Comparison of Fat Harvested from Flank and Falciform Regions for Stem Cell Therapy in Dogs
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Recruitment and Imaging Evaluation
2.2. Fat Harvest and Culture of MSCs
2.3. Stromal Vascular Fraction (SVF) and Colony Forming Units (CFU)
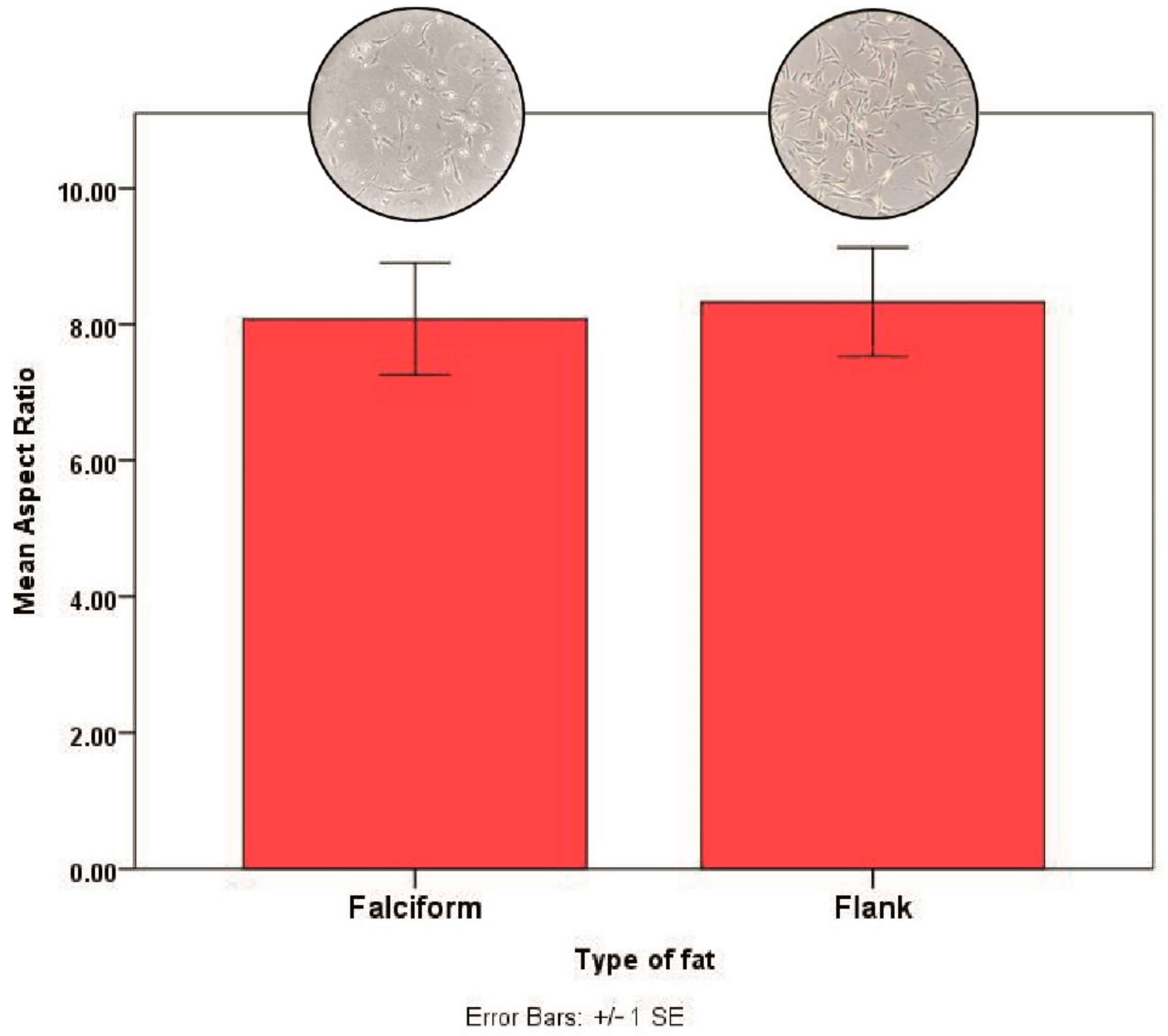
2.4. Cell Morphology
2.5. Statistical Analysis
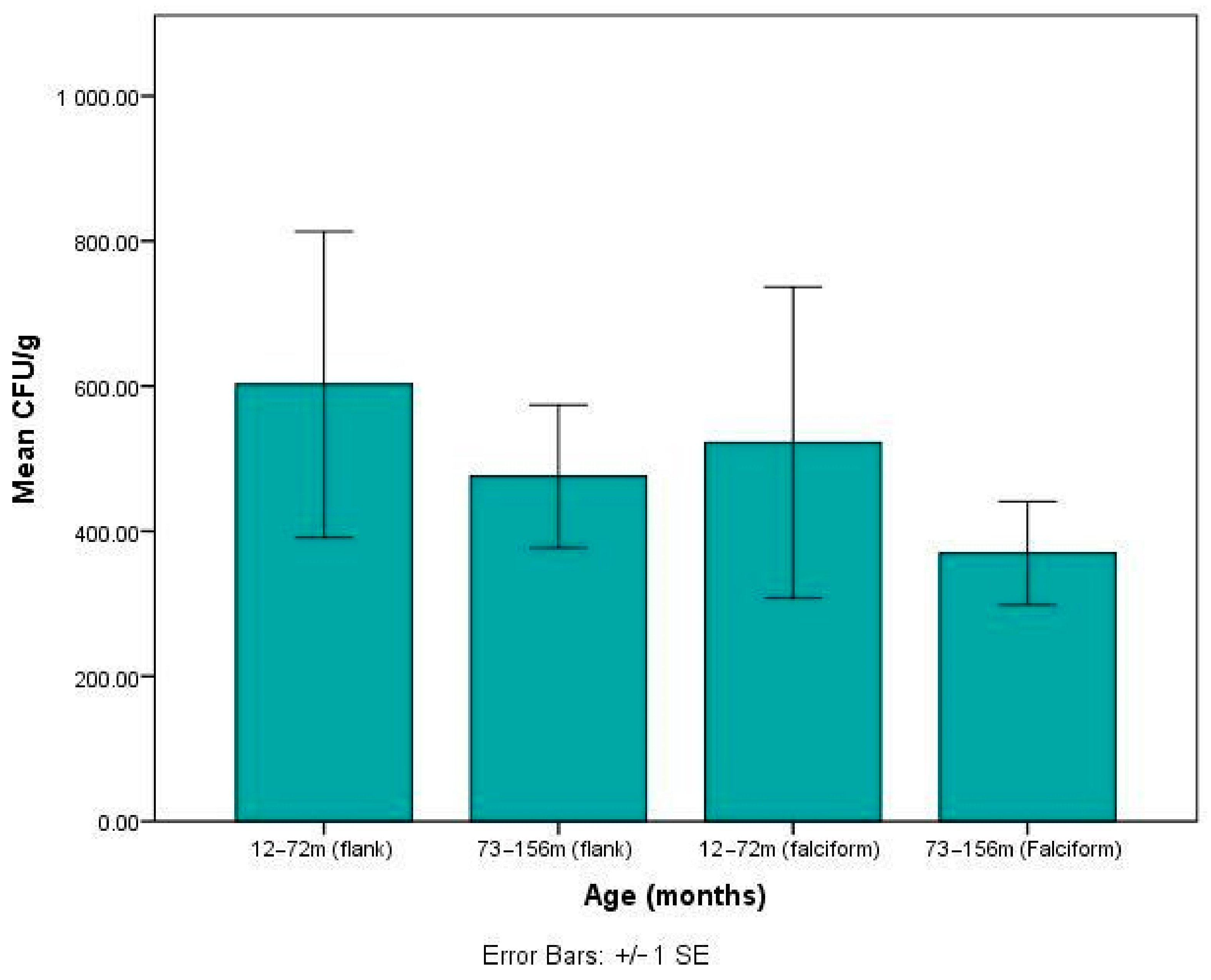
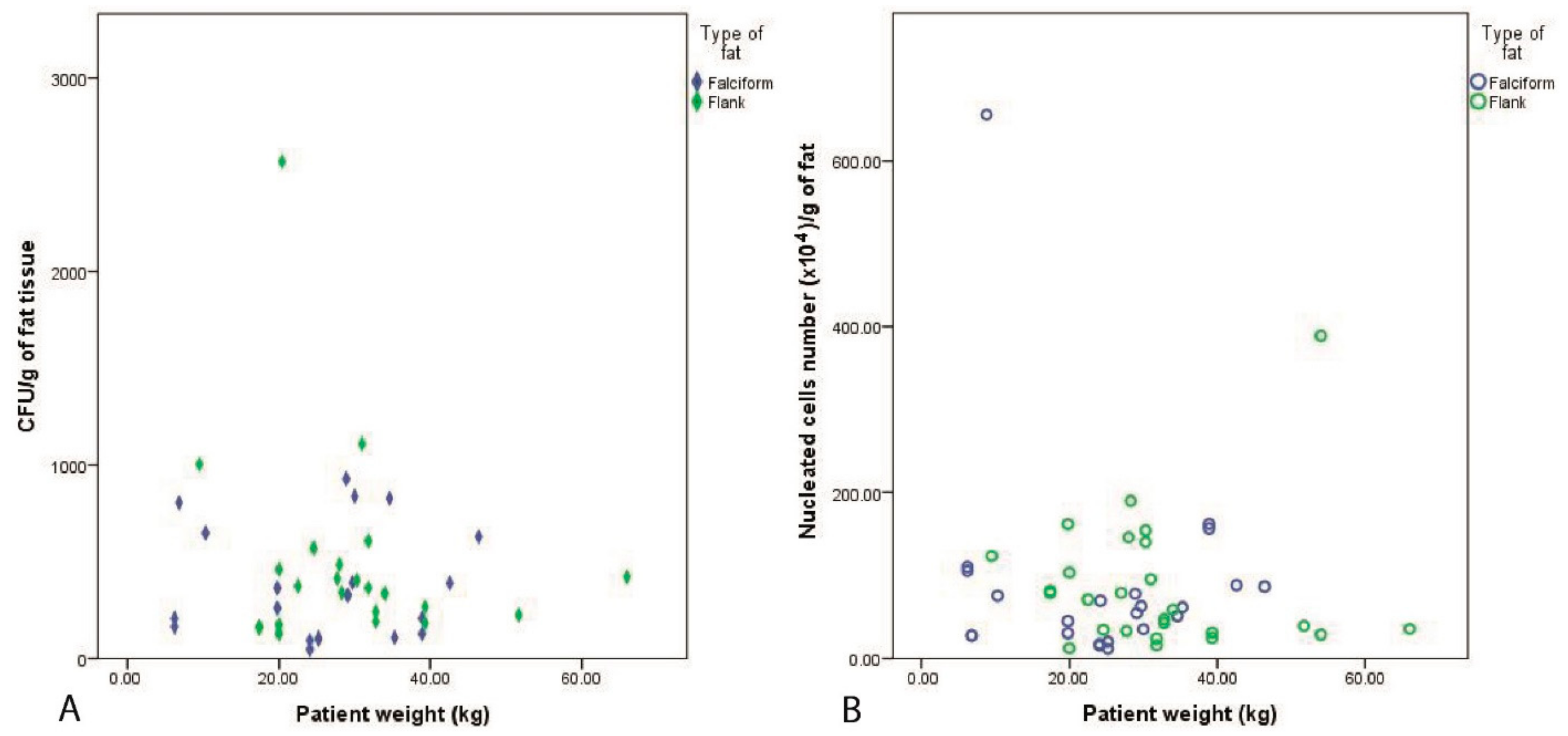
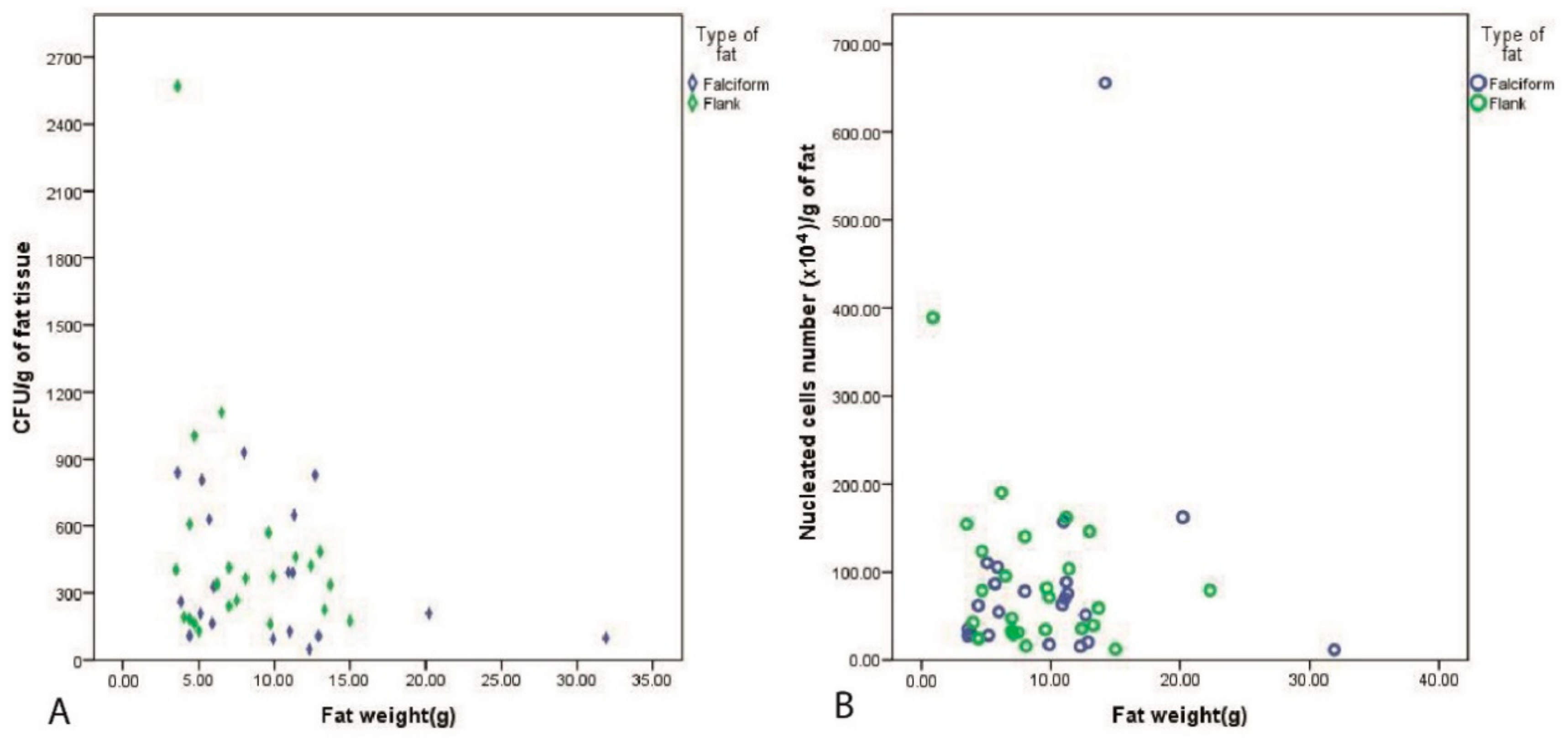
3. Results
3.1. Patient Recruitment and Imaging Evaluation
3.2. Fat Harvest and Culture of MSCs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cabon, Q.; Febre, M.; Gomez, N.; Cachon, T.; Pillard, P.; Carozzo, C.; Saulnier, N.; Robert, C.; Livet, V.; Rakic, R.; et al. Long-term safety and efficacy of single or repeated intra-articular injection of allogeneic neonatal mesenchymal stromal cells for managing pain and lameness in moderate to severe canine osteoarthritis without anti-inflammatory pharmacological support: Pilot clinical study. Front. Veter. Sci. 2019, 6, 10. [Google Scholar] [CrossRef]
- Guercio, A.; Di Bella, S.; Casella, S.; Di Marco, P.; Russo, C.; Piccione, G. Canine mesenchymal stem cells (MSC s): Characterization in relation to donor age and adipose tissue-harvesting site. Cell Biol. Int. 2013, 37, 789–798. [Google Scholar] [CrossRef]
- Caplan, A.I.; Correa, D. The MSC: An injury drugstore. Cell Stem Cell 2011, 9, 11–15. [Google Scholar] [CrossRef]
