Diagnosis and Treatment of Snake Envenomation in Dogs in Queensland, Australia
Abstract
1. Introduction
2. Materials and Methods
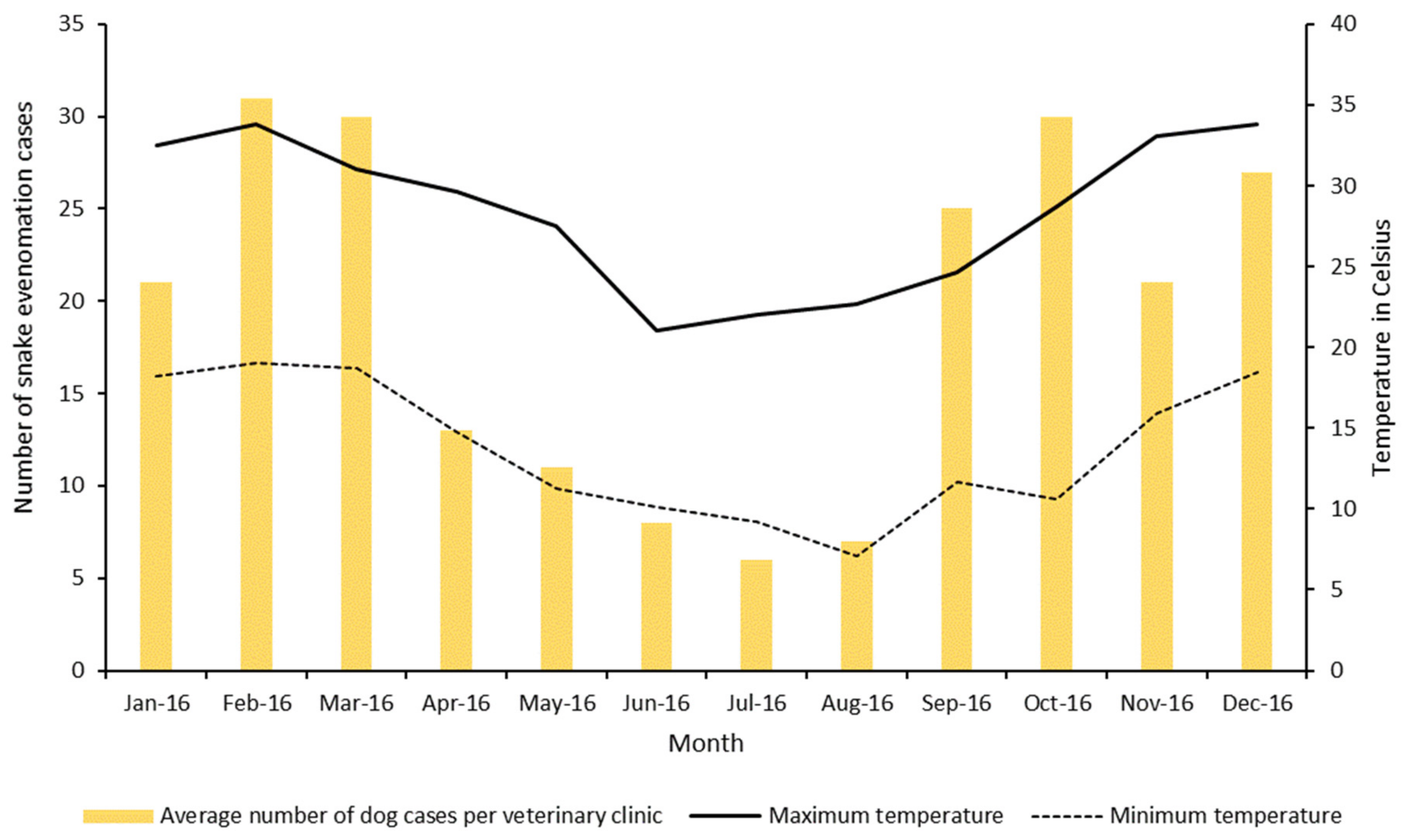
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shea, G.M. The distribution and identification of dangerously venomous Australian terrestrial snakes. Aust. Vet. J. 1999, 77, 791–798. [Google Scholar] [CrossRef][Green Version]
- Hodgson, W.C.; Wickramaratna, J.C. Snake venoms and their toxins: An Australian perspective. Toxicon 2006, 48, 931–940. [Google Scholar] [CrossRef] [PubMed]
- Kasturiratne, A.; Wickremasinghe, A.R.; de Silva, N.; Gunawardena, N.K.; Pathmeswaran, A.; Premaratna, R.; Savioli, L.; Lalloo, D.G.; de Silva, H.J. The global burden of snakebite: A literature analysis and modelling based on regional estimates of envenoming and deaths. PLoS Med. 2008, 5, e218. [Google Scholar] [CrossRef] [PubMed]
- Welton, R.E.; Liew, D.; Braitberg, G. Incidence of fatal snake bite in Australia: A coronial based retrospective study (2000–2016). Toxicon 2017, 131, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Johnston, C.I.; Ryan, N.M.; Page, C.B.; Buckley, N.A.; Brown, S.G.A.; O’Leary, M.A.; Isbister, G.K. Can Australians identify snakes? The Australian Snakebite Project, 2005–2015 (ASP-20). Med. J. Aust. 2017, 207, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Mirtschin, P.J.; Masci, P.; Paton, D.C.; Kuchel, T. Snake bites recorded by veterinary practices in Australia. Aust. Vet. J. 1998, 76, 195–198. [Google Scholar] [CrossRef] [PubMed]
- Heller, J.; Mellor, D.J.; Hodgson, J.L.; Reid, S.W.; Hodgson, D.R.; Bosward, K.L. Elapid snake envenomation in dogs in New South Wales: A review. Aust. Vet. J. 2007, 85, 469–479. [Google Scholar] [CrossRef] [PubMed]
