Abstract
Canine degenerative myelopathy (DM), recognized as a spontaneous model of amyotrophic lateral sclerosis, is known as a late-onset progressive degenerative disease of the spinal cord. Because of the progressive nature of DM, many dogs are elected to be euthanized, resulting in limited information on the end-stage clinical presentation. We investigated the long-term clinical course from diagnosis to natural death to further deepen our understanding of the entire clinical picture of this disease. Because curcumin was administered in some cases, the therapeutic effect of curcumin on DM was also examined. Forty dogs included in this study were client-owned Pembroke Welsh Corgis with a definitive diagnosis of DM by necropsy and histopathology. Dogs were excluded from this study if they died from another disease or were elected to be euthanized. Information on the long-term clinical symptoms of DM was investigated based on a questionnaire, which was collected from the dog owners. Urinary incontinence and respiratory disorder were observed in most dogs, as was respiratory impairment-correlated death. In contrast, signs consistent with brainstem dysfunction were noticed at the terminal stage in a small portion of dogs. Although further studies with more cases are needed, the results of this study suggest that administration of curcumin is effective in slowing the progression of DM.
1. Introduction
Canine degenerative myelopathy (DM) is a slowly progressive fatal neurodegenerative disorder that affects the spinal cord [1]. A previous study reported that DM was caused by mutations in the gene encoding Cu/Zn superoxide dismutase 1 (SOD1), a key antioxidant enzyme [2]. Further, DM-affected dogs are homozygous for the A allele of a SOD1 missense mutation, SOD1: c.118G>A and c.52A>T, which predicts a p.E40K and T18S amino acid substitution [2,3]. DM has been recently considered a spontaneous model of familial amyotrophic lateral sclerosis (ALS) [4]. ALS is a neurodegenerative disorder characterized by progressive muscle weakness, and 20% of patients with familial ALS have SOD1 gene mutations similar to those found in DM-affected dogs [5,6,7]. In DM and ALS, the exact molecular mechanisms underlying mutant SOD1-mediated neurodegeneration are unclear. Collectively, study results suggest that the cytotoxicity to motor neurons appears to result from a gain of toxic SOD1 function [7,8,9,10], although it has also been proposed that loss-of-function might play a modifying role in ALS [11].
The clinical signs of DM initially appear in the pelvic limbs as spastic upper motor neuron (UMN) paresis and general proprioceptive ataxia, which eventually progresses to flaccid tetraplegia [1]. Furthermore, affected dogs exhibit respiratory muscle disorder and respiratory dysfunction [12,13]. It is important to understand the long-term clinical course of DM in dogs to inform their symptomatic treatment by veterinarians. Understanding the long-term clinical course of DM in dogs could also determine their usefulness as an animal model for ALS in humans. This has not previously been documented in detail, largely because most dogs with DM are elected to be euthanized [14].
Although DM affects many dog breeds [15], an increasing number of Pembroke Welsh Corgis (PWCs) have been affected by this disease in Japan [16]. The increased prevalence of DM in PWCs is attributed to the high SOD1 mutation rate and the large number of animals raised. In addition, PWCs are easier to handle than other predisposed large breed dogs, allowing many owners to provide appropriate care for a longer duration. In the present study, we investigated the long-term clinical course, in particular the incidence and onset of clinical symptoms other than gait abnormality, in 40 DM-affected PWCs from diagnosis to natural death to deepen our understanding of the entire clinical presentation of this disease. In addition, some included cases received curcumin, which is effective for treating ALS [17]. Therefore, the effects of curcumin on DM were also investigated in this study.
2. Materials and Methods
2.1. Case Recruitment
We recruited dogs with DM through our website on the condition that their owner or attending physician allowed an autopsy of their dog and completed a questionnaire. Our study group included six dogs that attended our hospital after receiving required permissions. Data on these cases were collected from medical records. All the dogs included in this study were client-owned PWCs with a definitive diagnosis of DM by necropsy and histopathology at the Animal Medical Center of the Joint Department of Veterinary Medicine, Gifu University. All dogs were submitted for a necropsy by their owners or their veterinary clinics with the owner’s permission. Histopathological diagnosis was made by veterinary pathologists (HS and JS) using spinal cord sections. For all dogs, SOD1 genotypes were determined by real-time polymerase chain reaction as described in a previous study [16]. For inclusion, cases were required to satisfy the criteria that a neurological clinical manifestation should have been present and the owners should provide responses to a questionnaire detailing their dog’s clinical history. Dogs were excluded from this study if they died from another disease or were elected to be euthanized.