- Boxall, S.A.; Jones, E.A. Markers for characterization of bone marrow multipotential stromal cells. Stem Cells Int. 2012, 2012, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Bacigalupo, A.; Tong, J.; Podesta, M.; Piaggio, G.; Figari, O.; Colombo, P.; Sogno, G.; Tedone, E.; Moro, F.; Van Lint, M.T. Bone marrow harvest for marrow transplantation: Effect of multiple small (2 mL) or large (20 mL) aspirates. Bone Marrow Transplant. 1992, 9, 467–470. [Google Scholar] [PubMed]
- Hernigou, P.; Poignard, A.; Beaujean, F.; Rouard, H. Percutaneous autologous bone-marrow grafting for nonunions influence of the number and concentration of progenitor cells. J. Bone Jt. Surg. Am. Vol. 2005, 87, 1430–1437. [Google Scholar] [CrossRef]
- Meliga, E.; Strem, B.M.; Duckers, H.J.; Serruys, P.W. Adipose-derived cells. Cell Transpl. 2007, 16, 963–970. [Google Scholar] [CrossRef]
- Astor, D.E.; Hoelzler, M.G.; Harman, R.; Bastian, R.P. Patient factors influencing the concentration of stromal vascular fraction (SVF) for adipose-derived stromal cell (ASC) therapy in dogs. Can. J. Veter. Res. 2013, 77, 177–182. [Google Scholar]
- Pawitan, J.A. Prospect of adipose tissue derived mesenchymal stem cells in regenerative medicine. Cell Tissue Transpl. Ther. 2009, 2, 7–9. [Google Scholar] [CrossRef][Green Version]
- Engels, P.E.; Tremp, M.; Kingham, P.J.; Di Summa, P.G.; Largo, R.D.; Schaefer, D.J.; Kalbermatten, D.F. Harvest site influences the growth properties of adipose derived stem cells. Cytotechnology 2012, 65, 437–445. [Google Scholar] [CrossRef]
- Black, L.L.; Gaynor, J.; Gahring, D.; Adams, C.; Aron, D.; Harman, S.; Gingerich, D.A.; Harman, R.J. Effect of adipose-derived mesenchymal stem and regenerative cells on lameness in dogs with chronic osteoarthritis of the coxofemoral joints: A randomized, double-blinded, multicenter, controlled trial. Veter. Ther. Res. Appl. Veter. Med. 2007, 8, 272. [Google Scholar]
