Adult-Onset Neuronal Ceroid Lipofuscinosis in a Shikoku Inu
Abstract
:1. Introduction
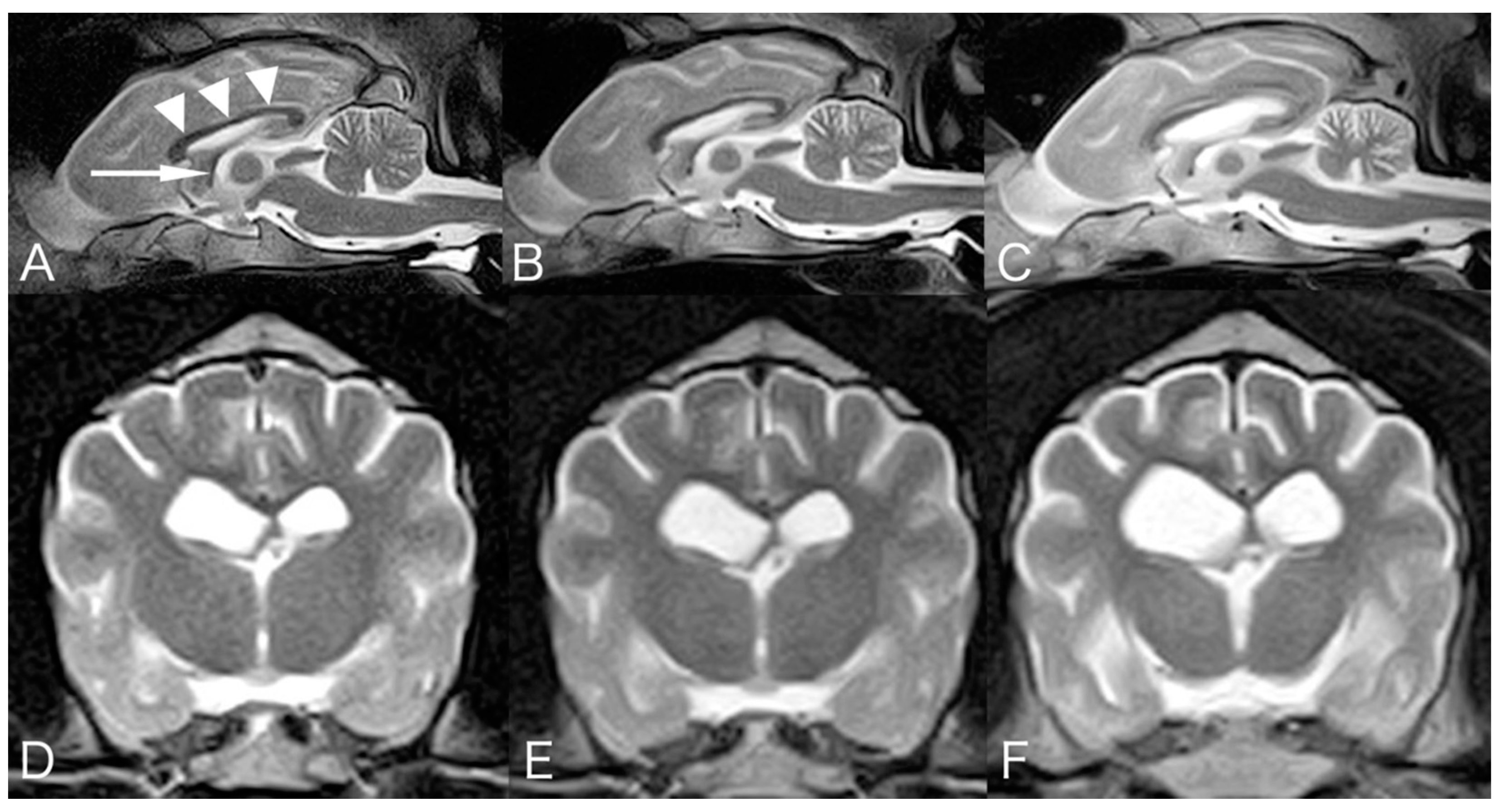
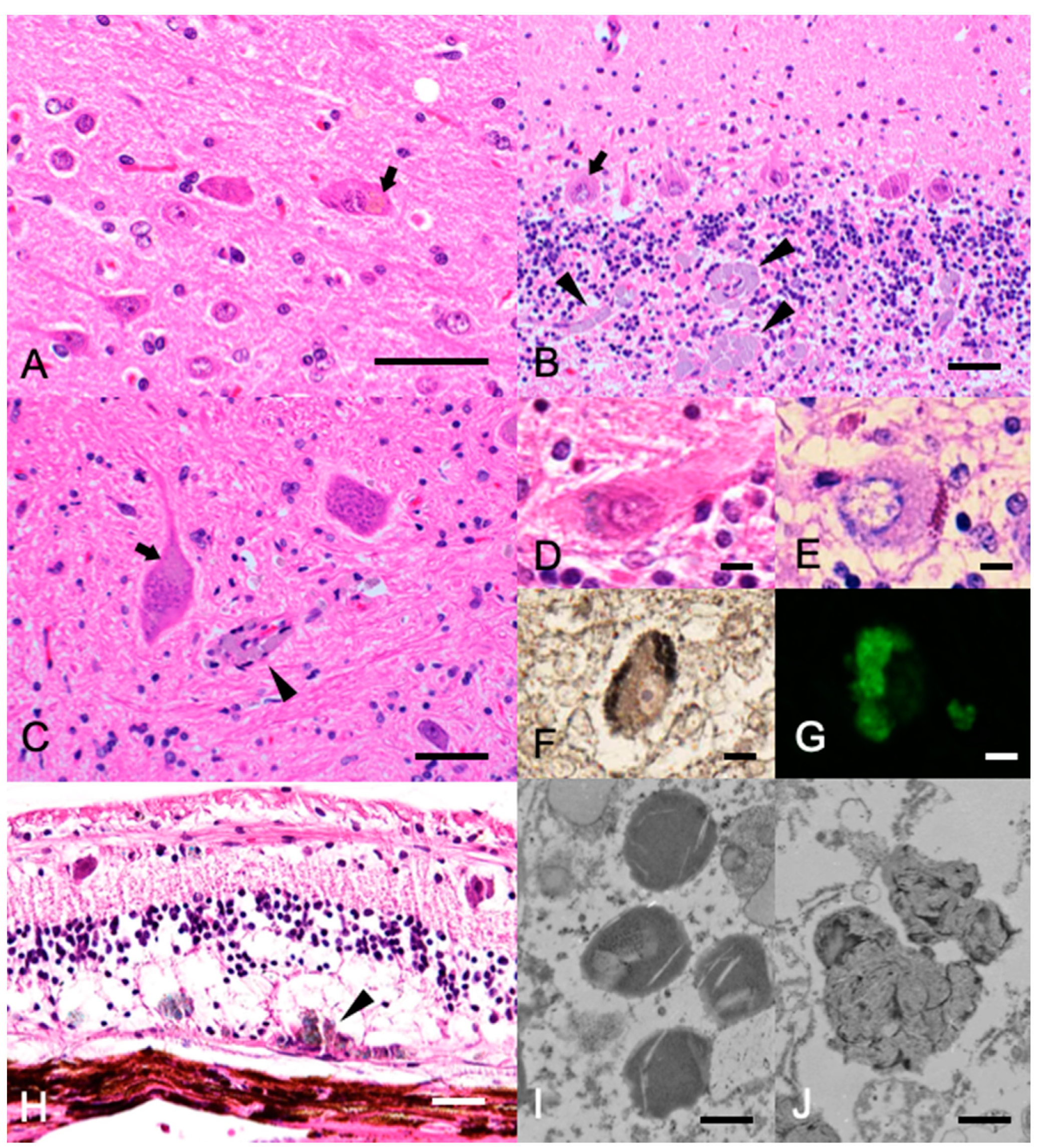
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Katz, M.L.; Rustad, E.; Robinson, G.O.; Whiting, R.E.; Student, J.T.; Coates, J.R.; Narfstrom, K. Canine neuronal ceroid lipofuscinoses: Promising models for preclinical testing of therapeutic interventions. Neurobiol. Dis. 2017, 108, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Katz, M.L.; Khan, S.; Awano, T.; Shahid, S.A.; Siakotos, A.N.; Johnson, G.S. A mutation in the CLN8 gene in English Setter dogs with neuronal ceroid-lipofuscinosis. Biochem. Biophys. Res. Commun. 2005, 327, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Asakawa, M.; MacKillop, E.; Olby, N.J.; Robertson, I.D.; Cullen, J.M. IMAGING DIAGNOSIS-NEURONAL CEROID LIPOFUSCINOSIS WITH A CHRONIC SUBDURAL HEMATOMA. Vet. Radiol. Ultrasound 2010, 51, 155–158. [Google Scholar] [CrossRef] [PubMed]
- Sanders, D.N.; Farias, F.H.; Johnson, G.S.; Chiang, V.; Cook, J.R.; O’Brien, D.P.; Hofmann, S.L.; Lu, J.-Y.; Katz, M.L. A mutation in canine PPT1 causes early onset neuronal ceroid lipofuscinosis in a Dachshund. Mol. Genet. Metab. 2010, 100, 349–356. [Google Scholar] [CrossRef] [Green Version]
- Guo, J.; O’Brien, D.P.; Mhlanga-Mutangadura, T.; Olby, N.J.; Taylor, J.F.; Schnabel, R.D.; Katz, M.L.; Johnson, G.S. A rare homozygous MFSD8 single-base-pair deletion and frameshift in the whole genome sequence of a Chinese Crested dog with neuronal ceroid lipofuscinosis. BMC Vet. Res. 2014, 10, 960. [Google Scholar] [CrossRef] [Green Version]
- Lingaas, F.; Guttersrud, O.-A.; Arnet, E.; Espenes, A. Neuronal ceroid lipofuscinosis in Salukis is caused by a single base pair insertion inCLN8. Anim. Genet. 2018, 49, 52–58. [Google Scholar] [CrossRef]
- Rossmeisl, J.J.H.; Duncan, R.; Fox, J.; Herring, E.S.; Inzana, K.D. Neuronal Ceroid-Lipofuscinosis in a Labrador Retriever. J. Vet. Diagn. Investig. 2003, 15, 457–460. [Google Scholar] [CrossRef] [Green Version]
- Koie, H.; Shibuya, H.; Sato, T.; Sato, A.; Nawa, K.; Nawa, Y.; Kitagawa, M.; Sakai, M.; Takahashi, T.; Yamaya, Y.; et al. Magnetic Resonance Imaging of Neuronal Ceroid Lipofuscinosis in a Border Collie. J. Vet. Sci. 2004, 66, 1453–1456. [Google Scholar] [CrossRef] [Green Version]
