Pluripotency and Growth Factors in Early Embryonic Development of Mammals: A Comparative Approach
Abstract
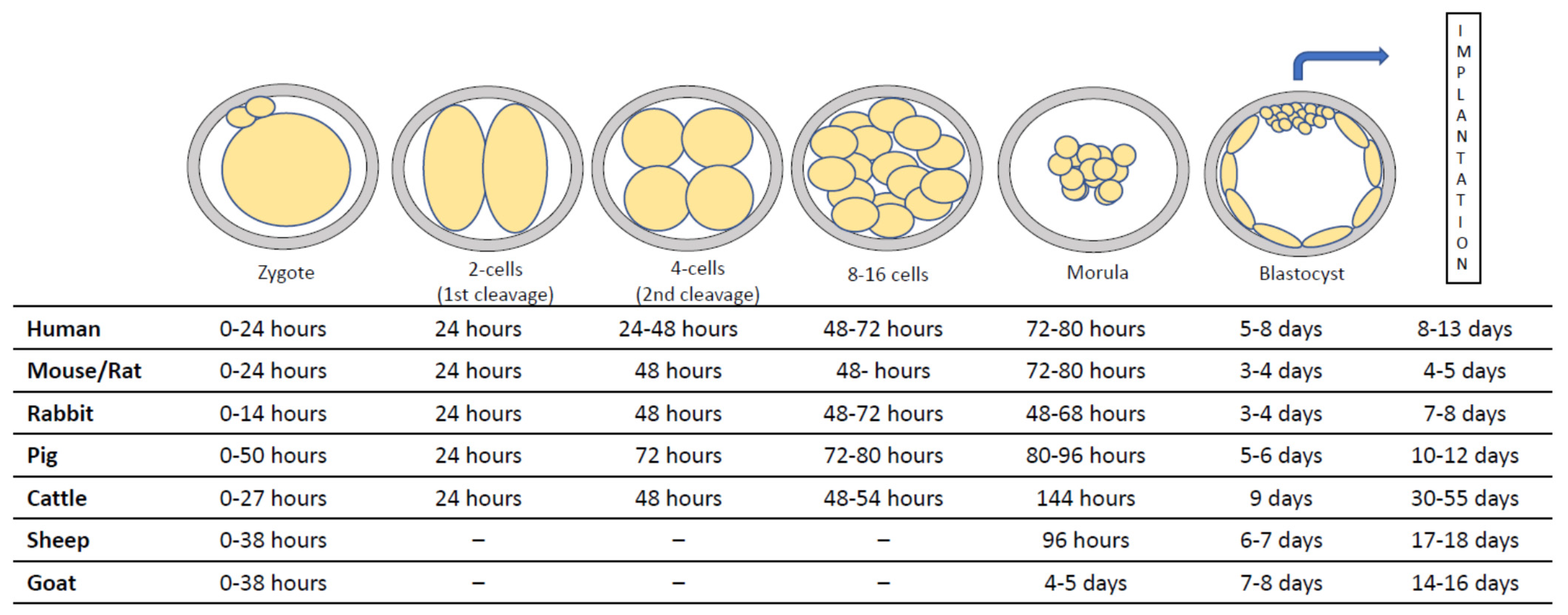
:1. Introduction
2. Pluripotency Transcription Factors
3. Growth Factors and Early Development
3.1. Vascular Endothelial Growth Factor (VEGF)
3.2. Transforming Growth Factor-Beta (TGF-β) Superfamily
3.3. Fibroblast Growth Factor (FGF) Family
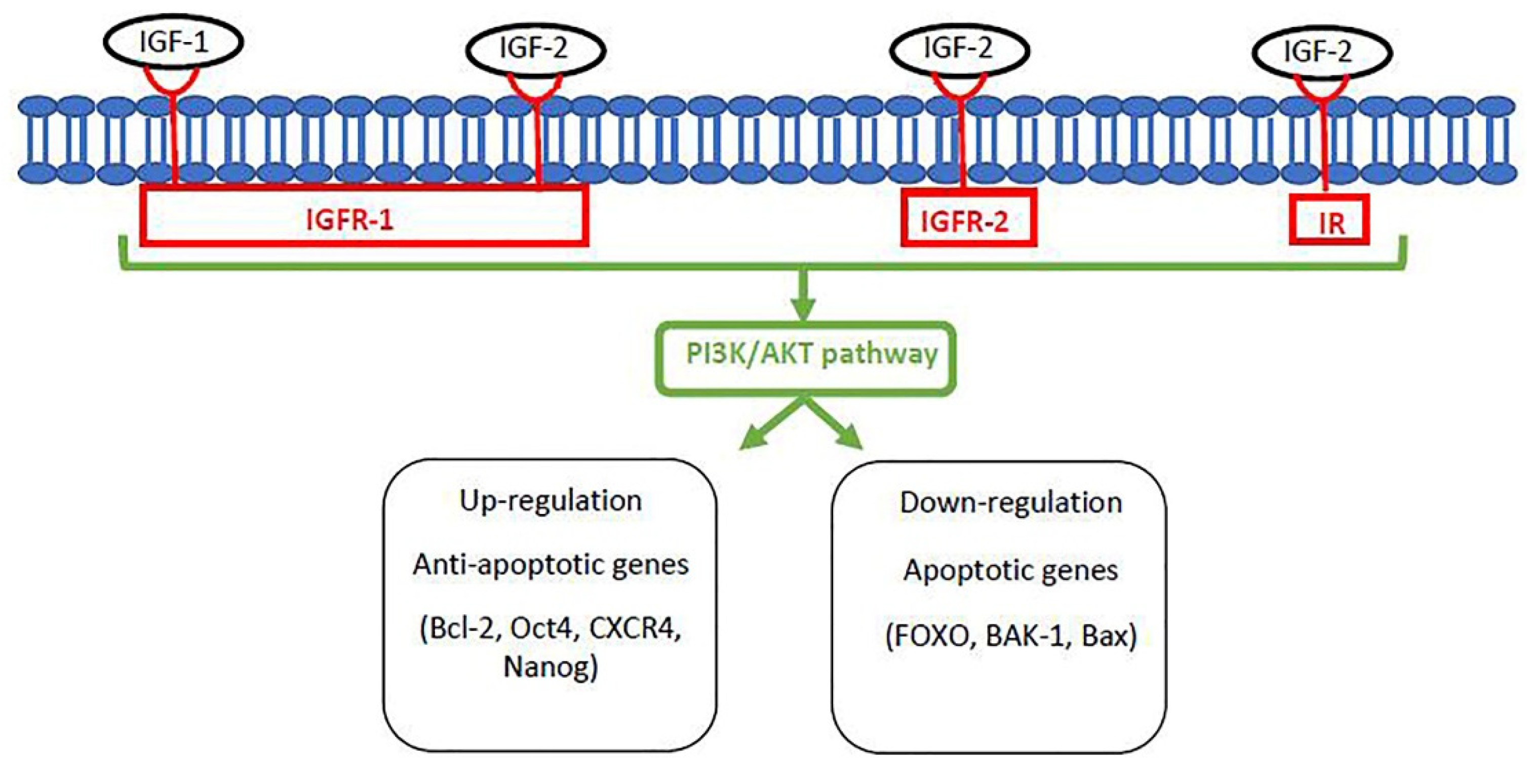
3.4. Insulin-Like Growth Factor (IGF) System
3.5. Epidermal Growth Factor (EGF) Family
3.6. Other Growth Factors
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Madeja, Z.E.; Pawlak, P.; Piliszek, A. Beyond the Mouse: Non-Rodent Animal Models for Study of Early Mammalian Development and Biomedical Research. Int. J. Dev. Biol. 2019, 63, 187–201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lonergan, P.; Fair, T.; Forde, N.; Rizos, D. Embryo Development in Dairy Cattle. Theriogenology 2016, 86, 270–277. [Google Scholar] [CrossRef]
- Flood, P.F.; Betteridge, K.J.; Diocee, M.S. Transmission Electron Microscopy of Horse Embryos 3-16 Days after Ovulation. J. Reprod. Fertil. Suppl. 1982, 32, 319–327. [Google Scholar] [PubMed]
- Hall, V.J. Early Development of the Porcine Embryo: The Importance of Cell Signalling in Development of Pluripotent Cell Lines. Reprod. Fertil. Dev. 2012, 25, 94–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- HosseinNia, P.; Hajian, M.; Tahmoorespur, M.; Hosseini, S.M.; Ostadhosseini, S.; Nasiri, M.R.; Nasr-Esfahani, M.H. Expression Profile of Developmentally Important Genes in Preand Peri-Implantation Goat Embryos Produced In Vitro. Int. J. Fertil. Steril. 2016, 10, 310–319. [Google Scholar] [CrossRef] [PubMed]
- Mclaren, A.; Smith, R. Functional Test of Tight Junctions in the Mouse Blastocyst. Nature 1977, 267, 351–353. [Google Scholar] [CrossRef]
- Niakan, K.K.; Eggan, K. Analysis of Human Embryos from Zygote to Blastocyst Reveals Distinct Gene Expression Patterns Relative to the Mouse. Dev. Biol. 2013, 375, 54–64. [Google Scholar] [CrossRef] [Green Version]
- Saenz-de-Juano, M.D.; Peñaranda, D.S.; Marco-Jiménez, F.; Llobat, L.; Vicente, J.S. Differential MRNA Expression in Rabbit in Vivo Pre-Implantatory Embryos. Reprod. Domest. Anim. 2011, 46, 567–572. [Google Scholar] [CrossRef]
- Bazer, F.W.; Johnson, G.A. Pig Blastocyst-Uterine Interactions. Differentiation 2014, 87, 52–65. [Google Scholar] [CrossRef]
- Stout, T.A.E.; Meadows, S.; Allen, W.R. Stage-Specific Formation of the Equine Blastocyst Capsule Is Instrumental to Hatching and to Embryonic Survival in Vivo. Anim. Reprod. Sci. 2005, 87, 269–281. [Google Scholar] [CrossRef]
- Piliszek, A.; Madeja, Z.E. Pre-Implantation Development of Domestic Animals. Curr. Top. Dev. Biol. 2018, 128, 267–294. [Google Scholar] [CrossRef] [PubMed]
- Brinster, R.L. Embryo Development. J. Anim. Sci. 1974, 38, 1003–1012. [Google Scholar] [CrossRef] [PubMed]
- Simmet, K.; Zakhartchenko, V.; Wolf, E. Comparative Aspects of Early Lineage Specification Events in Mammalian Embryos—Insights from Reverse Genetics Studies. Cell Cycle 2018, 17, 1688–1695. [Google Scholar] [CrossRef] [Green Version]
- Boyer, L.A.; Mathur, D.; Jaenisch, R. Molecular Control of Pluripotency. Curr. Opin. Genet. Dev. 2006, 16, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Madeja, Z.E.; Hryniewicz, K.; Orsztynowicz, M.; Pawlak, P.; Perkowska, A. WNT/β-Catenin Signaling Affects Cell Lineage and Pluripotency-Specific Gene Expression in Bovine Blastocysts: Prospects for Bovine Embryonic Stem Cell Derivation. Stem Cells Dev. 2015, 24, 2437–2454. [Google Scholar] [CrossRef]
- Degrelle, S.A.; Campion, E.; Cabau, C.; Piumi, F.; Reinaud, P.; Richard, C.; Renard, J.-P.; Hue, I. Molecular Evidence for a Critical Period in Mural Trophoblast Development in Bovine Blastocysts. Dev. Biol. 2005, 288, 448–460. [Google Scholar] [CrossRef] [Green Version]
- Silva, P.G.C.; Moura, M.T.; Silva, R.L.O.; Nascimento, P.S.; Silva, J.B.; Ferreira-Silva, J.C.; Cantanhêde, L.F.; Chaves, M.S.; Benko-Iseppon, A.M.; Oliveira, M.A.L. Temporal Expression of Pluripotency-Associated Transcription Factors in Sheep and Cattle Preimplantation Embryos. Zygote 2018, 26, 270–278. [Google Scholar] [CrossRef]
- Van Eijk, M.J.; van Rooijen, M.A.; Modina, S.; Scesi, L.; Folkers, G.; van Tol, H.T.; Bevers, M.M.; Fisher, S.R.; Lewin, H.A.; Rakacolli, D.; et al. Molecular Cloning, Genetic Mapping, and Developmental Expression of Bovine POU5F1. Biol. Reprod. 1999, 60, 1093–1103. [Google Scholar] [CrossRef] [Green Version]
- Simmet, K.; Zakhartchenko, V.; Philippou-Massier, J.; Blum, H.; Klymiuk, N.; Wolf, E. OCT4/POU5F1 Is Required for NANOG Expression in Bovine Blastocysts. Proc. Natl. Acad. Sci. USA 2018, 115, 2770–2775. [Google Scholar] [CrossRef] [Green Version]
- Naddafpour, A.; Ghazvini Zadegan, F.; Hajian, M.; Hosseini, S.M.; Jafarpour, F.; Rahimi, M.; Habibi, R.; Nasr Esfahani, M.H. Effects of Abundances of OCT-4 MRNA Transcript on Goat Pre-Implantation Embryonic Development. Anim. Reprod. Sci. 2020, 215, 106286. [Google Scholar] [CrossRef]
- Habibi, R.; Hosseini, S.M.; Zadegan, F.G.; Hajian, M.; Ostadhosseini, S.; Vash, N.T.; Naddafpour, A.; Nasr Esfahani, M.H. Functional Characterization of NANOG in Goat Pre-Implantation Embryonic Development. Theriogenology 2018, 120, 33–39. [Google Scholar] [CrossRef]
- Kumar, D.; Sarkhel, B.C. Differential Expression Pattern of Key Regulatory Developmental Genes in Pre-Implant Zona Free Cloned vs. in Vitro Fertilized Goat Embryos. Gene Expr. Patterns 2017, 25–26, 118–123. [Google Scholar] [CrossRef]
- Llobat, L. Embryo Gene Expression in Pig Pregnancy. Reprod. Domest. Anim. 2020. [Google Scholar] [CrossRef] [PubMed]
- Ozawa, M.; Sakatani, M.; Yao, J.; Shanker, S.; Yu, F.; Yamashita, R.; Wakabayashi, S.; Nakai, K.; Dobbs, K.B.; Sudano, M.J.; et al. Global Gene Expression of the Inner Cell Mass and Trophectoderm of the Bovine Blastocyst. BMC Dev. Biol 2012, 12, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ortega, M.S.; Kelleher, A.M.; O’Neil, E.; Benne, J.; Cecil, R.; Spencer, T.E. NANOG Is Required to Form the Epiblast and Maintain Pluripotency in the Bovine Embryo. Mol. Reprod. Dev. 2020, 87, 152–160. [Google Scholar] [CrossRef]
- García, E.V.; Miceli, D.C.; Rizo, G.; Valdecantos, P.A.; Barrera, A.D. Effect of Early Addition of Bone Morphogenetic Protein 5 (BMP5) to Embryo Culture Medium on in Vitro Development and Expression of Developmentally Important Genes in Bovine Preimplantation Embryos. Theriogenology 2015, 84, 589–599. [Google Scholar] [CrossRef] [PubMed]