- Jacoby-Alner, T.E.; Stephens, N.; Davern, K.M.; Balmer, L.; Brown, S.G.; Swindells, K. Histopathological analysis and in situ localisation of Australian tiger snake venom in two clinically envenomed domestic animals. Toxicon 2011, 58, 304–314. [Google Scholar] [CrossRef]
- Boller, M.; Kelers, K.; Stevenson, M.A.; Winkel, K.D.; Hardjo, S.; Heller, J.; Judge, P.R.; Ong, H.M.; Padula, A.M.; Reddrop, C.; et al. SnakeMap: Four years of experience with a national small animal snake envenomation registry. Aust. Vet. J. 2020, 98, 442–448. [Google Scholar] [CrossRef]
- Survey Monkey. Available online: https://www.surveymonkey.com/ (accessed on 5 January 2021).
- Snake Bite Research Australia. Available online: http://snakebiteresearch.com.au/ (accessed on 5 January 2021).
- Australian Veterinary Association. Available online: https://www.ava.com.au/ (accessed on 5 January 2021).
- Reeves, M.P. A retrospective report of 90 dogs with suspected cane toad (Bufo marinus) toxicity. Aust. Vet. J. 2004, 82, 608–611. [Google Scholar] [CrossRef]
- Lervik, J.B.; Lilliehöök, I.; Frendin, J.H. Clinical and biochemical changes in 53 Swedish dogs bitten by the European adder-Vipera berus. Acta Vet. Scand. 2010, 52, 26. [Google Scholar] [CrossRef]
- Indrawirawan, Y.; Sheridan, G.; McAlees, T. Clinical features of mainland Tiger and Eastern Brown Snake envenomation in dogs and cats in Melbourne. Aust. Vet. Pract. 2014, 44, 704–712. [Google Scholar]
- Morrison, J.J.; Pearn, J.H.; Covacevich, J.; Nixon, J. Can Australians Identify Snakes. Med. J. Aust. 1983, 2, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Hill, F.W. Snake bite in dogs. Aust. Vet. J. 1979, 55, 82–85. [Google Scholar] [CrossRef] [PubMed]
- Australian Museum. Reptiles. Available online: https://australian.museum/learn/animals/reptiles (accessed on 5 January 2021).
