Acute Cytauxzoon felis Cases in Domestic Cats from Eastern Kansas, a Retrospective Case-Control Study (2006–2019)
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethic Approval
2.2. Study Design
2.3. Statistical Analyses
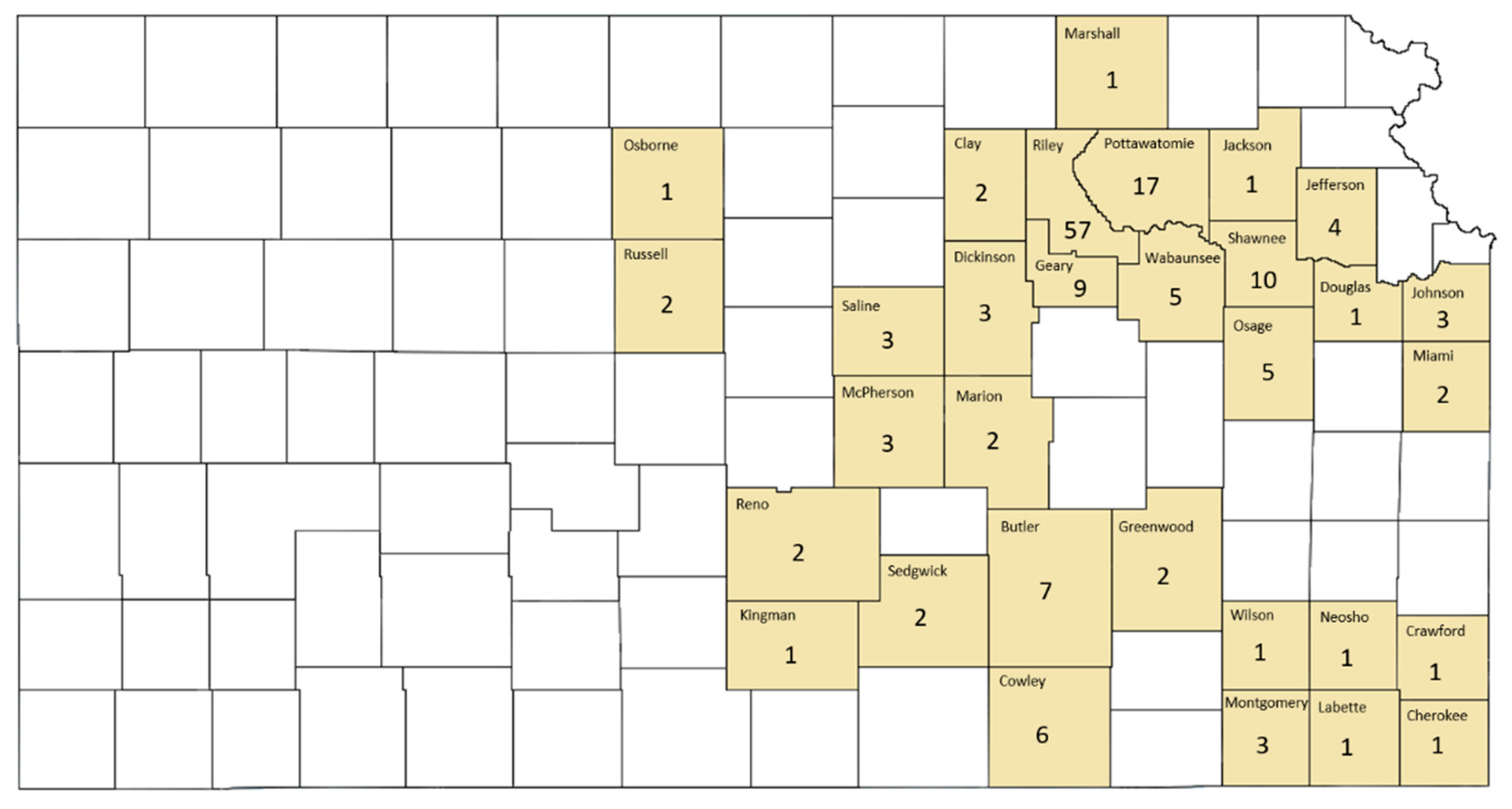
3. Results
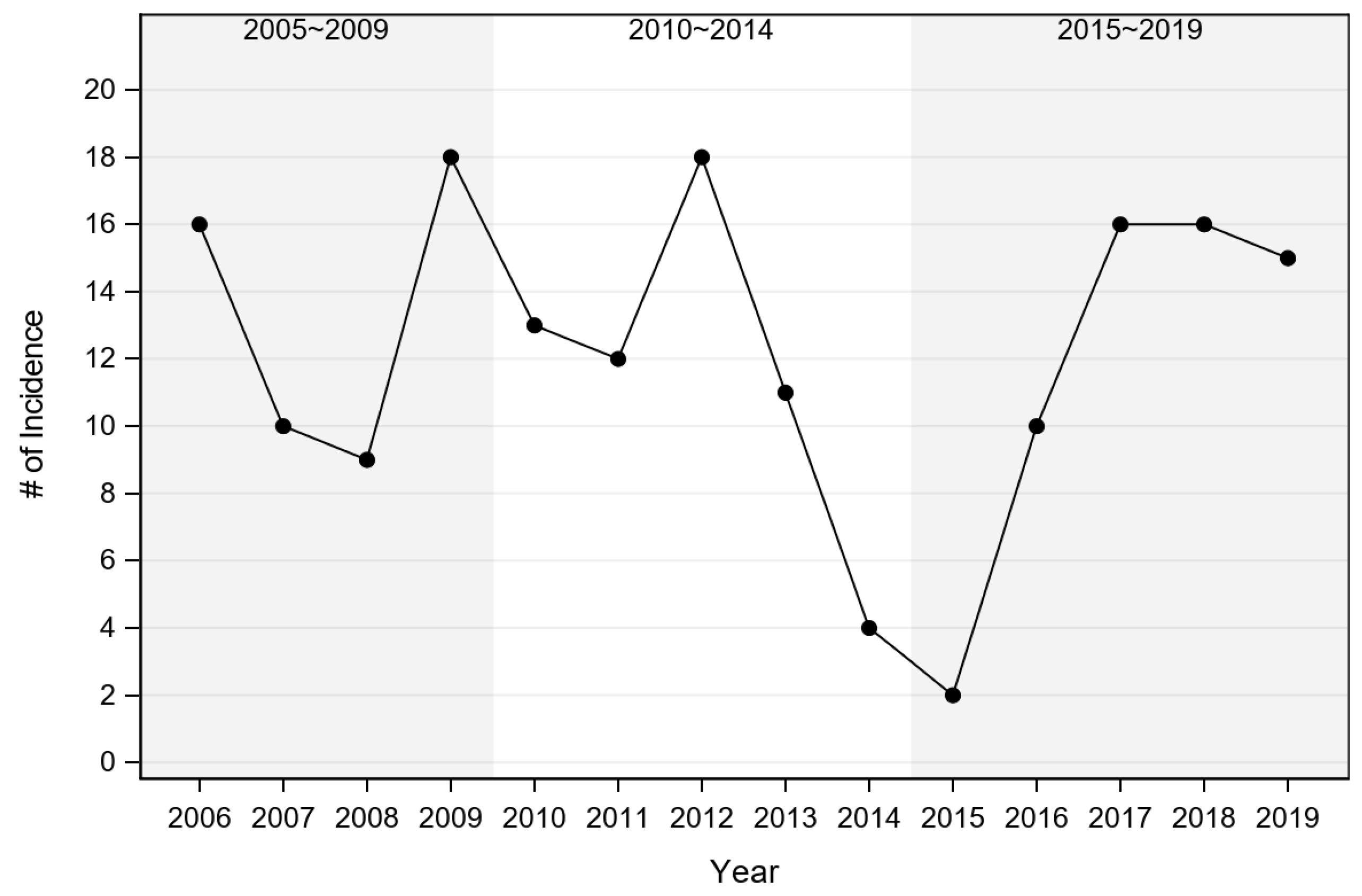
3.1. Annual Acute Cytauxzoonosis Incidence in Domestic Cats from Eastern Kansas
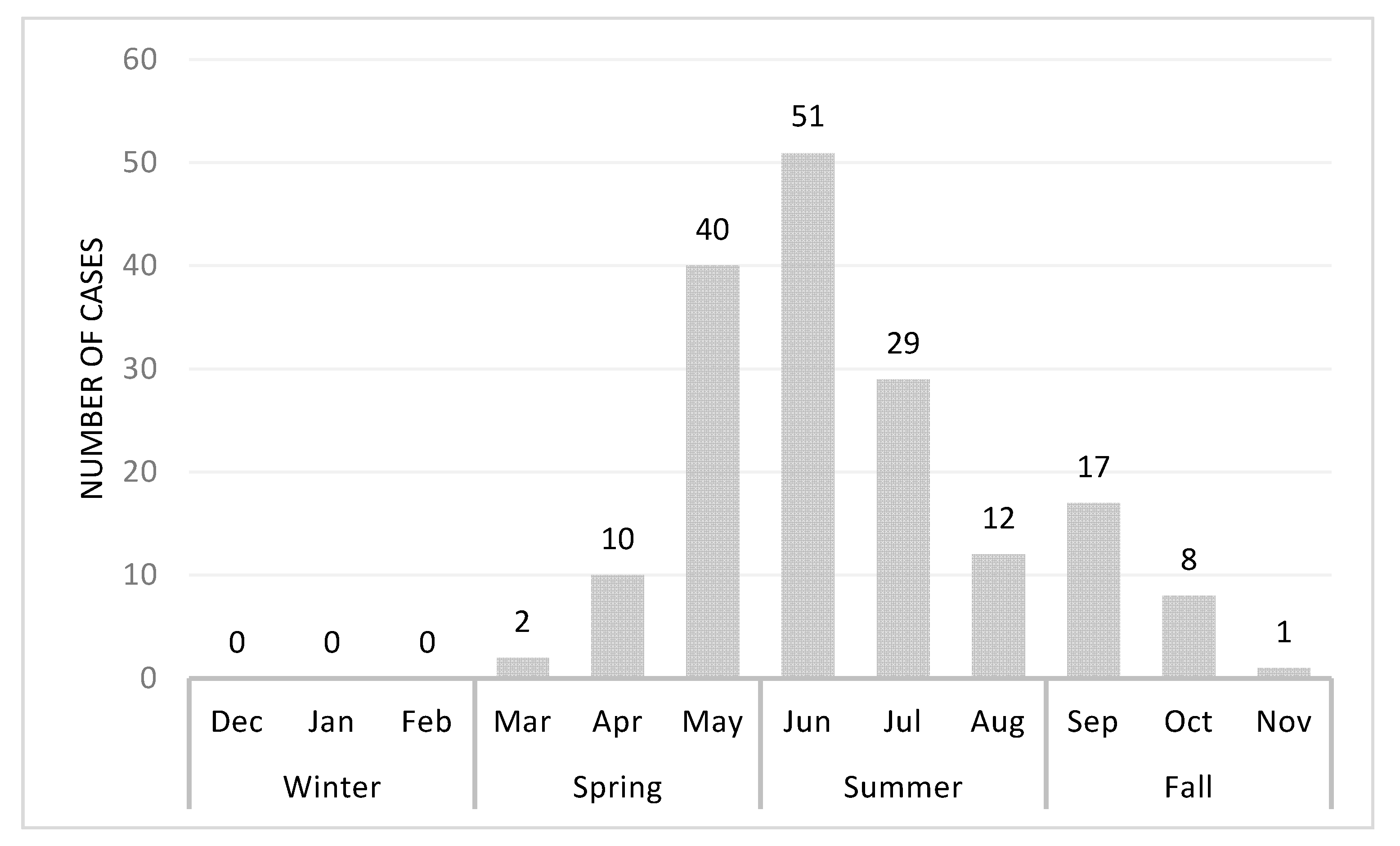