- Lichtner, B.; Knaus, P.; Lehrach, H.; Adjaye, J. BMP10 as a Potent Inducer of Trophoblast Differentiation in Human Embryonic and Induced Pluripotent Stem Cells. Biomaterials 2013, 34, 9789–9802. [Google Scholar] [CrossRef]
- Berg, D.K.; Smith, C.S.; Pearton, D.J.; Wells, D.N.; Broadhurst, R.; Donnison, M.; Pfeffer, P.L. Trophectoderm Lineage Determination in Cattle. Dev. Cell 2011, 20, 244–255. [Google Scholar] [CrossRef] [Green Version]
- Fogarty, N.M.E.; McCarthy, A.; Snijders, K.E.; Powell, B.E.; Kubikova, N.; Blakeley, P.; Lea, R.; Elder, K.; Wamaitha, S.E.; Kim, D.; et al. Genome Editing Reveals a Role for OCT4 in Human Embryogenesis. Nature 2017, 550, 67–73. [Google Scholar] [CrossRef]
- Kuijk, E.W.; van Tol, L.T.A.; Van de Velde, H.; Wubbolts, R.; Welling, M.; Geijsen, N.; Roelen, B.A.J. The Roles of FGF and MAP Kinase Signaling in the Segregation of the Epiblast and Hypoblast Cell Lineages in Bovine and Human Embryos. Development 2012, 139, 871–882. [Google Scholar] [CrossRef] [Green Version]
- Piliszek, A.; Madeja, Z.E.; Plusa, B. Suppression of ERK Signalling Abolishes Primitive Endoderm Formation but Does Not Promote Pluripotency in Rabbit Embryo. Development 2017, 144, 3719–3730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plusa, B.; Piliszek, A.; Frankenberg, S.; Artus, J.; Hadjantonakis, A.-K. Distinct Sequential Cell Behaviours Direct Primitive Endoderm Formation in the Mouse Blastocyst. Development 2008, 135, 3081–3091. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bontovics, B.; Maraghechi, P.; Lázár, B.; Anand, M.; Németh, K.; Fábián, R.; Vašíček, J.; Makarevich, A.V.; Gócza, E.; Chrenek, P. The Effect of Dual Inhibition of Ras-MEK-ERK and GSK3β Pathways on Development of in Vitro Cultured Rabbit Embryos. Zygote 2020, 28, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, A.; Allegrucci, C.; Alberio, R. Modulation of Pluripotency in the Porcine Embryo and IPS Cells. PLoS ONE 2012, 7, e49079. [Google Scholar] [CrossRef]
- Roode, M.; Blair, K.; Snell, P.; Elder, K.; Marchant, S.; Smith, A.; Nichols, J. Human Hypoblast Formation is Not Dependent on FGF Signalling. Dev. Biol. 2012, 361, 358–363. [Google Scholar] [CrossRef] [Green Version]
- Bernardo, A.S.; Jouneau, A.; Marks, H.; Kensche, P.; Kobolak, J.; Freude, K.; Hall, V.; Feher, A.; Polgar, Z.; Sartori, C.; et al. Mammalian Embryo Comparison Identifies Novel Pluripotency Genes Associated with the Naïve or Primed State. Biol. Open 2018, 7. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.-M.; Cui, L.-S.; Hao, H.-S.; Wang, H.-Y.; Zhao, S.-J.; Du, W.-H.; Wang, D.; Liu, Y.; Zhu, H.-B. Transcriptome Analyses of Inner Cell Mass and Trophectoderm Cells Isolated by Magnetic-Activated Cell Sorting from Bovine Blastocysts Using Single Cell RNA-Seq. Reprod. Domest. Anim. 2016, 51, 726–735. [Google Scholar] [CrossRef]
- Wei, Q.; Li, R.; Zhong, L.; Mu, H.; Zhang, S.; Yue, L.; Xiang, J.; Li, E.; Zhi, M.; Cao, S.; et al. Lineage Specification Revealed by Single-Cell Gene Expression Analysis in Porcine Preimplantation Embryos. Biol. Reprod. 2018, 99, 283–292. [Google Scholar] [CrossRef] [Green Version]
- Ferrara, N.; Chen, H.; Davis-Smyth, T.; Gerber, H.P.; Nguyen, T.N.; Peers, D.; Chisholm, V.; Hillan, K.J.; Schwall, R.H. Vascular Endothelial Growth Factor Is Essential for Corpus Luteum Angiogenesis. Nat. Med. 1998, 4, 336–340. [Google Scholar] [CrossRef]
- Valdés, G.; Erices, R.; Chacón, C.; Corthorn, J. Angiogenic, Hyperpermeability and Vasodilator Network in Utero-Placental Units along Pregnancy in the Guinea-Pig (Cavia Porcellus). Reprod. Biol. Endocrinol. 2008, 6, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrara, N. Vascular Endothelial Growth Factor: Molecular and Biological Aspects. Curr. Top. Microbiol. Immunol. 1999, 237, 1–30. [Google Scholar] [CrossRef]
- Yancopoulos, G.D.; Davis, S.; Gale, N.W.; Rudge, J.S.; Wiegand, S.J.; Holash, J. Vascular-Specific Growth Factors and Blood Vessel Formation. Nature 2000, 407, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Cheung, C.Y.; Anderson, D.F.; Brace, R.A. Transport-Associated Pathway Responses in Ovine Fetal Membranes to Changes in Amniotic Fluid Dynamics. Physiol. Rep. 2017, 5, e13455. [Google Scholar] [CrossRef] [PubMed]
- Gabler, C.; Einspanier, A.; Schams, D.; Einspanier, R. Expression of Vascular Endothelial Growth Factor (VEGF) and Its Corresponding Receptors (Flt-1 and Flk-1) in the Bovine Oviduct. Mol. Reprod. Dev. 1999, 53, 376–383. [Google Scholar] [CrossRef]
- Kaczynski, P.; Goryszewska, E.; Baryla, M.; Waclawik, A. Prostaglandin F2α Stimulates Angiogenesis at the Embryo-Maternal Interface during Early Pregnancy in the Pig. Theriogenology 2019, 142, 169–176. [Google Scholar] [CrossRef]
- Kalkunte, S.S.; Mselle, T.F.; Norris, W.E.; Wira, C.R.; Sentman, C.L.; Sharma, S. Vascular Endothelial Growth Factor C Facilitates Immune Tolerance and Endovascular Activity of Human Uterine NK Cells at the Maternal-Fetal Interface. J. Immunol. 2009, 182, 4085–4092. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kazi, A.A.; Koos, R.D. Estrogen-Induced Activation of Hypoxia-Inducible Factor-1alpha, Vascular Endothelial Growth Factor Expression, and Edema in the Uterus Are Mediated by the Phosphatidylinositol 3-Kinase/Akt Pathway. Endocrinology 2007, 148, 2363–2374. [Google Scholar] [CrossRef] [PubMed]
- Llobat, L.; Marco-Jiménez, F.; Peñaranda, D.S.; Thieme, R.; Navarrete, A.; Vicente, J.S. MRNA Expression in Rabbit Blastocyst and Endometrial Tissue of Candidate Gene Involved in Gestational Losses. Reprod. Domest. Anim. 2012, 47, 281–287. [Google Scholar] [CrossRef]
- Pfarrer, C.D.; Ruziwa, S.D.; Winther, H.; Callesen, H.; Leiser, R.; Schams, D.; Dantzer, V. Localization of Vascular Endothelial Growth Factor (VEGF) and Its Receptors VEGFR-1 and VEGFR-2 in Bovine Placentomes from Implantation until Term. Placenta 2006, 27, 889–898. [Google Scholar] [CrossRef]
- Złotkowska, A.; Adamczyk, S.; Andronowska, A. Presence of Trophoblast in the Uterine Lumen Affects VEGF-C Expression in Porcine Endometrium. Theriogenology 2019, 125, 216–223. [Google Scholar] [CrossRef]
- Hunter, M.G.; Robinson, R.S.; Mann, G.E.; Webb, R. Endocrine and Paracrine Control of Follicular Development and Ovulation Rate in Farm Species. Anim. Reprod. Sci. 2004, 82–83, 461–477. [Google Scholar] [CrossRef]
- Anchordoquy, J.M.; Anchordoquy, J.P.; Testa, J.A.; Sirini, M.Á.; Furnus, C.C. Influence of Vascular Endothelial Growth Factor and Cysteamine on in Vitro Bovine Oocyte Maturation and Subsequent Embryo Development. Cell Biol. Int. 2015, 39, 1090–1098. [Google Scholar] [CrossRef]