- Jurgens, W.J.F.M.; Oedayrajsingh-Varma, M.J.; Helder, M.N.; ZandiehDoulabi, B.; Schouten, T.E.; Kuik, D.J.; Ritt, M.J.P.F.; Van Milligen-Kummer, F. Effect of tissue-harvesting site on yield of stem cells derived from adipose tissue: Implications for cell-based therapies. Cell Tissue Res. 2008, 332, 415–426. [Google Scholar] [CrossRef]
- Peterson, B.; Zhang, J.; Iglesias, R.; Kabo, M.; Hedrick, M.; Benhaim, P.; Lieberman, J.R. Healing of critically sized femoral defects, using genetically modified mesenchymal stem cells from human adipose tissue. Tissue Eng. 2005, 11, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Prunet-Marcassus, B.; Cousin, B.; Caton, D.; André, M.; Penicaud, L.; Casteilla, L. From heterogeneity to plasticity in adipose tissues: Site-specific differences. Exp. Cell Res. 2006, 312, 727–736. [Google Scholar] [CrossRef] [PubMed]
- Fraser, J.K.; Wulur, I.; Alfonso, Z.; Hedrick, M.H. Fat tissue: An underappreciated source of stem cells for biotechnology. Trends Biotechnol. 2006, 24, 150–154. [Google Scholar] [CrossRef]
- Strem, B.M.; Hicok, K.C.; Zhu, M.; Wulur, I.; Alfonso, Z.; Schreiber, R.E.; Fraser, J.K.; Hedrick, M.H. Multipotential differentiation of adipose tissue-derived stem cells. Keio J. Med. 2005, 54, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Koch, T.G.; Berg, L.C.; Betts, D.H. Current and future regenerative medicine—Principles, concepts, and therapeutic use of stem cell therapy and tissue engineering in equine medicine. Can. Veter J. 2009, 50, 155–165. [Google Scholar]
