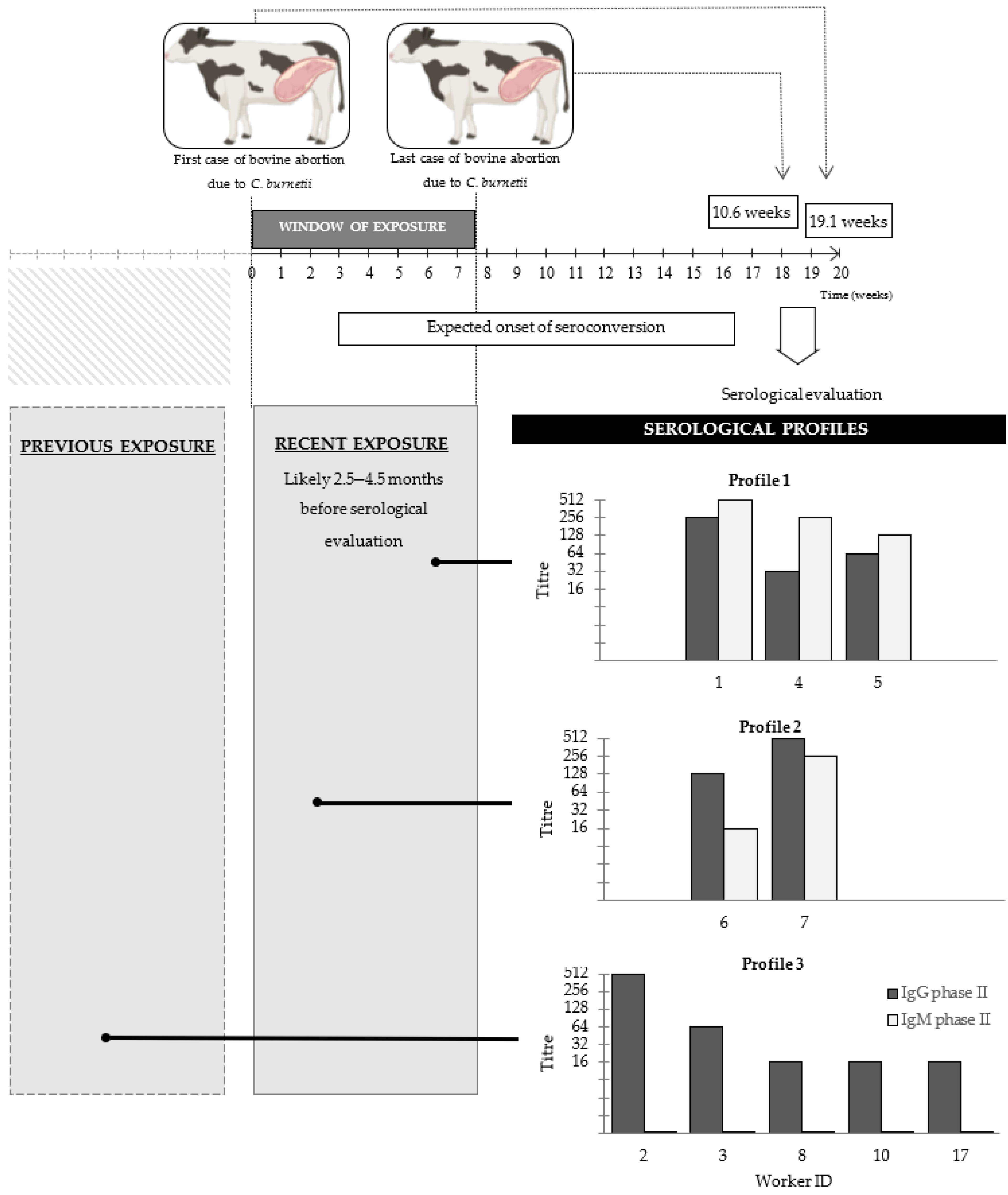
2.1. Bovine Abortions and Window of Workers Exposure
2.2. Farm and Laboratory Workers’ Data and Consent
2.3. Review of Case Records from the Veterinary Diagnostic Laboratory
2.4. Indirect Fluorescent Antibody Test
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marrie, T.J. Epidemiology of Q fever. In Q Fever, The Disease, 2nd ed.; Marrie, T.J., Ed.; CRC Press, Inc.: Boca Raton, FL, USA, 1990; Volume 1, pp. 49–70. [Google Scholar]
- Maurin, M.; Raoult, D.F. Q fever. Clin. Microbiol. Rev. 1999, 12, 518–553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Signs, K.A.; Stobierski, M.G.; Gandhi, T.N. Q fever cluster among raw milk drinkers in Michigan, 2011. Clin. Infect. Dis. 2012, 55, 1387–1389. [Google Scholar] [CrossRef] [Green Version]
- Hatchette, T.F.; Hudson, R.C.; Schlech, W.F.; Campbell, N.A.; Hatchette, J.E.; Ratnam, S.; Raoult, D.; Donovan, C.; Marrie, T.J. Goat-associated Q fever: A new disease in Newfoundland. Emerg. Infect. Dis. 2001, 7, 413–419. [Google Scholar] [CrossRef]
- Maltezou, H.C.; Constantopoulou, I.; Kallergi, C.; Vlahou, V.; Georgakopoulos, D.; Kafetzis, D.A.; Raoult, D. Q fever in children in Greece. Am. J. Trop. Med. Hyg. 2004, 70, 540–544. [Google Scholar] [CrossRef]
- Krumbiegel, E.R.; Wisniewski, H.J. Consumption of infected raw milk by human volunteers. Arch. Environ. Health 1970, 21, 63–65. [Google Scholar] [CrossRef]
- Rabaza, A.; Fraga, M.; Corbellini, L.G.; Turner, K.M.; Riet-Correa, F.; Eisler, M.C. Molecular prevalence of Coxiella burnetii in bulk-tank milk from bovine dairy herds: Systematic review and meta-analysis. One Health 2021, 12, 100208. [Google Scholar] [CrossRef] [PubMed]
- Georgiev, M.; Afonso, A.; Neubauer, H.; Needham, H.; Thiéry, R.; Rodolakis, A.; Roest, H.J.; Stärk, K.D.; Stegeman, J.A.; Vellema, P.; et al. Q fever in humans and farm animals in four European countries, 1982 to 2010. Eurosurveillance 2013, 18, 20407. [Google Scholar] [CrossRef] [PubMed]
- Porter, S.R.; Czaplicki, G.; Mainil, J.; Guatteo, R.; Saegerman, C. Q Fever: Current state of knowledge and perspectives of research of a neglected zoonosis. Int. J. Microbiol. 2011, 11, 248418. [Google Scholar] [CrossRef]