2.2. Clinical Symptoms and Treatment
Information was obtained from a questionnaire mainly consisting of items related to clinical symptoms. The following information was obtained via the questionnaire: sex, age of onset, disease duration (from onset to death), and presence and onset of clinical manifestations (Appendix A). Questionnaire data such as the clinical symptoms of the six dogs that visited our hospital were obtained from medical records with the permission of the owners. Eight of the 40 cases had been administered an oral supplement containing curcumin (Neuroact, Veterinarian Medical Development Company Limited, Saitama, Japan). The curcumin dose was approximately 13 mg, which was administered in 1 to 2 divided doses. Their outcomes were compared to the disease progression of cases with no curcumin supplementation. Continuous variables are reported as the median and range.
2.3. Statistical Analyses
The Spearman rank-order correlation was used to evaluate the relationship between the onset of dyspnea and survival time. The median age at onset of DM and the onset timing of each symptom were compared across the two treatment groups (curcumin versus control) using the Mann–Whitney U-test. The Kaplan–Meier survival curve, together with a log-rank test, was used to compare the overall survival of the two treatment groups. The confidence interval was set to 95%, and significance was set at p < 0.05. All statistical analyses were performed with the statistical software program (EZR, Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface for a certain software (R, The R Foundation for Statistical Computing, Vienna, Austria).
3. Results
3.1. Cases
In total, 40 dogs were included in this study. Among them, 26 dogs were male, 21 of which were castrated, and 14 dogs were female, nine of which were spayed. The median age of onset was 10 years and eight months old (range: seven years and seven months to 14 years old). The median age at death was 13 years and nine months old (range: 10 years and three months to 16 years and one month old), and the median survival time (date of symptom onset until date of death) was 36 months (range: 18 to 52 months). All dogs were homozygous for the c.118G>A mutation in the SOD1 gene.
3.2. Clinical Symptom
Without exception, all cases initially presented with progressive generalized proprioceptive ataxia of the pelvic limbs as spastic UMN paresis, which eventually progressed to flaccid tetraplegia. The time course for gait impairment among the 40 dogs with DM is shown in Figure 1. Furthermore, the incidence and onset of symptoms other than gait disturbance is shown in Table 1 and Appendix B. Urinary incontinence was evident in 31 dogs, and 17 of these 31 dogs also showed fecal incontinence. Fecal incontinence was observed simultaneously or subsequent to the onset of urinary incontinence. Eleven dogs showed dysphonia, in particular hoarseness, but the timing of onset differed substantially between individuals. Four cases exhibited dysphagia, and three of four dogs died within two months of the onset of dysphagia; the remaining dog survived for seven months after the onset of dysphagia. Tongue spasms occurred in five dogs. One dog demonstrated a decline in the perception of the face, which suggested trigeminal nerve paralysis. Another dog had loss of facial movement, which indicated facial nerve paralysis. Tongue spasms and facial and trigeminal nerve paralysis arose in the patients who lived longer than the median survival time.
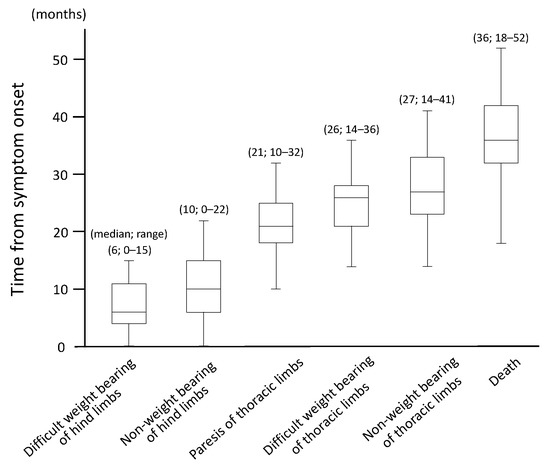
Figure 1.
Time course of gait impairment in 40 dogs with degenerative myelopathy.

Table 1.
Prevalence and time from symptom onset other than gait disorder.