- Nixon, A.J.; Dahlgren, L.A.; Haupt, J.L.; Yeager, A.E.; Ward, D.L. Effect of adipose-derived nucleated cell fractions on tendon repair in horses with collagenase-induced tendinitis. Am. J. Veter. Res. 2008, 69, 928–937. [Google Scholar] [CrossRef]
- Johnston, S.A. Osteoarthritis: Joint anatomy, physiology, and pathobiology. Vet. Clin. N. Am. Small Anim. Pract. 1997, 27, 699–723. [Google Scholar] [CrossRef]
- Black, L.L.; Gaynor, J.; Adams, C.; Dhupa, S.; Sams, A.E.; Taylor, R.; Harman, S.; Gingerich, D.A.; Harman, R.J. Effect of intraarticular injection of autologous adipose-derived mesenchymal stem and regenerative cells on clinical signs of chronic osteoarthritis of the elbow joint in dogs. Veter. Ther. Res. Appl. Veter. Med. 2008, 9, 192–200. [Google Scholar]
- Arnhold, S.; Wenisch, S. Adipose tissue derived mesenchymal stem cells for musculoskeletal repair in veterinary medicine. Am. J. Stem Cells 2015, 4, 1. [Google Scholar]
- Harasen, G. Stimulating bone growth in the small animal patient: Grafts and beyond! Can. Veter. J. 2011, 52, 199–200. [Google Scholar]
- Alstrup, T.; Eijken, M.; Bohn, A.B.; Møller, B.; Damsgaard, T.E. Isolation of adipose tissue-derived stem cells: Enzymatic digestion in combination with mechanical distortion to increase adipose tissue-derived stem cell yield from human aspirated fat. Curr. Protoc. Stem Cell Biol. 2019, 48, e68. [Google Scholar] [CrossRef] [PubMed]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Pochampally, R. Colony Forming Unit Assays for MSCs. Adv. Struct. Saf. Stud. 2008, 449, 83–91. [Google Scholar] [CrossRef]