- Tilburg, J.J.; Roest, H.J.I.; Buffet, S.; Nabuurs-Franssen, M.H.; Horrevorts, A.M.; Raoult, D.; Klaassen, C.H.W. Epidemic genotype of Coxiella burnetii among goats, sheep, and humans in the Netherlands. Emerg. Infect. Dis. 2012, 18, 887. [Google Scholar] [CrossRef] [PubMed]
- Roest, H.I.J.; Bossers, A.; Rebel, J.M.J. Q fever diagnosis and control in domestic ruminants. Dev. Biol. 2013, 135, 183–189. [Google Scholar] [CrossRef]
- Cygan, Z.; Buczek, J.; Modzelewska, A.; Guzik, Z. Q-fever diagnosed in dairy cows on the basis of serological tests. Med. Weter. 1983, 39, 536–538. [Google Scholar]
- Mikołajczyk, E.Z.; Lewińska, R.; Lojewska, R.; Rumin, W.; Kruszewska, D. Serologic reaction in humans during the outbreaks of Q fever. Przegląd. Epidemiol. 1986, 40, 342–348. [Google Scholar]
- Hellenbrand, W.; Breuer, T.; Petersen, L. Changing epidemiology of Q fever in Germany, 1947–1999. Emerg. Infect. Dis. 2001, 7, 789–796. [Google Scholar] [CrossRef] [PubMed]
- Chmielewski, T.; Tylewska-Wierzbanowska, S. Q fever outbreaks in Poland during 2005–2011. Med. Sci. Mon. Int. Med. J. Exp. Clin. Res. 2013, 19, 1073–1079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hechemy, K.E. History and prospects of Coxiella burnetii research. In Coxiella burnetii: Recent Advances and New Perspectives in Research of the Q Fever Bacterium, 2nd ed.; Toman, R., Heinzen, R.A., Samuel, J.E., Mege, J.L., Eds.; Springer: New York, NY, USA, 2012; pp. 1–11. [Google Scholar]
- Raoult, D.; Marrie, T.J.; Mege, J.L. Natural history and pathophysiology of Q fever. Lancet Infect. Dis. 2005, 5, 219–226. [Google Scholar] [CrossRef]
- Raoult, D.; Tissot-Dupont, H.; Foucault, C.; Gouvernet, J.; Fournier, P.E.; Bernit, E.; Stein, A.; Nesri, M.; Harle, J.R.; Weiller, P.J. Q fever 1985–1998. Clinical and epidemiologic features of 1383 infections. Medicine 2000, 79, 109–123. [Google Scholar] [CrossRef] [PubMed]
- Fenollar, F.; Fournier, P.E.; Carrieri, M.P.; Habib, G.; Messana, T.; Raoult, D. Risks factors and prevention of Q fever endocarditis. Clin. Infect. Dis. 2001, 33, 312–316. [Google Scholar] [CrossRef]
- Landais, C.; Fenollar, F.; Thuny, F.; Raoult, D. From acute Q fever to endocarditis: Serological follow-up strategy. Clin. Infect. Dis. 2007, 44, 1337–1340. [Google Scholar] [CrossRef] [Green Version]