- Mizukami, K.; Chang, H.-S.; Yabuki, A.; Kawamichi, T.; Kawahara, N.; Hayashi, D.; Hossain, M.A.; Rahman, M.M.; Uddin, M.M.; Yamato, O. Novel rapid genotyping assays for neuronal ceroid lipofuscinosis in Border Collie dogs and high frequency of the mutant allele in Japan. J. Vet. Diagn. Investig. 2011, 23, 1131–1139. [Google Scholar] [CrossRef] [Green Version]
- Mizukami, K.; Kawamichi, T.; Koie, H.; Tamura, S.; Matsunaga, S.; Imamoto, S.; Saito, M.; Hasegawa, D.; Matsuki, N.; Tamahara, S.; et al. Neuronal Ceroid Lipofuscinosis in Border Collie Dogs in Japan: Clinical and Molecular Epidemiological Study (2000–2011). Sci. World J. 2012, 2012, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Minatel, L.; Underwood, S.C.; Carfagnini, J.C. Ceroid-lipofuscinosis in a Cocker Spaniel dog. Vet. Pathol. 2000, 37, 488–490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evans, J.; Katz, M.L.; Levesque, D.; Shelton, G.D.; de Lahunta, A.; O’Brien, D.P. A variant form of neuronal ceroid lipofuscinosis in American Bulldogs. J. Vet. Intern. Med. 2005, 19, 44–51. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, D.; Katz, M. Neuronal Ceroid Lipofuscinosis in 3 Australian Shepherd Littermates. J. Vet. Intern. Med. 2008, 22, 472–475. [Google Scholar] [CrossRef]
- Katz, M.L.; Farias, F.H.; Sanders, U.N.; Zeng, R.; Khan, S.; Johnson, G.S.; O’Brien, D.P. A Missense Mutation in CanineCLN6in an Australian Shepherd with Neuronal Ceroid Lipofuscinosis. J. Biomed. Biotechnol. 2010, 2011, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakamoto, Y.; Yamato, O.; Uchida, K.; Nibe, K.; Tamura, S.; Ozawa, T.; Ueoka, N.; Nukaya, A.; Yabuki, A.; Nakaichi, M. Neuronal Ceroid-Lipofuscinosis in Longhaired Chihuahuas: Clinical, Pathologic, and MRI Findings. J. Am. Anim. Hosp. Assoc. 2011, 47, e64–e70. [Google Scholar] [CrossRef] [PubMed]
- Ashwini, A.; D’Angelo, A.; Yamato, O.; Giordano, C.; Cagnotti, G.; Harcourt-Brown, T.; Mhlanga-Mutangadura, T.; Guo, J.; Johnson, G.S.; Katz, M.L. Neuronal ceroid lipofuscinosis associated with an MFSD8 mutation in Chihuahuas. Mol. Genet. Metab. 2016, 118, 326–332. [Google Scholar] [CrossRef]