- Meeson, R.L.; Sanghani-Keri, A.; Coathup, M.; Blunn, G. VEGF with AMD3100 endogenously mobilizes mesenchymal stem cells and improves fracture healing. J. Orthop. Res. 2019, 37, 1294–1302. [Google Scholar] [CrossRef]
- Alt, E.; Yan, Y.; Gehmert, S.; Song, Y.-H.; Altman, A.; Gehmert, S.; Vykoukal, D.; Bai, X. Fibroblasts share mesenchymal phenotypes with stem cells, but lack their differentiation and colony-forming potential. Biol. Cell 2011, 103, 197–208. [Google Scholar] [CrossRef]
- Yao, X.; Peng, R.; Ding, J. Effects of aspect ratios of stem cells on lineage commitments with and without induction media. Biomaterials 2013, 34, 930–939. [Google Scholar] [CrossRef] [PubMed]
- Vidal, M.A.; Bs, G.E.K.; Lopez, M.J.; Johnson, J.R.; Moore, R.M.; Gimble, J.M. Characterization of equine adipose tissue-derived stromal cells: Adipogenic and osteogenic capacity and comparison with bone marrow-derived mesenchymal stromal cells. Veter. Surg. 2007, 36, 613–622. [Google Scholar] [CrossRef]
- Kern, S.; Eichler, H.; Stoeve, J.; Klüter, H.; Bieback, K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells 2006, 24, 1294–1301. [Google Scholar] [CrossRef]
- Sasaki, A.; Mizuno, M.; Ozeki, N.; Katano, H.; Otabe, K.; Tsuji, K.; Koga, H.; Mochizuki, M.; Sekiya, I. Canine mesenchymal stem cells from synovium have a higher chondrogenic potential than those from infrapatellar fat pad, adipose tissue, and bone marrow. PLoS ONE 2018, 13, e0202922. [Google Scholar] [CrossRef] [PubMed]