- Biswas, D.; So, K.H.; Hwang, S.U.; Yoon, J.D.; Kim, M.; Kim, D.Y.; Hyun, S.H. Embryotropic Effects of Vascular Endothelial Growth Factor on Porcine Embryos Produced by in Vitro Fertilization. Theriogenology 2018, 120, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Hao, Y.; Li, Z.; Zhou, J.; Zhu, H.; Bu, G.; Liu, Z.; Hou, X.; Zhang, X.; Miao, Y.-L. Maternal Cytokines CXCL12, VEGFA, and WNT5A Promote Porcine Oocyte Maturation via MAPK Activation and Canonical WNT Inhibition. Front. Cell Dev. Biol. 2020, 8, 578. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, G.C.; Alves, L.A.; Betarelli, R.P.; Guimarães, C.S.O.; Helmo, F.R.; Pereira Júnior, C.D.; Corrêa, R.R.M.; Zangeronimo, M.G. Expression of Vascular Endothelial Growth Factor (VEGF) and Factor VIII in the Gilt Placenta and Its Relation to Fetal Development. Theriogenology 2017, 92, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Chiumia, D.; Hankele, A.-K.; Groebner, A.E.; Schulke, K.; Reichenbach, H.-D.; Giller, K.; Zakhartchenko, V.; Bauersachs, S.; Ulbrich, S.E. Vascular Endothelial Growth Factor A and VEGFR-1 Change during Preimplantation in Heifers. Int. J. Mol. Sci. 2020, 21, 544. [Google Scholar] [CrossRef] [Green Version]
- Mess, A.M.; Carreira, A.C.O.; Marinovic de Oliveira, C.; Fratini, P.; Favaron, P.O.; Barreto, R. da S.N.; Pfarrer, C.; Meirelles, F.V.; Miglino, M.A. Vascularization and VEGF Expression Altered in Bovine Yolk Sacs from IVF and NT Technologies. Theriogenology 2017, 87, 290–297. [Google Scholar] [CrossRef]
- Berisha, B.; Schams, D.; Kosmann, M.; Amselgruber, W.; Einspanier, R. Expression and Tissue Concentration of Vascular Endothelial Growth Factor, Its Receptors, and Localization in the Bovine Corpus Luteum during Estrous Cycle and Pregnancy. Biol. Reprod. 2000, 63, 1106–1114. [Google Scholar] [CrossRef]
- Sugino, N.; Suzuki, T.; Sakata, A.; Miwa, I.; Asada, H.; Taketani, T.; Yamagata, Y.; Tamura, H. Angiogenesis in the Human Corpus Luteum: Changes in Expression of Angiopoietins in the Corpus Luteum throughout the Menstrual Cycle and in Early Pregnancy. J. Clin. Endocrinol. Metab. 2005, 90, 6141–6148. [Google Scholar] [CrossRef] [Green Version]
- Wheeler, K.C.; Jena, M.K.; Pradhan, B.S.; Nayak, N.; Das, S.; Hsu, C.-D.; Wheeler, D.S.; Chen, K.; Nayak, N.R. VEGF May Contribute to Macrophage Recruitment and M2 Polarization in the Decidua. PLoS ONE 2018, 13, e0191040. [Google Scholar] [CrossRef] [PubMed]
- Hedger, M.P.; Winnall, W.R.; Phillips, D.J.; de Kretser, D.M. The Regulation and Functions of Activin and Follistatin in Inflammation and Immunity. Vitam Horm. 2011, 85, 255–297. [Google Scholar] [CrossRef]
- Akpan, I.; Pro, B.; Platanias, L.C. Transforming Growth Factor Superfamily Ligands and Links to Tumorigenesis. Leuk Lymphoma 2018, 59, 1282–1283. [Google Scholar] [CrossRef] [PubMed]
- Bloise, E.; Ciarmela, P.; Dela Cruz, C.; Luisi, S.; Petraglia, F.; Reis, F.M. Activin A in Mammalian Physiology. Physiol. Rev. 2019, 99, 739–780. [Google Scholar] [CrossRef] [PubMed]
- Paria, B.C.; Dey, S.K. Preimplantation Embryo Development in Vitro: Cooperative Interactions among Embryos and Role of Growth Factors. Proc. Natl. Acad. Sci. USA 1990, 87, 4756–4760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pauken, C.M.; Capco, D.G. Regulation of Cell Adhesion during Embryonic Compaction of Mammalian Embryos: Roles for PKC and Beta-Catenin. Mol. Reprod. Dev. 1999, 54, 135–144. [Google Scholar] [CrossRef]
- Yoon, J.D.; Hwang, S.-U.; Kim, M.; Lee, G.; Jeon, Y.; Hyun, S.-H. GDF8 Enhances SOX2 Expression and Blastocyst Total Cell Number in Porcine IVF Embryo Development. Theriogenology 2019, 129, 70–76. [Google Scholar] [CrossRef]
- Jaeger, L.A.; Spiegel, A.K.; Ing, N.H.; Johnson, G.A.; Bazer, F.W.; Burghardt, R.C. Functional Effects of Transforming Growth Factor Beta on Adhesive Properties of Porcine Trophectoderm. Endocrinology 2005, 146, 3933–3942. [Google Scholar] [CrossRef] [Green Version]
- Hajian, M.; Hosseini, S.M.; Ostadhosseini, S.; Nasr-Esfahani, M.H. Targeting the Transforming Growth Factor-β Signaling during Pre-Implantation Development in Embryos of Cattle, Sheep and Goats. Growth Factors 2016, 34, 141–148. [Google Scholar] [CrossRef]
- Sudiman, J.; Sutton-McDowall, M.L.; Ritter, L.J.; White, M.A.; Mottershead, D.G.; Thompson, J.G.; Gilchrist, R.B. Bone Morphogenetic Protein 15 in the Pro-Mature Complex Form Enhances Bovine Oocyte Developmental Competence. PLoS ONE 2014, 9, e103563. [Google Scholar] [CrossRef] [Green Version]
- Barrera, A.D.; García, E.V.; Miceli, D.C. Effect of Exogenous Transforming Growth Factor Β1 (TGF-Β1) on Early Bovine Embryo Development. Zygote 2018, 26, 232–241. [Google Scholar] [CrossRef]
- Weiss, A.; Attisano, L. The TGFbeta Superfamily Signaling Pathway. Wiley Interdiscip Rev. Dev. Biol. 2013, 2, 47–63. [Google Scholar] [CrossRef]
- Zhang, K.; Rajput, S.K.; Lee, K.-B.; Wang, D.; Huang, J.; Folger, J.K.; Knott, J.G.; Zhang, J.; Smith, G.W. Evidence Supporting a Role for SMAD2/3 in Bovine Early Embryonic Development: Potential Implications for Embryotropic Actions of Follistatin. Biol. Reprod. 2015, 93, 86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashry, M.; Rajput, S.K.; Folger, J.K.; Knott, J.G.; Hemeida, N.A.; Kandil, O.M.; Ragab, R.S.; Smith, G.W. Functional Role of AKT Signaling in Bovine Early Embryonic Development: Potential Link to Embryotrophic Actions of Follistatin. Reprod. Biol. Endocrinol. 2018, 16, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Böttcher, R.T.; Niehrs, C. Fibroblast Growth Factor Signaling during Early Vertebrate Development. Endocr. Rev. 2005, 26, 63–77. [Google Scholar] [CrossRef] [PubMed]
- Cooke, F.N.T.; Pennington, K.A.; Yang, Q.; Ealy, A.D. Several Fibroblast Growth Factors Are Expressed during Pre-Attachment Bovine Conceptus Development and Regulate Interferon-Tau Expression from Trophectoderm. Reproduction 2009, 137, 259–269. [Google Scholar] [CrossRef] [Green Version]
- Michael, D.D.; Alvarez, I.M.; Ocón, O.M.; Powell, A.M.; Talbot, N.C.; Johnson, S.E.; Ealy, A.D. Fibroblast Growth Factor-2 Is Expressed by the Bovine Uterus and Stimulates Interferon-Tau Production in Bovine Trophectoderm. Endocrinology 2006, 147, 3571–3579. [Google Scholar] [CrossRef] [Green Version]
- Ocón-Grove, O.M.; Cooke, F.N.T.; Alvarez, I.M.; Johnson, S.E.; Ott, T.L.; Ealy, A.D. Ovine Endometrial Expression of Fibroblast Growth Factor (FGF) 2 and Conceptus Expression of FGF Receptors during Early Pregnancy. Domest. Anim. Endocrinol. 2008, 34, 135–145. [Google Scholar] [CrossRef]