- Kolicheski, A.; Johnson, G.; O’Brien, D.; Mhlanga-Mutangadura, T.; Gilliam, D.; Guo, J.; Anderson-Sieg, T.; Schnabel, R.; Taylor, J.; Lebowitz, A.; et al. Australian Cattle Dogs with Neuronal Ceroid Lipofuscinosis are Homozygous for a CLN5 Nonsense Mutation Previously Identified in Border Collies. J. Vet. Intern. Med. 2016, 30, 1149–1158. [Google Scholar] [CrossRef] [Green Version]
- Schmutz, I.; Jagannathan, V.; Bartenschlager, F.; Stein, V.M.; Gruber, A.D.; Leeb, T.; Katz, M.L. ATP13A2 missense variant in Australian Cattle Dogs with late onset neuronal ceroid lipofuscinosis. Mol. Genet. Metab. 2019, 127, 95–106. [Google Scholar] [CrossRef]
- Katz, M.L.; Narfström, K.; Johnson, G.S.; O’Brien, D.P. Assessment of retinal function and characterization of lysosomal storage body accumulation in the retinas and brains of Tibetan terriers with ceroid-lipofuscinosis. Am. J. Vet. Res. 2005, 66, 67–76. [Google Scholar] [CrossRef] [Green Version]
- Farias, F.H.G.; Zeng, R.; Johnson, G.S.; Wininger, F.A.; Taylor, J.F.; Schnabel, R.D.; McKay, S.D.; Sanders, D.N.; Lohi, H.; Seppälä, E.H.; et al. A truncating mutation in ATP13A2 is responsible for adult-onset neuronal ceroid lipofuscinosis in Tibetan terriers. Neurobiol. Dis. 2011, 42, 468–474. [Google Scholar] [CrossRef]
- Wöhlke, A.; Philipp, U.; Bock, P.; Beineke, A.; Lichtner, P.; Meitinger, T.; Distl, O. A One Base Pair Deletion in the Canine ATP13A2 Gene Causes Exon Skipping and Late-Onset Neuronal Ceroid Lipofuscinosis in the Tibetan Terrier. PLoS Genet. 2011, 7, e1002304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drögemüller, C.; Wöhlke, A.; Distl, O. Evaluation of the canine TPP1 gene as a candidate for neuronal ceroid lipofuscinosis in Tibetan Terrier and Polish Owczarek Nizinny dogs. Anim. Genet. 2005, 36, 178–179. [Google Scholar] [CrossRef]
- Abitbol, M.; Thibaud, J.-L.; Olby, N.; Hitte, C.; Puech, J.-P.; Maurer, M.; Pilot-Storck, F.; Hedan, B.; Dreano, S.; Brahimi, S.; et al. A canine Arylsulfatase G (ARSG) mutation leading to a sulfatase deficiency is associated with neuronal ceroid lipofuscinosis. Proc. Natl. Acad. Sci. USA 2010, 107, 14775–14780. [Google Scholar] [CrossRef] [Green Version]