- Koga, H.; Muneta, T.; Nagase, T.; Nimura, A.; Ju, Y.-J.; Mochizuki, T.; Sekiya, I. Comparison of mesenchymal tissues-derived stem cells for in vivo chondrogenesis: Suitable conditions for cell therapy of cartilage defects in rabbit. Cell Tissue Res. 2008, 333, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Baglioni, S.; Francalanci, M.; Squecco, R.; Lombardi, A.; Cantini, G.; Angeli, R.; Gelmini, S.; Guasti, D.; Benvenuti, S.; Annunziato, F.; et al. Characterization of human adult stem-cell populations isolated from visceral and subcutaneous adipose tissue. FASEB J. 2009, 23, 3494–3505. [Google Scholar] [CrossRef] [PubMed]
- Metcalf, G.L.; McClure, S.R.; Hostetter, J.M.; Martinez, R.F.; Wang, C. Evaluation of adipose-derived stromal vascular fraction from the lateral tailhead, inguinal region, and mesentery of horses. Can. J. Vet. Res. 2016, 80, 294–301. [Google Scholar]
- Yaneselli, K.M.; Kuhl, C.P.; Terraciano, P.B.; De Oliveira, F.S.; Pizzato, S.B.; Pazza, K.; Magrisso, A.B.; Torman, V.; Rial, A.; Moreno, M.; et al. Comparison of the characteristics of canine adipose tissue-derived mesenchymal stem cells extracted from different sites and at different passage numbers. J. Veter. Sci. 2018, 19, 13–20. [Google Scholar] [CrossRef]
- Requicha, J.F.; Viegas, C.A.; Albuquerque, C.; De Azevedo, J.T.; Reis, R.L.; Gomes, M.E. Effect of anatomical origin and cell passage number on the stemness and osteogenic differentiation potential of canine adipose-derived stem cells. Stem Cell Rev. Rep. 2012, 8, 1211–1222. [Google Scholar] [CrossRef]