- Dichmann, D.S.; Miller, C.P.; Jensen, J.; Scott Heller, R.; Serup, P. Expression and Misexpression of Members of the FGF and TGFbeta Families of Growth Factors in the Developing Mouse Pancreas. Dev. Dyn. 2003, 226, 663–674. [Google Scholar] [CrossRef]
- Hillege, M.M.G.; Galli Caro, R.A.; Offringa, C.; de Wit, G.M.J.; Jaspers, R.T.; Hoogaars, W.M.H. TGF-β Regulates Collagen Type I Expression in Myoblasts and Myotubes via Transient Ctgf and Fgf-2 Expression. Cells 2020, 9, 375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moore, J.W.; Dionne, C.; Jaye, M.; Swain, J.L. The MRNAs Encoding Acidic FGF, Basic FGF and FGF Receptor Are Coordinately Downregulated during Myogenic Differentiation. Development 1991, 111, 741–748. [Google Scholar] [CrossRef]
- Nishimura, T.; Nakatake, Y.; Konishi, M.; Itoh, N. Identification of a Novel FGF, FGF-21, Preferentially Expressed in the Liver. Biochim Biophys Acta 2000, 1492, 203–206. [Google Scholar] [CrossRef]
- Soulet, L.; Chevet, E.; Lemaitre, G.; Blanquaert, F.; Meddahi, A.; Barritault, D. FGFs and Their Receptors, in Vitro and in Vivo Studies: New FGF Receptor in the Brain, FGF-1 in Muscle, and the Use of Functional Analogues of Low-Affinity Heparin-Binding Growth Factor Receptors in Tissue Repair. Mol. Reprod. Dev. 1994, 39, 49–54, discussion 54–55. [Google Scholar] [CrossRef]
- Zhang, Y.; Madiai, F.; Hackshaw, K.V. Cloning and Characterization of a Novel Form of Mouse Fibroblast Growth Factor-1 (FGF-1) MRNA, FGF-1.G: Differential Expression of FGF-1 and FGF-1.G MRNAs during Embryonic Development and in Postnatal Tissues. Biochim. Biophys. Acta 2001, 1521, 45–58. [Google Scholar] [CrossRef]
- Yang, L.; Soonpaa, M.H.; Adler, E.D.; Roepke, T.K.; Kattman, S.J.; Kennedy, M.; Henckaerts, E.; Bonham, K.; Abbott, G.W.; Linden, R.M.; et al. Human Cardiovascular Progenitor Cells Develop from a KDR+ Embryonic-Stem-Cell-Derived Population. Nature 2008, 453, 524–528. [Google Scholar] [CrossRef] [PubMed]
- Yook, J.-Y.; Kim, M.-J.; Son, M.J.; Lee, S.; Nam, Y.; Han, Y.-M.; Cho, Y.S. Combinatorial Activin Receptor-like Kinase/Smad and Basic Fibroblast Growth Factor Signals Stimulate the Differentiation of Human Embryonic Stem Cells into the Cardiac Lineage. Stem Cells Dev. 2011, 20, 1479–1490. [Google Scholar] [CrossRef] [PubMed]
- Guzzetta, A.; Koska, M.; Rowton, M.; Sullivan, K.R.; Jacobs-Li, J.; Kweon, J.; Hidalgo, H.; Eckart, H.; Hoffmann, A.D.; Back, R.; et al. Hedgehog-FGF Signaling Axis Patterns Anterior Mesoderm during Gastrulation. Proc. Natl. Acad. Sci. USA 2020, 117, 15712–15723. [Google Scholar] [CrossRef]
- Kunath, T.; Yamanaka, Y.; Detmar, J.; MacPhee, D.; Caniggia, I.; Rossant, J.; Jurisicova, A. Developmental Differences in the Expression of FGF Receptors between Human and Mouse Embryos. Placenta 2014, 35, 1079–1088. [Google Scholar] [CrossRef]
- Taniguchi, F.; Harada, T.; Iwabe, T.; Ohama, Y.; Takenaka, Y.; Terakawa, N. Aberrant Expression of Keratinocyte Growth Factor Receptor in Ovarian Surface Epithelial Cells of Endometrioma. Fertil. Steril. 2008, 89, 478–480. [Google Scholar] [CrossRef] [PubMed]
- Barros, R.G.; Lima, P.F.; Soares, A.C.S.; Sanches, L.; Price, C.A.; Buratini, J. Fibroblast Growth Factor 2 Regulates Cumulus Differentiation under the Control of the Oocyte. J. Assist. Reprod. Genet. 2019, 36, 905–913. [Google Scholar] [CrossRef]
- Diógenes, M.N.; Guimarães, A.L.S.; Leme, L.O.; Dode, M.A.N. Bovine in Vitro Embryo Production: The Effects of Fibroblast Growth Factor 10 (FGF10). J. Assist. Reprod. Genet. 2017, 34, 383–390. [Google Scholar] [CrossRef] [Green Version]
- Mor, A.; Mondal, S.; Reddy, I.J.; Nandi, S.; Gupta, P. Molecular Cloning and Expression of FGF2 Gene in Pre-Implantation Developmental Stages of in Vitro-Produced Sheep Embryos. Reprod. Domest. Anim. 2018, 53, 895–903. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Wang, M.; Baloch, A.R.; Zhang, Q.; Wang, J.; Ma, R.; Xu, G.; Kashif, J.; Wang, L.; Fan, J.; et al. FGF10 Enhances Yak Oocyte Fertilization Competence and Subsequent Blastocyst Quality and Regulates the Levels of CD9, CD81, DNMT1, and DNMT3B. J. Cell Physiol. 2019, 234, 17677–17689. [Google Scholar] [CrossRef]
- Da Silva, R.B.; Yang, M.Y.; Caixeta, E.S.; Castilho, A.C.; Amorim, R.L.; Price, C.A.; Fortune, J.E.; Buratini, J. Fibroblast Growth Factor 18 Regulates Steroidogenesis in Fetal Bovine Ovarian Tissue in Vitro. Mol. Reprod. Dev. 2019, 86, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Akizawa, H.; Nagatomo, H.; Odagiri, H.; Kohri, N.; Yamauchi, N.; Yanagawa, Y.; Nagano, M.; Takahashi, M.; Kawahara, M. Conserved Roles of Fibroblast Growth Factor Receptor 2 Signaling in the Regulation of Inner Cell Mass Development in Bovine Blastocysts. Mol. Reprod. Dev. 2016, 83, 516–525. [Google Scholar] [CrossRef]
- Valdez Magaña, G.; Rodríguez, A.; Zhang, H.; Webb, R.; Alberio, R. Paracrine Effects of Embryo-Derived FGF4 and BMP4 during Pig Trophoblast Elongation. Dev. Biol. 2014, 387, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Madiai, F.; Hackshaw, K. Expression of the Mouse FGF-1 and FGF-1.A MRNAs during Embryonic Development and in the Aging Heart. Res. Commun. Mol. Pathol. Pharmacol. 2002, 112, 139–144. [Google Scholar]
- Ferriani, R.A.; Ahmed, A.; Sharkey, A.; Smith, S.K. Colocalization of Acidic and Basic Fibroblast Growth Factor (FGF) in Human Placenta and the Cellular Effects of BFGF in Trophoblast Cell Line JEG-3. Growth Factors 1994, 10, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-S.; Li, J.-Y.; Jin, S.-H.; Wang, H.-Y. The expression of basic fibroblast growth factor 1 during human embryonic yolk sac hematopoiesis. Zhonghua Xue Ye Xue Za Zhi 2008, 29, 535–539. [Google Scholar]
- Fu, Y.M.; Spirito, P.; Yu, Z.X.; Biro, S.; Sasse, J.; Lei, J.; Ferrans, V.J.; Epstein, S.E.; Casscells, W. Acidic Fibroblast Growth Factor in the Developing Rat Embryo. J. Cell Biol. 1991, 114, 1261–1273. [Google Scholar] [CrossRef] [Green Version]
- Gupta, A.; Bazer, F.W.; Jaeger, L.A. Immunolocalization of Acidic and Basic Fibroblast Growth Factors in Porcine Uterine and Conceptus Tissues. Biol. Reprod. 1997, 56, 1527–1536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niswander, L.; Martin, G.R. Fgf-4 Expression during Gastrulation, Myogenesis, Limb and Tooth Development in the Mouse. Development 1992, 114, 755–768. [Google Scholar] [CrossRef] [PubMed]
- Laufer, E.; Nelson, C.E.; Johnson, R.L.; Morgan, B.A.; Tabin, C. Sonic Hedgehog and Fgf-4 Act through a Signaling Cascade and Feedback Loop to Integrate Growth and Patterning of the Developing Limb Bud. Cell 1994, 79, 993–1003. [Google Scholar] [CrossRef]