Dyspnea was recognized in a high percentage of the dogs observed (34 of 40 dogs; 85.0%). The median survival time from the onset of dyspnea was 1.5 months (range: 0–22 months), and 11 out of the 34 dogs died within one month of the onset of dyspnea. Meanwhile, five of 34 cases lived more than a year after the onset of dyspnea. The correlation coefficient between the onset of dyspnea and survival time was 0.76 (p < 0.0001; Figure 2), suggesting that respiratory impairment was correlated with death. Symptomatic treatment such as oxygen inhalation was provided in these cases when deemed necessary, as judged by their regular veterinarian.
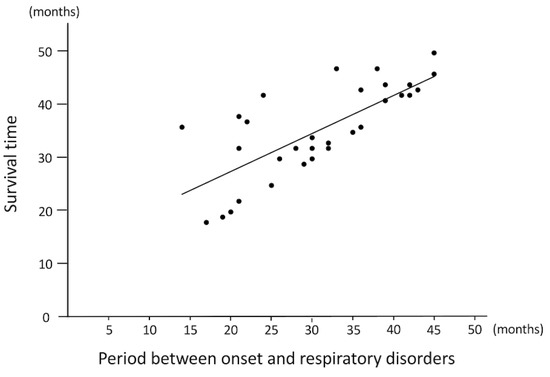
Figure 2.
The relationship between the onset of dyspnea and survival time (n = 34).
All cases first exhibited progressive paresis of the pelvic limbs, which eventually progressed to flaccid tetraplegia.
These results suggest that the onset of dyspnea is correlated with survival time (R = 0.72, p < 0.0001).
3.3. Treatment
Dogs in the curcumin-treated group included one intact and four neutered males and one intact and two spayed females. No adverse events associated with treatment were observed throughout the study. The dogs treated with curcumin had a significantly longer survival time than those who had not received treatment (Figure 3); Table 2 compares the disease progression of DM in dogs in the curcumin-treated and control groups. The timing of the onset of thoracic limb paresis was not significantly different between the curcumin and control groups; however, nonweight bearing of the hind/thoracic limbs was prolonged in the curcumin-treated dogs when compared with the control dogs. Owing to the small number of cases presenting clinical symptoms other than gait disturbance, analyzing whether there was a difference between the curcumin-treated and control groups was not possible. However, as respiratory disorder was a common symptom, we compared the timing of respiratory disorder onset between the two groups but found no significant difference.
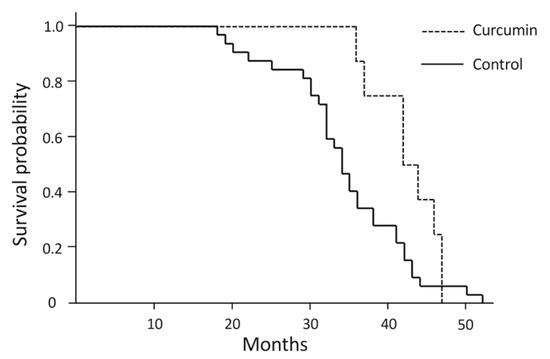
Figure 3.
A Kaplan–Meyer survival curve for curcumin-treated and control dogs.

Table 2.
Comparison of disease progression of degenerative myelopathy between curcumin-treated and control dogs.
The survival time of the curcumin-treated group (n = 8) was significantly longer than that of the control group (n = 32; p = 0.04 by log-rank test).
4. Discussion
Canine DM is a fatal disease, hence almost all dogs in previous studies characterizing its clinical evolution were euthanized [1,14]. Therefore, to date, the median survival time and the later clinical course of DM have not been documented in detail. This study is the first report indicating the natural clinical course of DM. The age of disease onset and sex distribution of PWC dogs included in this study was not notably different from those of previous studies [1,14,15]. One previous study demonstrated that the total duration of clinical signs before euthanasia was 19 months [14], whereas the present study exhibited a median survival time of 36 months.
For all DM cases, symptoms began as hind limb weakness, with the affected dogs eventually developing tetraplegia; the symptoms of patients with ALS tend to vary depending on the type of gene mutation [18]. Dogs with DM have an SOD1 missense mutation (E40K or T18S), and all cases in this study only exhibited an E40K mutation; therefore, all cases may have shown similar symptoms. The clinical progression of DM was different across patients, but the progression duration of gait abnormality was similar to that of a previous description [1].