- Dupuis, G.; Péter, O.; Peacock, M.; Burgdorfer, W.; Haller, E. Immunoglobulin responses in acute Q fever. J. Clin. Microbiol. 1985, 22, 484–487. [Google Scholar] [CrossRef] [Green Version]
- Tozer, S.J.; Lambert, S.B.; Sloots, T.P.; Nissen, M.D. Q fever sero-prevalence in metropolitan samples is similar to rural/remote samples in Queensland, Australia. Eur. J. Clin. Microbiol. 2011, 30, 1287–1293. [Google Scholar] [CrossRef] [PubMed]
- Dal Pozzo, F.; Martinelle, L.; Léonard, P.; Renaville, B.; Renaville, R.; Thys, C.; Smeets, F.; Czaplicki, G.; Van Esbroeck, M.; Saegerman, C. Q Fever serological survey and associated risk factors in veterinarians, southern Belgium, 2013. Transbound. Emerg. Dis. 2017, 64, 959–966. [Google Scholar] [CrossRef] [Green Version]
- Eldin, C.; Melenotte, C.; Mediannikov, O.; Ghigo, E.; Million, M.; Edouard, S.; Mege, J.L.; Maurin, M.; Raoult, D. From Q Fever to Coxiella burnetii infection: A paradigm change. Clin. Microbiol. Rev. 2017, 30, 115–190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fournier, P.E.; Marrie, T.J.; Raoult, D. Diagnosis of Q Fever. J. Clin. Microbiol. 1998, 36, 1823–1834. [Google Scholar] [CrossRef] [Green Version]
- Tissot-Dupont, H.; Thirion, X.; Raoult, D. Q fever serology: Cut off determination for microimmunofluorescence. Clin. Vaccine Immunol. 1994, 1, 189–196. [Google Scholar] [CrossRef]
- Macías-Rioseco, M.; Riet-Correa, F.; Miller, M.M.; Sondgeroth, K.; Fraga, M.; Silveira, C.; Uzal, F.A.; Giannitti, F. Bovine abortion caused by Coxiella burnetii: Report of a cluster of cases in Uruguay and review of the literature. J. Vet. Diagn. Invest. 2019, 31, 634–639. [Google Scholar] [CrossRef]
- Edlinger, E. Immunofluorescence serology: A tool for prognosis of Q-fever. Diagn. Micr. Infc. Dis. 1985, 3, 343–351. [Google Scholar] [CrossRef]
- Wegdam-Blans, M.C.A.; Wielders, C.C.H.; Meekelenkamp, J.; Korbeeck, J.M.; Herremans, T.; Tjhie, H.T.; Bijilmer, H.A.; Koopmans, M.P.G.; Schneeberger, P.M. Evaluation of commonly used serological tests for detection of Coxiella burnetii antibodies in well-defined acute and follow-up sera. Clin. Vaccine Immunol. 2012, 19, 1110–1115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- RStudio Team. RStudio: Integrated Development for R. RStudio; PBC: Boston, MA, USA, 2020; Available online: http://www.rstudio.org/ (accessed on 18 November 2020).