- Pizette, S.; Coulier, F.; Birnbaum, D.; DeLapeyrière, O. FGF6 Modulates the Expression of Fibroblast Growth Factor Receptors and Myogenic Genes in Muscle Cells. Exp. Cell Res. 1996, 224, 143–151. [Google Scholar] [CrossRef]
- Pfarrer, C.; Weise, S.; Berisha, B.; Schams, D.; Leiser, R.; Hoffmann, B.; Schuler, G. Fibroblast Growth Factor (FGF)-1, FGF2, FGF7 and FGF Receptors Are Uniformly Expressed in Trophoblast Giant Cells during Restricted Trophoblast Invasion in Cows. Placenta 2006, 27, 758–770. [Google Scholar] [CrossRef]
- Cormier, S.; Leroy, C.; Delezoide, A.-L.; Silve, C. Expression of Fibroblast Growth Factors 18 and 23 during Human Embryonic and Fetal Development. Gene Expr. Patterns 2005, 5, 569–573. [Google Scholar] [CrossRef]
- Rinderknecht, E.; Humbel, R.E. The Amino Acid Sequence of Human Insulin-like Growth Factor I and Its Structural Homology with Proinsulin. J. Biol. Chem. 1978, 253, 2769–2776. [Google Scholar] [CrossRef]
- Roberts, C.T.; Owens, J.A.; Sferruzzi-Perri, A.N. Distinct Actions of Insulin-like Growth Factors (IGFs) on Placental Development and Fetal Growth: Lessons from Mice and Guinea Pigs. Placenta 2008, 29 (Suppl. A), S42–S47. [Google Scholar] [CrossRef] [PubMed]
- Germain-Lee, E.L.; Janicot, M.; Lammers, R.; Ullrich, A.; Casella, S.J. Expression of a Type I Insulin-like Growth Factor Receptor with Low Affinity for Insulin-like Growth Factor II. Biochem. J. 1992, 281 Pt 2, 413–417. [Google Scholar] [CrossRef] [Green Version]
- Ullrich, A.; Gray, A.; Tam, A.W.; Yang-Feng, T.; Tsubokawa, M.; Collins, C.; Henzel, W.; Le Bon, T.; Kathuria, S.; Chen, E. Insulin-like Growth Factor I Receptor Primary Structure: Comparison with Insulin Receptor Suggests Structural Determinants That Define Functional Specificity. EMBO J. 1986, 5, 2503–2512. [Google Scholar] [CrossRef]
- Liu, J.P.; Baker, J.; Perkins, A.S.; Robertson, E.J.; Efstratiadis, A. Mice Carrying Null Mutations of the Genes Encoding Insulin-like Growth Factor I (Igf-1) and Type 1 IGF Receptor (Igf1r). Cell 1993, 75, 59–72. [Google Scholar] [CrossRef]
- Charnock, J.C.; Dilworth, M.R.; Aplin, J.D.; Sibley, C.P.; Westwood, M.; Crocker, I.P. The Impact of a Human IGF-II Analog ([Leu27]IGF-II) on Fetal Growth in a Mouse Model of Fetal Growth Restriction. Am. J. Physiol. Endocrinol. Metab. 2016, 310, E24–E31. [Google Scholar] [CrossRef] [Green Version]
- Fan, B.; Onteru, S.K.; Du, Z.-Q.; Garrick, D.J.; Stalder, K.J.; Rothschild, M.F. Genome-Wide Association Study Identifies Loci for Body Composition and Structural Soundness Traits in Pigs. PLoS ONE 2011, 6, e14726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ouni, M.; Gunes, Y.; Belot, M.-P.; Castell, A.-L.; Fradin, D.; Bougnères, P. The IGF1 P2 Promoter Is an Epigenetic QTL for Circulating IGF1 and Human Growth. Clin. Epigenetics 2015, 7, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spiroski, A.M.; Oliver, M.H.; Jaquiery, A.L.; Prickett, T.C.R.; Espiner, E.A.; Harding, J.E.; Bloomfield, F.H. Postnatal Effects of Intrauterine Treatment of the Growth-Restricted Ovine Fetus with Intra-Amniotic Insulin-like Growth Factor-1. J. Physiol. 2018, 596, 5925–5945. [Google Scholar] [CrossRef] [Green Version]
- Sutter, N.B.; Bustamante, C.D.; Chase, K.; Gray, M.M.; Zhao, K.; Zhu, L.; Padhukasahasram, B.; Karlins, E.; Davis, S.; Jones, P.G.; et al. A Single IGF1 Allele Is a Major Determinant of Small Size in Dogs. Science 2007, 316, 112–115. [Google Scholar] [CrossRef] [Green Version]
- Clemmons, D.R.; Maile, L.A. Interaction between Insulin-like Growth Factor-I Receptor and AlphaVbeta3 Integrin Linked Signaling Pathways: Cellular Responses to Changes in Multiple Signaling Inputs. Mol. Endocrinol. 2005, 19, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kitamura, T.; Kahn, C.R.; Accili, D. Insulin Receptor Knockout Mice. Annu. Rev. Physiol. 2003, 65, 313–332. [Google Scholar] [CrossRef]
- Ipsa, E.; Cruzat, V.F.; Kagize, J.N.; Yovich, J.L.; Keane, K.N. Growth Hormone and Insulin-Like Growth Factor Action in Reproductive Tissues. Front. Endocrinol. 2019, 10, 777. [Google Scholar] [CrossRef]
- Lighten, A.D.; Hardy, K.; Winston, R.M.L.; Moore, G.E. Expression of MRNA for the Insulin-like Growth Factors and Their Receptors in Human Preimplantation Embryos. Mol. Reprod. Dev. 1997, 47, 134–139. [Google Scholar] [CrossRef]
- Ozden-Akkaya, O.; Altunbas, K.; Yagcı, A. Effects of Methoxychlor on IGF-I Signaling Pathway in Rat Ovary. Biotech. Histochem. 2017, 92, 230–242. [Google Scholar] [CrossRef]
- Giroto, A.B.; Fontes, P.K.; Franchi, F.F.; Dos Santos, P.H.; Razza, E.M.; Nogueira, M.F.G.; Maioli, M.A.; Nogueira, G.P.; Nunes, G.B.; Mingoti, G.Z.; et al. Use of Pregnancy-Associated Plasma Protein-A during Oocyte in Vitro Maturation Increases IGF-1 and Affects the Transcriptional Profile of Cumulus Cells and Embryos from Nelore Cows. Mol. Reprod. Dev. 2019, 86, 1694–1704. [Google Scholar] [CrossRef] [PubMed]
- Lopes, A.C.; Palhão, M.P.; Fernandes, C.; Sudano, M.J.; Castilho, A.; Caixeta, E.S. Differential Expression of Insulin-like Growth Factor Family Members in Immature Cumulus-Oocyte Complexes from Dairy Cows with Different Genotypes. Reprod. Domest. Anim. 2017, 52, 1067–1073. [Google Scholar] [CrossRef] [PubMed]
- Balogh, O.; Müller, L.; Boos, A.; Kowalewski, M.P.; Reichler, I.M. Expression of Insulin-like Growth Factor 1 and Its Receptor in Preovulatory Follicles and in the Corpus Luteum in the Bitch. Gen. Comp. Endocrinol. 2018, 269, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Herrick, J.R.; Greene-Ermisch, A.F.; Schoolcraft, W.B.; Krisher, R.L. Exogenous Growth Factors Do Not Affect the Development of Individually Cultured Murine Embryos. J. Assist. Reprod. Genet. 2018, 35, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Javvaji, P.K.; Dhali, A.; Francis, J.R.; Kolte, A.P.; Roy, S.C.; Selvaraju, S.; Mech, A.; Sejian, V. IGF-1 Treatment during in Vitro Maturation Improves Developmental Potential of Ovine Oocytes through the Regulation of PI3K/Akt and Apoptosis Signaling. Anim. Biotechnol. 2020, 1–8. [Google Scholar] [CrossRef]
- Rodrigues, T.A.; Ispada, J.; Risolia, P.H.B.; Rodrigues, M.T.; Lima, R.S.; Assumpção, M.E.O.A.; Visintin, J.A.; Paula-Lopes, F.F. Thermoprotective Effect of Insulin-like Growth Factor 1 on in Vitro Matured Bovine Oocyte Exposed to Heat Shock. Theriogenology 2016, 86, 2028–2039. [Google Scholar] [CrossRef]