In dogs, DM leads to dysfunction of the cranial nerve, and urinary and fecal incontinence [1]; however, the timing of onset of these clinical signs has not been characterized. This study indicated that urinary and fecal incontinence was present in a high percentage of dogs with DM, which was similar to what was noted in patients with ALS [19]. The regulation of urination and defecation involves a complex neural control system in the brain, spinal cord, and peripheral autonomic neurons [20]. In dogs with DM, spinal cord lesions spread in ascending and descending orders from the caudal thoracic spinal cord [21], leading to the development of urinary and fecal incontinence. In this study, fecal incontinence had a lower incidence than urinary incontinence. Since the peripheral nervous system of the alimentary tract is more developed and autonomic than that of the urinary tract, automatic peristalsis may have occurred in many dogs with DM. Although this survey did not investigate how care was taken for incontinence, daily compression urination is required for cases with urinary incontinence. In addition, urinary and fecal incontinence induces dermatitis, making it necessary to instruct the owner to keep the skin clean. Furthermore, because a urinary tract infection is likely to occur in cases with incontinence, antibiotic treatment should be considered necessary.
Some dogs with DM presented symptoms of brainstem disorder, such as dysphonia, dysphagia, tongue spasms, and facial and trigeminal nerve paralysis. The nuclei of cranial nerve neurons in the medulla oblongata participate in swallowing, vocalization, movement of the tongue and face, and the sense of touch. As such, we expected that these symptoms would simultaneously develop; however, dysphonia was shown earlier than the other brainstem disorder symptoms. The action of barking requires the movement of the larynx and vibration of the vocal cords by breathing; thus, respiratory dysfunction may have been related to the early expression of dysphonia.
In patients with ALS, death is usually caused by respiratory failure owing to the loss of motor neurons supplying innervation to the diaphragm and chest wall muscles [22]. Moreover, dogs affected by DM are known to exhibit respiratory muscle disorders and respiratory dysfunction [12,13]. Our results demonstrate that respiratory dysfunction occurs in many dogs with DM, and this is the first report to indicate a relationship between the survival time and breathing impairment of dogs with DM.
Various therapeutic protocols have been tested as interventions to slow the disease progression of ALS. Many studies have developed novel therapeutic agents for ALS using rodent models; however, they have had poor therapeutic success when translated to human patients with ALS. It was presumed that the poor translation of the therapies was due to these ALS transgenic animals overexpressing specific human gene mutations [23]. Compared with the SOD1 rodent models, dogs with DM are more similar to ALS patients in terms of the size, structure, and complexity of their nervous systems as well as in terms of the duration of the disease. For these reasons, dogs with DM can be considered a good disease model for the evaluation of potential therapeutic interventions for ALS [4].
We found that dogs with DM treated with curcumin had a significantly longer survival time than those who did not receive this treatment. Curcumin is a biologically potent polyphenolic compound that has various effects on the central nervous system, including neuroprotective effects such as antioxidant and anti-inflammatory activities [24]. Furthermore, a previous study suggested that curcumin molecules bound strongly to the aggregation-prone regions of the mutant SOD1 proteins and blocked the exposed aggregation site, leading to the inhibition of the formation of SOD1 unstructured aggregates [25]. In addition, there are three known beneficial effects of curcumin on muscles: (1) promotion of protein synthesis; (2) reduction of muscle protein degradation; and (3) reduction of exercise muscle injuries [26,27]. There were no statistically significant differences in the duration from onset to the paresis of thoracic limbs in the curcumin and control groups. Nevertheless, the duration from onset to the nonweight bearing of hind/thoracic limbs was longer in dogs from the curcumin group. This suggests that curcumin may be efficient in preventing neurodegeneration and muscle atrophy in dogs with DM. Overall, the results of this study suggest that the administration of curcumin has a neuroprotective and muscle-protective potential against DM, and perhaps against ALS as well. However, dog owners who choose to administer curcumin to their dogs, despite the unproven effectiveness of curcumin in DM, may be more engaged in conservative care such as physical therapy. Therefore, extended survival may be because of the dog owner’s approach to care and not because of the therapeutic effects of curcumin. Furthermore, curcumin possess anti-inflammatory and antioxidant effects on different organs other than the nerves and muscles [28]. Therefore, improvement in motor function may be attributed to the effect of curcumin on joints [29] rather than to the pathology of DM itself.