- Kolicheski, A.; Heller, H.B.; Arnold, S.; Schnabel, R.; Taylor, J.; Knox, C.; Mhlanga-Mutangadura, T.; O’Brien, D.; Johnson, G.; Dreyfus, J.; et al. HomozygousPPT1Splice Donor Mutation in a Cane Corso Dog with Neuronal Ceroid Lipofuscinosis. J. Vet. Intern. Med. 2016, 31, 149–157. [Google Scholar] [CrossRef]
- Hirz, M.; Drögemüller, M.; Schänzer, A.; Jagannathan, V.; Dietschi, E.; Goebel, H.H.; Hecht, W.; Laubner, S.; Schmidt, M.J.; Steffen, F.; et al. Neuronal ceroid lipofuscinosis (NCL) is caused by the entire deletion of CLN8 in the Alpenländische Dachsbracke dog. Mol. Genet. Metab. 2017, 120, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Johnson, G.S.; Cook, J.; Harris, O.K.; Mhlanga-Mutangadura, T.; Schnabel, R.D.; Jensen, C.A.; Katz, M.L. Neuronal ceroid lipofuscinosis in a German Shorthaired Pointer associated with a previously reported CLN8 nonsense variant. Mol. Genet. Metab. Rep. 2019, 21, 100521. [Google Scholar] [CrossRef]
- Gilliam, D.; Kolicheski, A.; Johnson, G.S.; Mhlanga-Mutangadura, T.; Taylor, J.F.; Schnabel, R.D.; Katz, M.L. Golden Retriever dogs with neuronal ceroid lipofuscinosis have a two-base-pair deletion and frameshift in CNL5. Mol. Genet. Metab. 2015, 115, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Jolly, R.D.; Palmer, D.N.; Studdert, V.P.; Sutton, R.H.; Kelly, W.R.; Koppang, N.; Dahme, G.; Hartley, W.J.; Patterson, J.S.; Riis, R.C. Canine ceroid-lipofuscinosis: A review and classification. J. Small Anim. Pract. 1994, 35, 299–306. [Google Scholar] [CrossRef]
- Bichsel, P.; Vandevelde, M. A case of ceroid-liposuscinosis in a Yugoslavian shepherd dog. Schweiz. Arch. Tierheilkd. 1982, 124, 413–418. [Google Scholar] [PubMed]
- Goebel, H.H.; Bilzer, T.; Malkusch, F. Morphological studies in canine (Dalmetian) neuronal ceroid-lipofuscinosis. Am. J. Med. Genet. Suppl. 1998, 5, 127–139. [Google Scholar]
- Villani, N.A.; Bullock, G.; Michaels, J.R.; Yamato, O.; O’Brien, D.P.; Mhlanga-Mutangadura, T.; Johnson, G.S.; Katz, M.L. A mixed breed dog with neuronal ceroid lipofuscinosis is homozygous for a CNL5 nonsense mutation previously identified in Broder Collies and Australian Cattle Dogs. Mol. Genet. Metab. 