- Bonometti, S.; Menarim, B.C.; Reinholt, B.M.; Ealy, A.D.; Johnson, S.E. Growth Factor Modulation of Equine Trophoblast Mitosis and Prostaglandin Gene Expression. J. Anim. Sci. 2019, 97, 865–873. [Google Scholar] [CrossRef]
- Wamaitha, S.E.; Grybel, K.J.; Alanis-Lobato, G.; Gerri, C.; Ogushi, S.; McCarthy, A.; Mahadevaiah, S.K.; Healy, L.; Lea, R.A.; Molina-Arcas, M.; et al. IGF1-Mediated Human Embryonic Stem Cell Self-Renewal Recapitulates the Embryonic Niche. Nat. Commun. 2020, 11, 764. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghanipoor-Samami, M.; Javadmanesh, A.; Burns, B.M.; Thomsen, D.A.; Nattrass, G.S.; Estrella, C.A.S.; Kind, K.L.; Hiendleder, S. Atlas of Tissue- and Developmental Stage Specific Gene Expression for the Bovine Insulin-like Growth Factor (IGF) System. PLoS ONE 2018, 13, e0200466. [Google Scholar] [CrossRef]
- Sessions-Bresnahan, D.R.; Heuberger, A.L.; Carnevale, E.M. Obesity in Mares Promotes Uterine Inflammation and Alters Embryo Lipid Fingerprints and Homeostasis. Biol. Reprod. 2018, 99, 761–772. [Google Scholar] [CrossRef]
- Zhang, J.-J.; Liu, X.; Chen, L.; Zhang, S.; Zhang, X.; Hao, C.; Miao, Y.-L. Advanced Maternal Age Alters Expression of Maternal Effect Genes That Are Essential for Human Oocyte Quality. Aging (Albany N. Y.) 2020, 12, 3950–3961. [Google Scholar] [CrossRef]
- Liu, H.C.; He, Z.Y.; Mele, C.A.; Veeck, L.L.; Davis, O.; Rosenwaks, Z. Human Endometrial Stromal Cells Improve Embryo Quality by Enhancing the Expression of Insulin-like Growth Factors and Their Receptors in Cocultured Human Preimplantation Embryos. Fertil. Steril. 1999, 71, 361–367. [Google Scholar] [CrossRef]
- Kowalik, A.; Liu, H.C.; He, Z.Y.; Mele, C.; Barmat, L.; Rosenwaks, Z. Expression of the Insulin-like Growth Factor-1 Gene and Its Receptor in Preimplantation Mouse Embryos; Is It a Marker of Embryo Viability? Mol. Hum. Reprod. 1999, 5, 861–865. [Google Scholar] [CrossRef] [PubMed]
- Korgun, E.T.; Dohr, G.; Desoye, G.; Demir, R.; Kayisli, U.A.; Hahn, T. Expression of Insulin, Insulin-like Growth Factor I and Glucocorticoid Receptor in Rat Uterus and Embryo during Decidualization, Implantation and Organogenesis. Reproduction 2003, 125, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Lonergan, P.; Gutiérrez-Adán, A.; Pintado, B.; Fair, T.; Ward, F.; Fuente, J.D.L.; Boland, M. Relationship between Time of First Cleavage and the Expression of IGF-I Growth Factor, Its Receptor, and Two Housekeeping Genes in Bovine Two-Cell Embryos and Blastocysts Produced in Vitro. Mol. Reprod. Dev. 2000, 57, 146–152. [Google Scholar] [CrossRef]
- Watson, A.J.; Watson, P.H.; Arcellana-Panlilio, M.; Warnes, D.; Walker, S.K.; Schultz, G.A.; Armstrong, D.T.; Seamark, R.F. A Growth Factor Phenotype Map for Ovine Preimplantation Development. Biol. Reprod. 1994, 50, 725–733. [Google Scholar] [CrossRef] [Green Version]
- Guo, X.; Yang, D.; Ao, X.; Wu, X.; Li, G.; Wang, L.; Bao, M.-T.; Xue, L.; Bou, S. Production of Transgenic Cashmere Goat Embryos Expressing Red Fluorescent Protein and Containing IGF1 Hair-Follicle-Cell Specific Expression Cassette by Somatic Cell Nuclear Transfer. Sci. China C Life Sci. 2009, 52, 390–397. [Google Scholar] [CrossRef]
- Kautz, E.; Gram, A.; Aslan, S.; Ay, S.S.; Selçuk, M.; Kanca, H.; Koldaş, E.; Akal, E.; Karakaş, K.; Findik, M.; et al. Expression of Genes Involved in the Embryo-Maternal Interaction in the Early-Pregnant Canine Uterus. Reproduction 2014, 147, 703–717. [Google Scholar] [CrossRef] [Green Version]
- Pandey, S.P.; Chandel, H.S.; Srivastava, S.; Selvaraj, S.; Jha, M.K.; Shukla, D.; Ebensen, T.; Guzman, C.A.; Saha, B. Pegylated Bisacycloxypropylcysteine, a Diacylated Lipopeptide Ligand of TLR6, Plays a Host-Protective Role against Experimental Leishmania Major Infection. J. Immunol. 2014, 193, 3632–3643. [Google Scholar] [CrossRef]
- Huang, B.; Ning, S.; Zhang, Q.; Chen, A.; Jiang, C.; Cui, Y.; Hu, J.; Li, H.; Fan, G.; Qin, L.; et al. Bisphenol A Represses Dopaminergic Neuron Differentiation from Human Embryonic Stem Cells through Downregulating the Expression of Insulin-like Growth Factor 1. Mol. Neurobiol. 2017, 54, 3798–3812. [Google Scholar] [CrossRef]
- Yucel Cicek, O.S.; Hekimoglu, E.R.; Turgal, M.; Atilla, P.; Cakar, A.N.; Usubutun, A.; Beksac, M.S. Differential Expression of Leukemia Inhibitory Factor and Insulin like Growth Factor-1 between Normal Pregnancies, Partial Hydatidiform Moles and Complete Hydatidiform Moles. Placenta 2018, 69, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Kidder, G.M.; Watson, A.J.; Schultz, G.A.; Armstrong, D.T. Possible Roles of Insulin and Insulin-like Growth Factors in Rat Preimplantation Development: Investigation of Gene Expression by Reverse Transcription-Polymerase Chain Reaction. J. Reprod. Fertil. 1994, 100, 375–380. [Google Scholar] [CrossRef] [Green Version]
- Bartolac, L.K.; Lowe, J.L.; Koustas, G.; Grupen, C.G.; Sjöblom, C. Vitrification, Not Cryoprotectant Exposure, Alters the Expression of Developmentally Important Genes in in Vitro Produced Porcine Blastocysts. Cryobiology 2018, 80, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Hernández, R.; Rodríguez, F.M.; Gareis, N.C.; Rey, F.; Barbeito, C.G.; Diessler, M.E. Abundance of Insulin-like Growth Factors 1 and 2, and Type 1 Insulin-like Growth Factor Receptor in Placentas of Dogs. Anim. Reprod. Sci. 2020, 221, 106554. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Huang, X.; Yang, X.; Liang, X. Maternal Obesity in Mice Not Only Affects Fresh Embryo Quality but Also Aggravates Injury Due to Vitrification. J. Assist. Reprod. Genet. 2016, 33, 1515–1523. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.M.; Feng, H.L.; Ma, Y.Z.; Cang, M.; Li, H.J.; Yan, Z.; Zhou, P.; Wen, J.X.; Bou, S.; Liu, D.J. Expression of IGF Receptors and Its Ligands in Bovine Oocytes and Preimplantation Embryos. Anim. Reprod. Sci. 2009, 114, 99–108. [Google Scholar] [CrossRef]
- Han, V.K.; Bassett, N.; Walton, J.; Challis, J.R. The Expression of Insulin-like Growth Factor (IGF) and IGF-Binding Protein (IGFBP) Genes in the Human Placenta and Membranes: Evidence for IGF-IGFBP Interactions at the Feto-Maternal Interface. J. Clin. Endocrinol. Metab. 1996, 81, 2680–2693. [Google Scholar] [CrossRef]
- Agaoglu, O.K.; Agaoglu, A.R.; Guzeloglu, A.; Aslan, S.; Kurar, E.; Kayis, S.A.; Schäfer-Somi, S. Gene Expression Profiles of Some Cytokines, Growth Factors, Receptors, and Enzymes (GM-CSF, IFNγ, MMP-2, IGF-II, EGF, TGF-β, IGF-IIR) during Pregnancy in the Cat Uterus. Theriogenology 2016, 85, 638–644. [Google Scholar] [CrossRef]