- McQuiston, J.H.; Childs, J.E.; Thompson, H.A. Q fever. J. Am. Vet. Med. Assoc. 2002, 221, 796–799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Todkill, D.; Fowler, T.; Hawker, J.I. Estimating the incubation period of acute Q fever, a systematic review. Epidemiol. Infect. 2018, 146, 665–672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fournier, P.E.; Raoult, D. Comparison of PCR and serology assays for early diagnosis of acute Q fever. J. Clin. Microbiol. 2003, 41, 5094–5098. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tissot-Dupont, H.; Amadei, M.A.; Nezri, M.; Raoult, D. Wind in November, Q fever in December. Emerg. Infect. Dis. 2004, 10, 1264–1269. [Google Scholar] [CrossRef] [PubMed]
- Wielders, C.C.; van Loenhout, J.A.; Morroy, G.; Rietveld, A.; Notermans, D.W.; Wever, P.C.; Renders, N.H.M.; Leenders, A.C.A.P.; van der Hoek, W.; Schneeberger, P.M. Long-term serological follow-up of acute Q-fever patients after a large epidemic. PLoS ONE 2015, 10, e0131848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guigno, D.; Coupland, B.; Smith, E.G.; Farrell, I.D.; Desselberger, U.; Caul, E.O. Primary humoral antibody response to Coxiella burnetii, the causative agent of Q fever. J. Clin. Microbiol. 1992, 30, 1958–1967. [Google Scholar] [CrossRef] [Green Version]
- Guatteo, R.; Beaudeau, F.; Joly, A.; Seegers, H. Coxiella burnetii shedding by dairy cows. Vet. Res. 2007, 38, 849–860. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leone, M.; Honstettre, A.; Lepidi, H.; Capo, C.; Bayard, F.; Raoult, D.; Mege, J.L. Effect of sex on Coxiella burnetii infection: Protective role of 17β-estradiol. J. Infect. Dis. 2004, 189, 339–345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pape, M.; Bouzalas, E.G.; Koptopoulos, G.S.; Mandraveli, K.; Arvanitidou-Vagiona, M.; Nikolaidis, P. The serological prevalence of Coxiella burnetii antibodies in sheep and goats in northern Greece. Clin. Microbiol. Infect. 2009, 15, 146–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bae, M.; Jin, C.E.; Park, J.H.; Kim, M.J.; Chong, Y.P.; Lee, S.O.; Choi, S.H.; Kim, Y.S.; Woo, J.H.; Shin, Y.; et al. Diagnostic usefulness of molecular detection of Coxiella burnetii from blood of patients with suspected acute Q fever. Medicine 2019, 98, e15724. [Google Scholar] [CrossRef] [PubMed]
- Hartzell, J.D.; Marrie, T.J.; Raoult, D. Coxiella burnetii (Q fever). In Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases, 9th ed.; Bennett, J.E., Dolin, R., Blaser, M.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 2360–2367. [Google Scholar]
- Marrie, T.J.; Pollak, P.T. Seroepidemiology of Q fever in Nova Scotia: Evidence for age dependent cohorts and geographical distribution. Eur. J. Epidemiol. 1995, 11, 47–54. [Google Scholar] [CrossRef]
- Salveraglio, F.J.; Bacigalupi, J.C.; Srulevich, S.; Viera, G. Comprobación epidemiológica y clínica de la fiebre Q en el Uruguay. An. Fac. Med. Montev. 1956, 41, 131–138. [Google Scholar]
| Worker ID | Age Range (Years) | Gender | Type of Work | Exposure Category | IgG Phase II Titre * | IgM Phase II Titre * | Phase II IgG/IgM Ratio | Symptomatic † |
|---|---|---|---|---|---|---|---|---|
| 1 | 41–50 | M | Bacteriologist | Laboratory | 1/256 | 1/512 | 0.5 | Yes |
| 2 | 21–30 | F | Veterinary diagnostician | Farm and laboratory | 1/512 | <1/16 | - | Yes |