2019, 127, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Story, B.D.; Miller, M.E.; Bradbury, A.M.; Million, E.D.; Duan, D.; Taghian, T.; Faissler, D.; Fernau, D.; Beecy, S.J.; Gray-Edwards, H.L. Canine Models of Inherited Musculoskeletal and Neurodegenerative Diseases. Front. Vet. Sci. 2020, 7, 80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Incerti, L. MRI in neuronal ceroid lipofuscinosis. Neurol. Sci. 2000, 21, S71–S73. [Google Scholar] [CrossRef]
- Autti, T.; Raininko, R.; Santavuori, P.; Vanhanen, S.L.; Poutanen, V.P.; Haltia, M. MRI of neuronal ceroid lipofuscinosis. II. Postmortem MRI and histopathological study of the brain in 16 cases of neuronal ceroid lipofuscinosis of juvenile or late infantile type. Neuroradiology 1997, 39, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Jadav, R.; Sinha, S.; Yasha, T.; Aravinda, H.; Rao, S.; Bindu, P.; Satishchandra, P. Magnetic Resonance Imaging in Neuronal Ceroid Lipofuscinosis and its Subtypes. Neuroradiol. J. 2012, 25, 755–761. [Google Scholar] [CrossRef]
- Hasegawa, D.; Tamura, S.; Nakamoto, Y.; Matsuki, N.; Takahashi, K.; Fujita, M.; Uchida, K.; Yamato, O. Magnetic Resonance Findings of the Corpus Callosum in Canine and Feline Lysosomal Storage Diseases. PLoS ONE 2013, 8, e83455. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tamura, S.; Tsuboi, M.; Ueoka, N.; Doi, S.; Tamura, Y.; Uchida, K.; Yabuki, A.; Yamato, O. Adult-Onset Neuronal Ceroid Lipofuscinosis in a Shikoku Inu. Vet. Sci. 2021, 8, 227. https://doi.org/10.3390/vetsci8100227
Tamura S, Tsuboi M, Ueoka N, Doi S, Tamura Y, Uchida K, Yabuki A, Yamato O. Adult-Onset Neuronal Ceroid Lipofuscinosis in a Shikoku Inu. Veterinary Sciences. 2021; 8(10):227. https://doi.org/10.3390/vetsci8100227
Chicago/Turabian StyleTamura, Shinji, Masaya Tsuboi, Naotami Ueoka, Shoko Doi, Yumiko Tamura, Kazuyuki Uchida, Akira Yabuki, and Osamu Yamato. 2021. "Adult-Onset Neuronal Ceroid Lipofuscinosis in a Shikoku Inu" Veterinary Sciences 8, no. 10: 227. https://doi.org/10.3390/vetsci8100227
APA StyleTamura, S., Tsuboi, M., Ueoka, N., Doi, S., Tamura, Y., Uchida, K., Yabuki, A., & Yamato, O. (2021). Adult-Onset Neuronal Ceroid Lipofuscinosis in a Shikoku Inu. Veterinary Sciences, 8(10), 227. https://doi.org/10.3390/vetsci8100227