This study had several limitations. First, the number of available treatment cases was small, thereby weakening the statistical analyses and increasing the chance of errors. Second, this study was retrospective in nature, and there were likely a variety of disease management practices employed by the dog owners. In terms of DM supportive therapy, only daily intensive physiotherapy has demonstrated some benefit, leading to a longer survival and longer maintenance of ambulation when compared to patients who received moderate physiotherapy or no physiotherapy [30]. In our study, the curcumin-treated group may have received more physical therapy and routine care than the control group. For example, aggressive physiotherapy moderates the decline in gait function, and appropriate repositioning for quadriplegic dogs prevents pressure ulcers and reduces the likelihood of aspiration. Similarly, if careful observation by the owner reveals respiratory abnormalities in the affected dog, oxygen replacement therapy can be immediately considered, which leads to a more effective respiratory function. Thus, the response of the owner, rather than curcumin administration, may have prolonged the survival of the curcumin-treated group. Furthermore, the time of onset of DM and time from symptom onset are subjective judgments by the owner and may therefore be affected by the personality of the owner and home environment. Finally, the blood concentration of curcumin was not uniform across dogs with DM, suggesting the possibility of differences in bioavailability. Furthermore, other neuroprotective substances, in addition to curcumin, may have contributed to or protected against the progression of DM. Additional prospective studies, with uniform conditions, are required to confirm the effectiveness of curcumin.
5. Conclusions
This study has improved the characterization of the end-stage clinical presentation of DM in dogs, particularly urinary and fecal incontinence, brainstem disorders, and dyspnea. Dyspnea may be related to the death of dogs with DM as well as that of patients with ALS. The characterization of longitudinal changes in the clinical presentation of DM will assist veterinary practitioners and owners in recognizing when and how to provide appropriate care to DM-affected dogs and will be helpful in determining target therapies.
Author Contributions
Conceptualization, Y.K. and H.K.; Formal Analysis, Y.K., K.N., H.S., J.S., O.Y. and M.S.I.; Data Curation, Y.K., S.T. and N.N.; Writing—Original Draft Preparation, Y.K.; Writing—Review & Editing, N.N. and H.K.; Supervision, S.M. and H.K.; Funding acquisition, Y.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by JSPS KAKENHI Grant Number JP19K15978 and Veterinarian Medical Development Company Limited.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Informed consent was obtained from the dog owners.
Data Availability Statement
All datasets obtained and analyzed during the experiment are available upon reasonable request from the respective author.
Acknowledgments
The authors would like to acknowledge Harumi Kamishina, Naho Yoshida, and Saeko Asada for their excellent technical assistance as well as the veterinary general practitioners and pet owners for their gracious collaboration.
Conflicts of Interest
The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Appendix A
The content of the questionnaire.
- Profile of the case
- Age at onset
- Disease duration (from onset to death)
- Sex
- Dysfunction of gait
- The time from symptom onset to difficult weight bearing of hind limbs
- The time from symptom onset to nonweight bearing of hind limbs
- The time from symptom onset to paresis of thoracic limbs
- The time from symptom onset to difficult weight bearing of thoracic limbs
- The time from symptom onset to nonweight bearing of thoracic limbs
- The other abnormality
- The time from symptom onset to urinary disorder
- The time from symptom onset to fecal disorder
- The time from symptom onset to respiratory disorder
- Other clinical symptoms (If your dog has other symptoms, please provide details of the symptoms and the time from symptom onset.)
- Survival time
- The time from symptom onset to death
- Treatment (If your dog was being treated, please fill this out).
Appendix B

Table A1.
Details of clinical symptoms and treatment for each case.
Table A1.
Details of clinical symptoms and treatment for each case.