- Waurich, R.; Ringleb, J.; Braun, B.C.; Jewgenow, K. Embryonic Gene Activation in in Vitro Produced Embryos of the Domestic Cat (Felis Catus). Reproduction 2010, 140, 531–540. [Google Scholar] [CrossRef]
- Schultz, G.A.; Hogan, A.; Watson, A.J.; Smith, R.M.; Heyner, S. Insulin, Insulin-like Growth Factors and Glucose Transporters: Temporal Patterns of Gene Expression in Early Murine and Bovine Embryos. Reprod. Fertil. Dev. 1992, 4, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Ramin, N.; Thieme, R.; Fischer, S.; Schindler, M.; Schmidt, T.; Fischer, B.; Navarrete Santos, A. Maternal Diabetes Impairs Gastrulation and Insulin and IGF-I Receptor Expression in Rabbit Blastocysts. Endocrinology 2010, 151, 4158–4167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arat, S.; Caputcu, A.T.; Cevik, M.; Akkoc, T.; Cetinkaya, G.; Bagis, H. Effect of Growth Factors on Oocyte Maturation and Allocations of Inner Cell Mass and Trophectoderm Cells of Cloned Bovine Embryos. Zygote 2016, 24, 554–562. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Pan, Y.; Cui, Y.; Wen, Z.; Liu, P.; He, H.; Li, Q.; Peng, X.; Zhao, T.; Yu, S. Insulin-like Growth Factor I Enhances the Developmental Competence of Yak Embryos by Modulating Aquaporin 3. Reprod. Domest. Anim. 2017, 52, 825–835. [Google Scholar] [CrossRef] [PubMed]
- Conceição, J.C.Z.; Moura, M.T.; Ferreira-Silva, J.C.; Cantanhêde, L.F.; Chaves, R.M.; Lima, P.F.; Oliveira, M.A.L. Incidence of Apoptosis after Retinoids and Insulin-like Growth Factor-I (IGF-I) Supplementation during Goat in Vitro Embryo Production. Zygote 2016, 24, 808–813. [Google Scholar] [CrossRef] [PubMed]
- Kelly, J.M.; Kleemann, D.O.; Maxwell, W.M.C.; Walker, S.K. Effects of Insulin-like Growth Factor-I, Epidermal Growth Factor and Cysteamine on the in Vitro Maturation and Development of Oocytes Collected from 6- to 8-Week-Old Merino Lambs. Reprod. Fertil. Dev. 2008, 20, 570–578. [Google Scholar] [CrossRef]
- Liu, H.-B.; Muhammad, T.; Guo, Y.; Li, M.-J.; Sha, Q.-Q.; Zhang, C.-X.; Liu, H.; Zhao, S.-G.; Zhao, H.; Zhang, H.; et al. RNA-Binding Protein IGF2BP2/IMP2 Is a Critical Maternal Activator in Early Zygotic Genome Activation. Adv. Sci. 2019, 6, 1900295. [Google Scholar] [CrossRef] [Green Version]
- Valleh, M.V.; Tahmoorespur, M.; Joupari, M.D.; Dehghani, H.; Rasmussen, M.A.; Hyttel, P.; Strøbech, L. Paternal Breed Effects on Expression of IGF-II, BAK1 and BCL2-L1 in Bovine Preimplantation Embryos. Zygote 2015, 23, 712–721. [Google Scholar] [CrossRef] [Green Version]
- Harris, L.K.; Pantham, P.; Yong, H.E.J.; Pratt, A.; Borg, A.J.; Crocker, I.; Westwood, M.; Aplin, J.; Kalionis, B.; Murthi, P. The Role of Insulin-like Growth Factor 2 Receptor-Mediated Homeobox Gene Expression in Human Placental Apoptosis, and Its Implications in Idiopathic Fetal Growth Restriction. Mol. Hum. Reprod. 2019, 25, 572–585. [Google Scholar] [CrossRef]
- Citri, A.; Yarden, Y. EGF-ERBB Signalling: Towards the Systems Level. Nat. Rev. Mol. Cell Biol. 2006, 7, 505–516. [Google Scholar] [CrossRef]
- Richani, D.; Gilchrist, R.B. The Epidermal Growth Factor Network: Role in Oocyte Growth, Maturation and Developmental Competence. Hum. Reprod. Update 2018, 24, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Da Rosa, P.R.A.; De Cesaro, M.P.; Pereira Dau, A.M.; Duggavathi, R.; Bordignon, V.; Gonçalves, P.B.D. Reversible Meiotic Arrest of Bovine Oocytes by EGFR Inhibition and Follicular Hemisections. Theriogenology 2017, 99, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Sugimura, S.; Yamanouchi, T.; Palmerini, M.G.; Hashiyada, Y.; Imai, K.; Gilchrist, R.B. Effect of Pre-in Vitro Maturation with CAMP Modulators on the Acquisition of Oocyte Developmental Competence in Cattle. J. Reprod. Dev. 2018, 64, 233–241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saraiva, M.V.A.; Rossetto, R.; Brito, I.R.; Celestino, J.J.H.; Silva, C.M.G.; Faustino, L.R.; Almeida, A.P.; Bruno, J.B.; Magalhães, D.M.; Matos, M.H.T.; et al. Dynamic Medium Produces Caprine Embryo from Preantral Follicles Grown in Vitro. Reprod. Sci. 2010, 17, 1135–1143. [Google Scholar] [CrossRef] [PubMed]
- Wasielak, M.; Więsak, T.; Bogacka, I.; Jalali, B.M.; Bogacki, M. Maternal Effect Gene Expression in Porcine Metaphase II Oocytes and Embryos in Vitro: Effect of Epidermal Growth Factor, Interleukin-1β and Leukemia Inhibitory Factor. Zygote 2017, 25, 120–130. [Google Scholar] [CrossRef]
- Mesalam, A.; Lee, K.-L.; Khan, I.; Chowdhury, M.M.R.; Zhang, S.; Song, S.-H.; Joo, M.-D.; Lee, J.-H.; Jin, J.-I.; Kong, I.-K. A Combination of Bovine Serum Albumin with Insulin-Transferrin-Sodium Selenite and/or Epidermal Growth Factor as Alternatives to Fetal Bovine Serum in Culture Medium Improves Bovine Embryo Quality and Trophoblast Invasion by Induction of Matrix Metalloproteinases. Reprod. Fertil. Dev. 2019, 31, 333–346. [Google Scholar] [CrossRef]
- Chediek Dall’Acqua, P.; Barros Nunes, G.; Rodrigues da Silva, C.; Fontes, P.K.; Fábio Gouveia Nogueira, M.; Lombardi Lopes, F.; Marinho, M.; Zoccal Mingoti, G. Differences in Embryonic Gene Expression and Quality Indicate the Benefit of Epidermal Growth Factor Receptor Inhibitor during Prematuration to Improve Competence in Bovine Oocytes. Reprod. Domest. Anim. 2019, 54, 666–677. [Google Scholar] [CrossRef] [PubMed]
- Cheng, E.-H.; Liu, J.-Y.; Lee, T.-H.; Huang, C.-C.; Chen, C.-I.; Huang, L.-S.; Lee, M.-S. Requirement of Leukemia Inhibitory Factor or Epidermal Growth Factor for Pre-Implantation Embryogenesis via JAK/STAT3 Signaling Pathways. PLoS ONE 2016, 11, e0153086. [Google Scholar] [CrossRef] [PubMed]
- Park, K.-M.; Wang, J.W.; Yoo, Y.-M.; Choi, M.J.; Hwang, K.C.; Jeung, E.-B.; Jeong, Y.W.; Hwang, W.S. Sphingosine-1-Phosphate (S1P) Analog Phytosphingosine-1-Phosphate (P1P) Improves the in Vitro Maturation Efficiency of Porcine Oocytes via Regulation of Oxidative Stress and Apoptosis. Mol. Reprod. Dev. 2019, 86, 1705–1719. [Google Scholar] [CrossRef]
- Flores, J.M.; Sánchez, M.A.; García, P.; Sánchez, B.; Nieto, A. Immunohistochemical Localization of Epidermal Growth Factor, Transforming Growth Factor-Alpha and Growth Factor-Beta s in the Caprine Peri-Implantation Period. Theriogenology 1998, 50, 931–944. [Google Scholar] [CrossRef]
- Gharib-Hamrouche, N.; Chêne, N.; Guillomot, M.; Martal, J. Localization and Characterization of EGF/TGF-Alpha Receptors on Peri-Implantation Trophoblast in Sheep. J. Reprod. Fertil. 1993, 98, 385–392. [Google Scholar] [CrossRef] [Green Version]
- Jeong, W.; Jung, S.; Bazer, F.W.; Song, G.; Kim, J. Epidermal Growth Factor: Porcine Uterine Luminal Epithelial Cell Migratory Signal during the Peri-Implantation Period of Pregnancy. Mol. Cell Endocrinol. 2016, 420, 66–74. [Google Scholar] [CrossRef]