- Couper, P.; Amey, A.P. Snakes of South-East Queensland: A Queensland Museum Pocket Wild Guide; Queensland Museum: Brisbane, Australia, 2007. [Google Scholar]
- Lewis, P.F. Common tiger snake envenomation in dogs and mice—Relationship between the amount of venom Injected and the onset of clinical signs. Aust. Vet. J. 1994, 71, 130–132. [Google Scholar] [CrossRef] [PubMed]
- Padula, A.M.; Leister, E. Eastern brown snake (Pseudonaja textilis) envenomation in dogs and cats: Clinical signs, coagulation changes, brown snake venom antigen levels and treatment with a novel caprylic acid fractionated bivalent whole IgG equine antivenom. Toxicon 2017, 138, 89–97. [Google Scholar] [CrossRef]
- Tibballs, J.; Sutherland, S.K.; Rivera, R.A.; Masci, P.P. The cardiovascular and haematological effects of purified prothrombin activator from the common brown snake (Pseudonaja textilis) and their antagonism with heparin. Anaesth. Intensive Care 1992, 20, 28–32. [Google Scholar] [CrossRef]
- Hardy, M.C.; Cochrane, J.; Allavena, R.E. Venomous and poisonous Australian animals of veterinary importance: A rich source of novel therapeutics. BioMed Res. Int. 2014, 2014, 671041. [Google Scholar] [CrossRef]
- Padula, A.M.; Ong, H.M.; Kelers, K. Snake envenomation in domestic animal species in Australia. In Clinical Toxinology in Australia, Europe, and Americas, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2018; pp. 505–536. [Google Scholar]
- Judge, P.R. Coastal taipan (Oxyuranus scutellatus) envenomation of a dog. Aust. Vet. J. 2015, 93, 412–416. [Google Scholar] [CrossRef]
- Aktas, M.; Auguste, D.; Lefebvre, H.P.; Toutain, P.L.; Braun, J.P. Creatine kinase in the dog: A review. Vet. Res. Commun. 1993, 17, 353–369. [Google Scholar] [CrossRef]
- Snake Venom Detection Kit. Available online: https://labeling.seqirus.com/SVDK/AU/Snake-Venom-Detection-Kit/EN/Snake-Venom-Detection-Kit.pdf (accessed on 5 January 2021).
- Cox, J.C.; Moisidis, A.V.; Shepherd, J.M.; Drane, D.P.; Jones, S.L. A novel format for a rapid sandwich EIA and its application to the identification of snake venoms. J. Immunol. Methods 1992, 146, 213–218. [Google Scholar] [CrossRef]
- Forbes, G.; Church, S. Detection of snake venom in equine urine and plasma: Validation of a commercially available snake venom detection kit in horses. Aust. Equine Vet. 2010, 29, 57–61. [Google Scholar]
- VetCompass Australia. Available online: https://www.vetcompass.com.au/ (accessed on 5 January 2021).

| Diagnostic Procedures Performed | Dogs (%, n) |
|---|---|
| ACT only | 35.0% (7) |
| ACT and PCV/TP | 10.0% (2) |
| ACT and SVDK | 10.0% (2) |
| ACT, PCV/TP, and blood smear | 5.0% (1) |
| ACT, PCV/TP, and CK | 5.0% (1) |
| SVDK, PTT/aPTT, and ECG | 5.0% (1) |
| Clinical signs only | 30.0% (6) |
| Total | 100.0% (20) |
| Treatment Administered | Dogs (%, n) |
|---|---|
| IV Fluids and Antivenom | 35.0% (7) |
| IV Fluids, Antivenom, and Antihistamines | 15.0% (3) |
| IV Fluids, Antivenom, Adrenalin, and Antihistamines | 25.0% (5) |
| IV Fluids, Antivenom, Antihistamines, and Vitamin C | 5.0% (1) |
| IV Fluids, Antivenom, Adrenalin, Frusemide, and Vitamin C | 5.0% (1) |
| IV Fluids, Adrenalin, Frusemide, and Antihistamines | 5.0% (1) |
| IV Fluids and Vitamin C | 5.0% (1) |
| No treatment | 5.0% (1) |
| Total | 100% (20) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valenza, L.; Allavena, R.; Haworth, M.; Cochrane, J.; Henning, J. Diagnosis and Treatment of Snake Envenomation in Dogs in Queensland, Australia. Vet. Sci. 2021, 8, 14. https://doi.org/10.3390/vetsci8020014
Valenza L, Allavena R, Haworth M, Cochrane J, Henning J. Diagnosis and Treatment of Snake Envenomation in Dogs in Queensland, Australia. Veterinary Sciences. 2021; 8(2):14. https://doi.org/10.3390/vetsci8020014
Chicago/Turabian StyleValenza, Ludovica, Rachel Allavena, Mark Haworth, Jonathon Cochrane, and Joerg Henning. 2021. "Diagnosis and Treatment of Snake Envenomation in Dogs in Queensland, Australia" Veterinary Sciences 8, no. 2: 14. https://doi.org/10.3390/vetsci8020014
APA StyleValenza, L., Allavena, R., Haworth, M., Cochrane, J., & Henning, J. (2021). Diagnosis and Treatment of Snake Envenomation in Dogs in Queensland, Australia. Veterinary Sciences, 8(2), 14. https://doi.org/10.3390/vetsci8020014