| No | Survival Time (Month) | Age at Death | Symptoms Other Than Gait Disorder | Administration of Curcumin |
|---|---|---|---|---|
| 1 | 25 | 15 years 6 months | Respiratory, Urinary, Fecal | |
| 2 | 50 | 14 years 2 months | Respiratory | |
| 3 | 34 | 14 years | Urinary, Fecal | |
| 4 | 22 | 14 years | Respiratory, | |
| 5 | 32 | 10 years 3 months | Respiratory, Urinary | |
| 6 | 33 | 14 years 11 months | Respiratory, Dysphonia | |
| 7 | 42 | 15 years 9 months | Respiratory, Urinary, Fecal | |
| 8 | 30 | 14 years 6 months | Respiratory, Urinary | |
| 9 | 52 | 15 years 9 months | Urinary | |
| 10 | 42 | 12 years 3 months | Respiratory | |
| 11 | 34 | 13 years | Respiratory, Urinary, Fecal | |
| 12 | 34 | 13 years 6 months | Urinary, Dysphagia | |
| 13 | 31 | 12 years 7 months | Urinary | |
| 14 | 36 | 15 years 4 months | Respiratory, Dysphonia | |
| 15 | 42 | 12 years 3 months | Respiratory, Urinary, Fecal, Dysphonia | curcumin |
| 16 | 36 | 13 years 4 months | Respiratory, Urinary, Fecal, Dysphagia | |
| 17 | 32 | 15 years | Respiratory, Urinary, Dysphonia | |
| 18 | 19 | 15 years 7 months | Respiratory | |
| 19 | 32 | 14 years 10 months | Respiratory, Urinary | |
| 20 | 38 | 14 years 4 months | Urinary, Fecal | |
| 21 | 43 | 13 years 6 months | Respiratory | |
| 22 | 41 | 14 years 6 months | Respiratory, Urinary, Fecal, Facial nerve | |
| 23 | 20 | 13 years 2 months | Respiratory | |
| 24 | 44 | 12 years 1 month | Respiratory, Urinary, Fecal | |
| 25 | 43 | 14 years 4 months | Respiratory, Urinary | |
| 26 | 29 | 12 years 1 month | Respiratory, Urinary, Fecal | |
| 27 | 41 | 12 years 9 months | Respiratory, Urinary, Fecal, Dysphonia | |
| 28 | 30 | 14 years 4 months | Respiratory, Urinary, Dysphonia | |
| 29 | 36 | 13 years 3 months | Respiratory, Urinary | curcumin |
| 30 | 32 | 13 years 1 month | Respiratory, Urinary, Fecal | |
| 31 | 35 | 13 years 1 month | Respiratory, Urinary | |
| 32 | 35 | 12 years 6 months | Urinary, Fecal | |
| 33 | 18 | 12 years | Respiratory, Urinary | |
| 34 | 38 | 12 years 8 months | Respiratory, Urinary, Fecal, Dysphagia | |
| 35 | 41 | 16 years 8 months | Respiratory, Urinary, Fecal, tongue | curcumin |
| 36 | 37 | 15 years 10 months | Respiratory, Urinary, Fecal, Dysphonia | curcumin |
| 37 | 47 | 13 years 6 months | Respiratory, Urinary, Fecal, Dysphonia, Tongue, Trigeminal nerve | curcumin |
| 38 | 46 | 16 years 1 month | Respiratory, Urinary, Tongue, Dysphonia | curcumin |
| 39 | 42 | 13 years 5 months | Respiratory, Urinary, Dysphonia, Tongue | curcumin |
| 40 | 44 | 14 years 8 months | Respiratory, Dysphonia, Tongue, Dysphagia | curcumin |
References
- Coates, J.R.; Wininger, F.A. Canine degenerative myelopathy. Vet. Clin. N. Am. Small Anim. Pract. 2010, 40, 929–950. [Google Scholar] [CrossRef]
- Awano, T.; Johnson, G.S.; Wade, C.M.; Katz, M.L.; Johnson, G.C.; Taylor, J.F.; Perloski, M.; Biagi, T.; Baranowska, I.; Long, S.; et al. Genome-wide association analysis reveals a SOD1 mutation in canine degenerative myelopathy that resembles amyotrophic lateral sclerosis. Proc. Natl. Acad. Sci. USA 2009, 106, 2794–2799. [Google Scholar] [CrossRef] [Green Version]
- Wininger, F.A.; Zeng, R.; Johnson, G.S.; Katz, M.L.; Johnson, G.C.; Bush, W.W.; Jarboe, J.M.; Coates, J.R. Degenerative myelopathy in a Bernese mountain Dog with a novel SOD1 missense mutation. J. Vet. Intern. Med. 2011, 25, 1166–1170. [Google Scholar] [CrossRef]
- Nardone, R.; Höller, Y.; Taylor, A.C.; Lochner, P.; Tezzon, F.; Golaszewski, S.; Brigo, F.; Trinka, E. Canine degenerative myelopathy: A model of human amyotrophic lateral sclerosis. Zoology 2016, 119, 64–73. [Google Scholar] [CrossRef]