- Ranera, B.; Lyahyai, J.; Romero, A.; Vázquez, F.J.; Remacha, A.R.; Bernal, M.L.; Zaragoza, P.; Rodellar, C.; Martín-Burriel, I. Immunophenotype and gene expression profiles of cell surface markers of mesenchymal stem cells derived from equine bone marrow and adipose tissue. Veter. Immunol. Immunopathol. 2011, 144, 147–154. [Google Scholar] [CrossRef]
- Marycz, K.; Kornicka, K.; Marędziak, M.; Golonka, P.; Nicpoń, J. Equine metabolic syndrome impairs adipose stem cells osteogenic differentiation by predominance of autophagy over selective mitophagy. J. Cell. Mol. Med. 2016, 20, 2384–2404. [Google Scholar] [CrossRef]
- Sanghani-Kerai, A.; Osagie, L.; Blunn, G.; Coathup, M. The influence of age and osteoporosis on bone marrow stem cells from rats. Bone Jt. Res. 2018, 7, 289–297. [Google Scholar] [CrossRef]
- Zaim, M.; Karaman, S.; Cetin, G.; Isik, S. Donor age and long-term culture affect differentiation and proliferation of human bone marrow mesenchymal stem cells. Ann. Hematol. 2012, 91, 1175–1186. [Google Scholar] [CrossRef]
- Via, A.G.; Frizziero, A.; Oliva, F. Biological properties of mesenchymal stem cells from different sources. Muscle Ligaments Tendons J. 2012, 2, 154–162. [Google Scholar]
- Zhang, N.; Dietrich, M.A.; Lopez, M.J. Canine intra-articular multipotent stromal cells (MSC) from adipose tissue have the highest in vitro expansion rates, multipotentiality, and MSC immunophenotypes. Veter. Surg. 2013, 42, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, M.O.; Gordon-Evans, W.J.; Fredericks, L.P.; Kiefer, K.; Conzemius, M.G.; Griffon, D.J. Comparison of mesenchymal stem cell surface markers from bone marrow aspirates and adipose stromal vascular fraction sites. Front. Veter. Sci. 2016, 2, 82. [Google Scholar] [CrossRef]