- Takeuchi, M.; Seki, M.; Furukawa, E.; Takahashi, A.; Saito, K.; Kobayashi, M.; Ezoe, K.; Fukui, E.; Yoshizawa, M.; Matsumoto, H. Improvement of Implantation Potential in Mouse Blastocysts Derived from IVF by Combined Treatment with Prolactin, Epidermal Growth Factor and 4-Hydroxyestradiol. Mol. Hum. Reprod. 2017, 23, 557–570. [Google Scholar] [CrossRef] [PubMed]
- Crispo, M.; Dos Santos-Neto, P.C.; Vilariño, M.; Mulet, A.P.; de León, A.; Barbeito, L.; Menchaca, A. RAPID COMMUNICATION: Nerve Growth Factor Influences Cleavage Rate and Embryo Development in Sheep. J. Anim. Sci. 2016, 94, 4447–4451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia-Garcia, R.M.; Arias-Alvarez, M.; Sanchez-Rodriguez, A.; Lorenzo, P.L.; Rebollar, P.G. Role of Nerve Growth Factor in the Reproductive Physiology of Female Rabbits: A Review. Theriogenology 2020, 150, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Garfield, A.S.; Cowley, M.; Smith, F.M.; Moorwood, K.; Stewart-Cox, J.E.; Gilroy, K.; Baker, S.; Xia, J.; Dalley, J.W.; Hurst, L.D.; et al. Distinct Physiological and Behavioural Functions for Parental Alleles of Imprinted Grb10. Nature 2011, 469, 534–538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, X.; Song, M.; Yang, X.; Liu, X.; Liu, K.; Jiao, C.; Wang, J.; Bai, C.; Su, G.; Liu, X.; et al. Establishment of Bovine Embryonic Stem Cells after Knockdown of CDX2. Sci. Rep. 2016, 6, 28343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gómez, E.; Carrocera, S.; Martin, D.; Sánchez-Calabuig, M.J.; Gutiérrez-Adán, A.; Murillo, A.; Muñoz, M. Hepatoma-Derived Growth Factor: Protein Quantification in Uterine Fluid, Gene Expression in Endometrial-Cell Culture and Effects on in Vitro Embryo Development, Pregnancy and Birth. Theriogenology 2017, 96, 118–125. [Google Scholar] [CrossRef]
- Hong, L.; He, Y.; Tan, C.; Wu, Z.; Yu, M. HAI-1 Regulates Placental Folds Development by Influencing Trophoblast Cell Proliferation and Invasion in Pigs. Gene 2020, 749, 144721. [Google Scholar] [CrossRef] [PubMed]
- Kohama, K.; Kawaguchi, M.; Fukushima, T.; Lin, C.-Y.; Kataoka, H. Regulation of Pericellular Proteolysis by Hepatocyte Growth Factor Activator Inhibitor Type 1 (HAI-1) in Trophoblast Cells. Hum. Cell 2012, 25, 100–110. [Google Scholar] [CrossRef]
- Robertson, S.A.; Sjöblom, C.; Jasper, M.J.; Norman, R.J.; Seamark, R.F. Granulocyte-Macrophage Colony-Stimulating Factor Promotes Glucose Transport and Blastomere Viability in Murine Preimplantation Embryos. Biol. Reprod. 2001, 64, 1206–1215. [Google Scholar] [CrossRef] [Green Version]
- Larson, R.C.; Ignotz, G.G.; Currie, W.B. Platelet Derived Growth Factor (PDGF) Stimulates Development of Bovine Embryos during the Fourth Cell Cycle. Development 1992, 115, 821–826. [Google Scholar] [CrossRef] [PubMed]
- Thibodeaux, J.K.; Del Vecchio, R.P.; Hansel, W. Role of Platelet-Derived Growth Factor in Development of in Vitro Matured and in Vitro Fertilized Bovine Embryos. J. Reprod. Fertil. 1993, 98, 61–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawamura, K.; Chen, Y.; Shu, Y.; Cheng, Y.; Qiao, J.; Behr, B.; Pera, R.A.R.; Hsueh, A.J.W. Promotion of Human Early Embryonic Development and Blastocyst Outgrowth in Vitro Using Autocrine/Paracrine Growth Factors. PLoS ONE 2012, 7, e49328. [Google Scholar] [CrossRef] [Green Version]
- Jannaman, E.A.; Xiao, Y.; Hansen, P.J. Actions of Colony-Stimulating Factor 3 on the Maturing Oocyte and Developing Embryo in Cattle. J. Anim. Sci. 2020, 98, skaa115. [Google Scholar] [CrossRef] [PubMed]
| FGF Family Member | Localization | Species |
|---|---|---|
| FGF-1 | Mesoderm | Mouse [96] |
| Late embryo (Day 12.5) | Mouse [82] | |
| Trophectoderm | Human [97] | |
| Mesoderm | Human [98] | |
| Mesoderm | Rat [99] | |
| Trophectoderm | Cattle [74] | |
| FGF-2 | Trophectoderm | Human [97] |
| Mesoderm | Rat [99] | |
| Trophectoderm | Cattle [74] | |
| Ectoderm | Pig [100] | |
| Mesoderm | Pig [100] | |
| Endoderm | Pig [100] | |
| FGF-4 | ICM | Mouse [101] |
| FGF-6 | Somites | Mouse [102] |
| Myoblasts | Mouse [103] | |
| FGF-7 | Trophectoderm | Cow [104] |
| FGF-10 | Trophectoderm | Cattle [75] |
| Teca cells | Human [88] | |
| FGF-18 | Late embryo (Day 30) | Human [105] |
| FGF-23 | Late embryo (Day 30) | Human [105] |
| IGF System Compound | Localization | Species |
|---|---|---|
| IGF-1 | Blastocyst | Human [132], mouse [133], rat [134], cattle [135], sheep [136], goat [137], rabbit [48], dog [138], buffalo [139] |
| Early embryo | Human [140], cattle [129], horse [130] | |
| Early placenta or pregnant endometrium | Human [141], rat [142], rabbit [48], pig [143], dog [144], horse [127] | |
| IGF-2 | Blastocyst | Human [119], mouse [145], rat [142], cattle [146], sheep [137], pig [143], rabbit [48], goat [22], dog [138], horse [130], buffalo [139] |
| Early embryo | Cattle [129] | |
| Early placenta or pregnant endometrium | Human [147], rat [142], rabbit [48], dog [144], cat [148] | |
| IGFR-1 | Blastocyst | Human [119], mouse [133], rat [134], cattle [135], sheep [136], rabbit [48], horse [130], cat [149], buffalo [139] |
| Early embryo | Cattle [129] | |
| Early placenta or pregnant endometrium | Dog [144], rabbit [48], rat [134] | |
| IGFR-2 | Blastocyst | Human [119], rat [134], cattle [146], pig [143], goat [22], rabbit [48], cat [149], horse [130], buffalo [139] |
| Early embryo | Cattle [129] | |
| Early placenta or pregnant endometrium | Rabbit [48], cat [148] | |
| IR | Blastocyst | Human [119], mouse [150], rat [134], rabbit [151], cattle [150], sheep [136] |
| Early embryo | Cattle [129] | |
| Early placenta or pregnant endometrium | Rat [134] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Llobat, L. Pluripotency and Growth Factors in Early Embryonic Development of Mammals: A Comparative Approach. Vet. Sci. 2021, 8, 78. https://doi.org/10.3390/vetsci8050078
Llobat L. Pluripotency and Growth Factors in Early Embryonic Development of Mammals: A Comparative Approach. Veterinary Sciences. 2021; 8(5):78. https://doi.org/10.3390/vetsci8050078
Chicago/Turabian StyleLlobat, Lola. 2021. "Pluripotency and Growth Factors in Early Embryonic Development of Mammals: A Comparative Approach" Veterinary Sciences 8, no. 5: 78. https://doi.org/10.3390/vetsci8050078
APA StyleLlobat, L. (2021). Pluripotency and Growth Factors in Early Embryonic Development of Mammals: A Comparative Approach. Veterinary Sciences, 8(5), 78. https://doi.org/10.3390/vetsci8050078