- Gubbay, S.S.; Kahana, E.; Zilber, N.; Cooper, G.; Pintov, S.; Leibowitz, Y. Amyotrophic lateral sclerosis. A study of its presentation and prognosis. J. Neurol. 1985, 232, 295–300. [Google Scholar] [CrossRef]
- Byrne, S.; Walsh, C.; Lynch, C.; Bede, P.; Elamin, M.; Kenna, K.; McLaughlin, R.; Hardiman, O. Rate of familial amyotrophic lateral sclerosis: A systematic review and meta-analysis. J. Neurol. Neurosurg. Psychiatry 2011, 82, 623–627. [Google Scholar] [CrossRef]
- Huai, J.; Zhang, Z. Structural properties and interaction partners of familial ALS-associated SOD1 mutants. Front. Neurol. 2019, 10, 527. [Google Scholar] [CrossRef] [Green Version]
- Rosen, D.R.; Siddique, T.; Patterson, D.; Figlewicz, D.A.; Sapp, P.; Hentati, A.; Donaldson, D.; Goto, J.; O’Regan, J.P.; Deng, H.X. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature 1993, 362, 59–62. [Google Scholar] [CrossRef]
- Deng, H.X.; Hentati, A.; Tainer, J.A.; Iqbal, Z.; Cayabyab, A.; Hung, W.Y.; Getzoff, E.D.; Hu, P.; Herzfeldt, B.; Roos, R.P. Amyotrophic lateral sclerosis and structural defects in Cu, Zn superoxide dismutase. Science 1993, 261, 1047–1051. [Google Scholar] [CrossRef]
- Crisp, M.J.; Beckett, J.; Coates, J.R.; Miller, T.M. Canine degenerative myelopathy: Biochemical characterization of superoxide dismutase 1 in the first naturally occurring non-human amyotrophic lateral sclerosis model. Exp. Neurol. 2013, 248, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Saccon, R.A.; Bunton-Stasyshyn, R.K.; Fisher, E.M.; Fratta, P. Is SOD1 loss of function involved in amyotrophic lateral sclerosis? Brain 2013, 136, 2342–2358. [Google Scholar] [CrossRef]
- Oyake, K.; Kobatake, Y.; Shibata, S.; Sakai, H.; Saito, M.; Yamato, O.; Kushida, K.; Maeda, S.; Kamishina, H. Changes in respiratory function in Pembroke Welsh Corgi dogs with degenerative myelopathy. J. Vet. Med. Sci. 2016, 78, 1323–1327. [Google Scholar] [CrossRef] [Green Version]
- Morgan, B.R.; Coates, J.R.; Johnson, G.C.; Bujnak, A.C.; Katz, M.L. Characterization of intercostal muscle pathology in canine degenerative myelopathy: A disease model for amyotrophic lateral sclerosis. J. Neurosci. Res. 2013, 91, 1639–1650. [Google Scholar] [CrossRef] [Green Version]
- Coates, J.R.; March, P.A.; Oglesbee, M.; Ruaux, C.G.; Olby, N.J.; Berghaus, R.D.; O’Brien, D.P.; Keating, J.H.; Johnson, G.S.; Williams, D.A. Clinical characterization of a familial degenerative myelopathy in Pembroke Welsh Corgi dogs. J. Vet. Intern. Med. 2007, 21, 1323–1331. [Google Scholar] [CrossRef]
- Zeng, R.; Coates, J.R.; Johnson, G.C.; Hansen, L.; Awano, T.; Kolicheski, A.; Ivansson, E.; Perloski, M.; Lindblad-Toh, K.; O’Brien, D.P.; et al. Breed distribution of SOD1 alleles previously associated with canine degenerative myelopathy. J. Vet. Intern. Med. 2014, 28, 515–521. [Google Scholar] [CrossRef] [Green Version]
- Chang, H.S.; Kamishina, H.; Mizukami, K.; Momoi, Y.; Katayama, M.; Rahman, M.M.; Uddin, M.M.; Yabuki, A.; Kohyama, M.; Yamato, O. Genotyping assays for the canine degenerative myelopathy-associated c.118G>A (p.E40K) mutation of the SOD1 gene using conventional and real-time PCR methods: A high prevalence in the Pembroke Welsh Corgi breed in Japan. J. Vet. Med. Sci. 2013, 75, 795–798. [Google Scholar] [CrossRef] [Green Version]