- Lee, R.H.; Kim, B.; Choi, I.; Kim, H.; Choi, H.S.; Suh, K.; Bae, Y.C.; Jung, J.S. Characterization and expression analysis of mesenchymal stem cells from human bone marrow and adipose tissue. Cell. Physiol. Biochem. 2004, 14, 311–324. [Google Scholar] [CrossRef] [PubMed]

| Harvest Site | Flank | Falciform |
|---|---|---|
| Dog numbers | 21 | 17 |
| Median age (months) | 66 | 114.5 |
| Mean patient body weight (kg) | 29.4 ± 12.0 | 26.1 ± 11.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jifcovici, A.; Solano, M.A.; Fitzpatrick, N.; Findji, L.; Blunn, G.; Sanghani-Kerai, A. Comparison of Fat Harvested from Flank and Falciform Regions for Stem Cell Therapy in Dogs. Vet. Sci. 2021, 8, 19. https://doi.org/10.3390/vetsci8020019
Jifcovici A, Solano MA, Fitzpatrick N, Findji L, Blunn G, Sanghani-Kerai A. Comparison of Fat Harvested from Flank and Falciform Regions for Stem Cell Therapy in Dogs. Veterinary Sciences. 2021; 8(2):19. https://doi.org/10.3390/vetsci8020019
Chicago/Turabian StyleJifcovici, Alexandra, Miguel A. Solano, Noel Fitzpatrick, Laurent Findji, Gordon Blunn, and Anita Sanghani-Kerai. 2021. "Comparison of Fat Harvested from Flank and Falciform Regions for Stem Cell Therapy in Dogs" Veterinary Sciences 8, no. 2: 19. https://doi.org/10.3390/vetsci8020019
APA StyleJifcovici, A., Solano, M. A., Fitzpatrick, N., Findji, L., Blunn, G., & Sanghani-Kerai, A. (2021). Comparison of Fat Harvested from Flank and Falciform Regions for Stem Cell Therapy in Dogs. Veterinary Sciences, 8(2), 19. https://doi.org/10.3390/vetsci8020019