| 3 | 21–30 | F | Veterinary diagnostician | Farm and laboratory | 1/64 | <1/16 | - | Yes |
| 4 | 31–40 | F | Veterinary diagnostician | Farm and laboratory | 1/32 | 1/256 | 0.1 | No |
| 5 | 31–40 | F | Farm veterinarian | Farm | 1/64 | 1/128 | 0.5 | Yes |
| 6 | 31–40 | M | Veterinary diagnostician | Farm and laboratory | 1/128 | 1/16 | 8 | Yes |
| 7 | 31–40 | M | Laboratory technician | Farm and laboratory | 1/512 | 1/256 | 2 | No |
| 8 | 31–40 | F | Veterinary diagnostician | Laboratory | 1/16 | <1/16 | - | No |
| 9 | 41–50 | M | Farmworker | Farm | <1/16 | <1/16 | - | No |
| 10 | 21–30 | M | Farmworker | Farm | 1/16 | <1/16 | - | Yes |
| 11 | 31–40 | F | Laboratory technician | Laboratory | <1/16 | <1/16 | - | Yes |
| 12 | 61–70 | M | Farmworker | Farm | <1/16 | <1/16 | - | No |
| 13 | 21–30 | M | Farmworker | Farm | <1/16 | <1/16 | - | Yes |
| 14 | 21–30 | M | Farm veterinarian | Farm | <1/16 | <1/16 | - | Yes |
| 15 | 31–40 | M | Farmworker | Farm | <1/16 | <1/16 | - | Yes |
| 16 | 51–60 | M | Farmworker | Farm | <1/16 | <1/16 | - | Yes |
| 17 | 21–30 | M | Farmworker | Farm | 1/16 | <1/16 | - | No |
| 18 | 41–50 | M | Farmworker | Farm | <1/16 | <1/16 | - | Yes |
| 19 | 31–40 | F | Farmworker | Farm | <1/16 | <1/16 | - | No |
| 20 | 51–60 | M | Farmworker | Farm | <1/16 | <1/16 | - | No |
| 21 | 41–50 | M | Farmworker | Farm | <1/16 | <1/16 | - | No |
| 22 | 31–40 | F | Veterinary diagnostician | Farm and laboratory | <1/16 | <1/16 | - | Yes |
| 23 | 31–40 | M | Veterinary diagnostician | Farm and laboratory | <1/16 | <1/16 | - | No |
| 24 | 21–30 | M | Veterinary diagnostician | Farm and laboratory | <1/16 | <1/16 | - | Yes |
| 25 | 51–60 | M | Farmworker | Farm | <1/16 | <1/16 | - | No |
| 26 | 41–50 | M | Farmworker | Farm | <1/16 | <1/16 | - | Yes |
| 27 | 21–30 | F | Laboratory technician | Laboratory | <1/16 | <1/16 | - | Yes |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rabaza, A.; Giannitti, F.; Fraga, M.; Macías-Rioseco, M.; Corbellini, L.G.; Riet-Correa, F.; Hirigoyen, D.; Turner, K.M.E.; Eisler, M.C. Serological Evidence of Human Infection with Coxiella burnetii after Occupational Exposure to Aborting Cattle. Vet. Sci. 2021, 8, 196. https://doi.org/10.3390/vetsci8090196
Rabaza A, Giannitti F, Fraga M, Macías-Rioseco M, Corbellini LG, Riet-Correa F, Hirigoyen D, Turner KME, Eisler MC. Serological Evidence of Human Infection with Coxiella burnetii after Occupational Exposure to Aborting Cattle. Veterinary Sciences. 2021; 8(9):196. https://doi.org/10.3390/vetsci8090196
Chicago/Turabian StyleRabaza, Ana, Federico Giannitti, Martín Fraga, Melissa Macías-Rioseco, Luis G. Corbellini, Franklin Riet-Correa, Darío Hirigoyen, Katy M. E. Turner, and Mark C. Eisler. 2021. "Serological Evidence of Human Infection with Coxiella burnetii after Occupational Exposure to Aborting Cattle" Veterinary Sciences 8, no. 9: 196. https://doi.org/10.3390/vetsci8090196
APA StyleRabaza, A., Giannitti, F., Fraga, M., Macías-Rioseco, M., Corbellini, L. G., Riet-Correa, F., Hirigoyen, D., Turner, K. M. E., & Eisler, M. C. (2021). Serological Evidence of Human Infection with Coxiella burnetii after Occupational Exposure to Aborting Cattle. Veterinary Sciences, 8(9), 196. https://doi.org/10.3390/vetsci8090196