- Ahmadi, M.; Agah, E.; Nafissi, S.; Jaafari, M.R.; Harirchian, M.H.; Sarraf, P.; Faghihi-Kashani, S.; Hosseini, S.J.; Ghoreishi, A.; Aghamollaii, V.; et al. Safety and efficacy of nanocurcumin as add-on therapy to Riluzole in patients with amyotrophic lateral sclerosis: A pilot randomized clinical trial. Neurotherapeutics 2018, 15, 430–438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cudkowicz, M.E.; McKenna-Yasek, D.; Sapp, P.E.; Chin, W.; Geller, B.; Hayden, D.L.; Schoenfeld, D.A.; Hosler, B.A.; Horvitz, H.R.; Brown, R.H. Epidemiology of mutations in superoxide dismutase in amyotrophic lateral sclerosis. Ann. Neurol. 1997, 41, 210–221. [Google Scholar] [CrossRef]
- Nübling, G.S.; Mie, E.; Bauer, R.M.; Hensler, M.; Lorenzl, S.; Hapfelmeier, A.; Irwin, D.E.; Borasio, G.D.; Winkler, A.S. Increased prevalence of bladder and intestinal dysfunction in amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. Frontotemporal. Degener. 2014, 15, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Granger, N.; Olby, N.J.; Nout-Lomas, Y.S. Canine Spinal Cord Injury Consortium (CANSORT-SCI). Bladder and bowel management in dogs with spinal cord injury. Front. Vet. Sci. 2020, 7, 949–968. [Google Scholar] [CrossRef]
- March, P.A.; Coates, J.R.; Abyad, R.J.; Williams, D.A.; O’Brien, D.P.; Olby, N.J.; Keating, J.H.; Oglesbee, M. Degenerative myelopathy in 18 Pembroke Welsh Corgi dogs. Vet. Pathol. 2009, 46, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Andrews, J.A.; Meng, L.; Kulke, S.F.; Rudnicki, S.A.; Wolff, A.A.; Bozik, M.E.; Malik, F.I.; Shefner, J.M. Association between decline in slow vital capacity and respiratory insufficiency, use of assisted ventilation, tracheostomy, or death in patients with amyotrophic lateral sclerosis. JAMA Neurol. 2018, 75, 58–64. [Google Scholar] [CrossRef] [Green Version]
- Alexander, G.M.; Erwin, K.L.; Byers, N.; Deitch, J.S.; Augelli, B.J.; Blankenhorn, E.P.; Heiman-Patterson, T.D. Effect of transgene copy number on survival in the G93A SOD1 transgenic mouse model of ALS. Brain. Res. Mol. Brain. Res. 2004, 130, 7–15. [Google Scholar] [CrossRef]
- Monroy, A.; Lithgow, G.J.; Alavez, S. Curcumin and neurodegenerative diseases. Biofactors 2013, 39, 122–132. [Google Scholar] [CrossRef]
- Bhatia, N.K.; Srivastava, A.; Katyal, N.; Jain, N.; Khan, M.A.; Kundu, B.; Deep, S. Curcumin binds to the pre-fibrillar aggregates of Cu/Zn superoxide dismutase (SOD1) and alters its amyloidogenic pathway resulting in reduced cytotoxicity. Biochim. Biophys. Acta 2015, 1854, 426–436. [Google Scholar] [CrossRef]
- Fang, W.; Nasir, Y. The effect of curcumin supplementation on recovery following exercise-induced muscle damage and delayed-onset muscle soreness: A systematic review and meta-analysis of randomized controlled trials. Phytother. Res. 2021, 35, 1768–1781. [Google Scholar] [CrossRef]
- Mañas-García, L.; Bargalló, N.; Gea, J.; Barreiro, E. Muscle phenotype, proteolysis, and atrophy signaling during reloading in mice: Effects of curcumin on the gastrocnemius. Nutrients 2020, 12, 388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferguson, J.J.A.; Abbott, K.A.; Garg, M.L. Anti-inflammatory effects of oral supplementation with curcumin: A systematic review and meta-analysis of randomized controlled trials. Nutr. Rev. 2021, 79, 1043–1066. [Google Scholar] [CrossRef]
- Shokri-Mashhadi, N.; Bagherniya, M.; Askari, G.; Sathyapalan, T.; Sahebkar, A. A systematic review of the clinical use of curcumin for the treatment of osteoarthritis. Adv. Exp. Med. Biol. 2021, 1291, 265–282. [Google Scholar]
- Kathmann, I.; Cizinauskas, S.; Doherr, M.G.; Steffen, F.; Jaggy, A. Daily controlled physiotherapy increases survival time in dogs with suspected degenerative myelopathy. J. Vet. Intern. Med. 2006, 20, 927–932. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).