3.2. Season Variation of Acute Cytauxzoonosis Incidence in Domestic Cats from Eastern Kansas
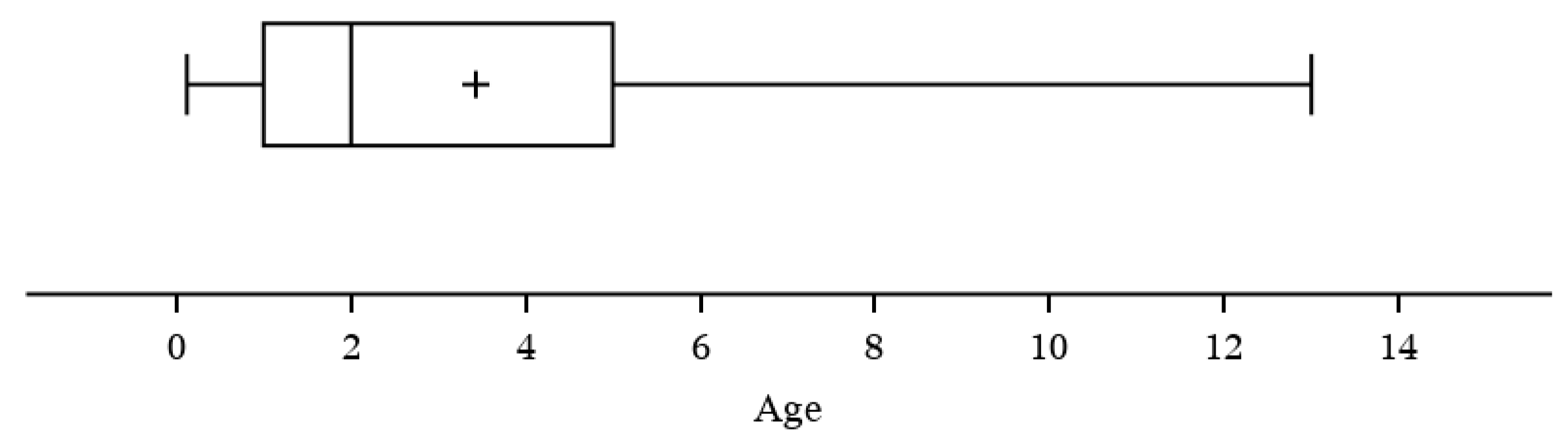
3.3. Evaluation of Acute Cytauxzoonosis Incidence among Cats with Different Ages, Sex, and Lifestyle
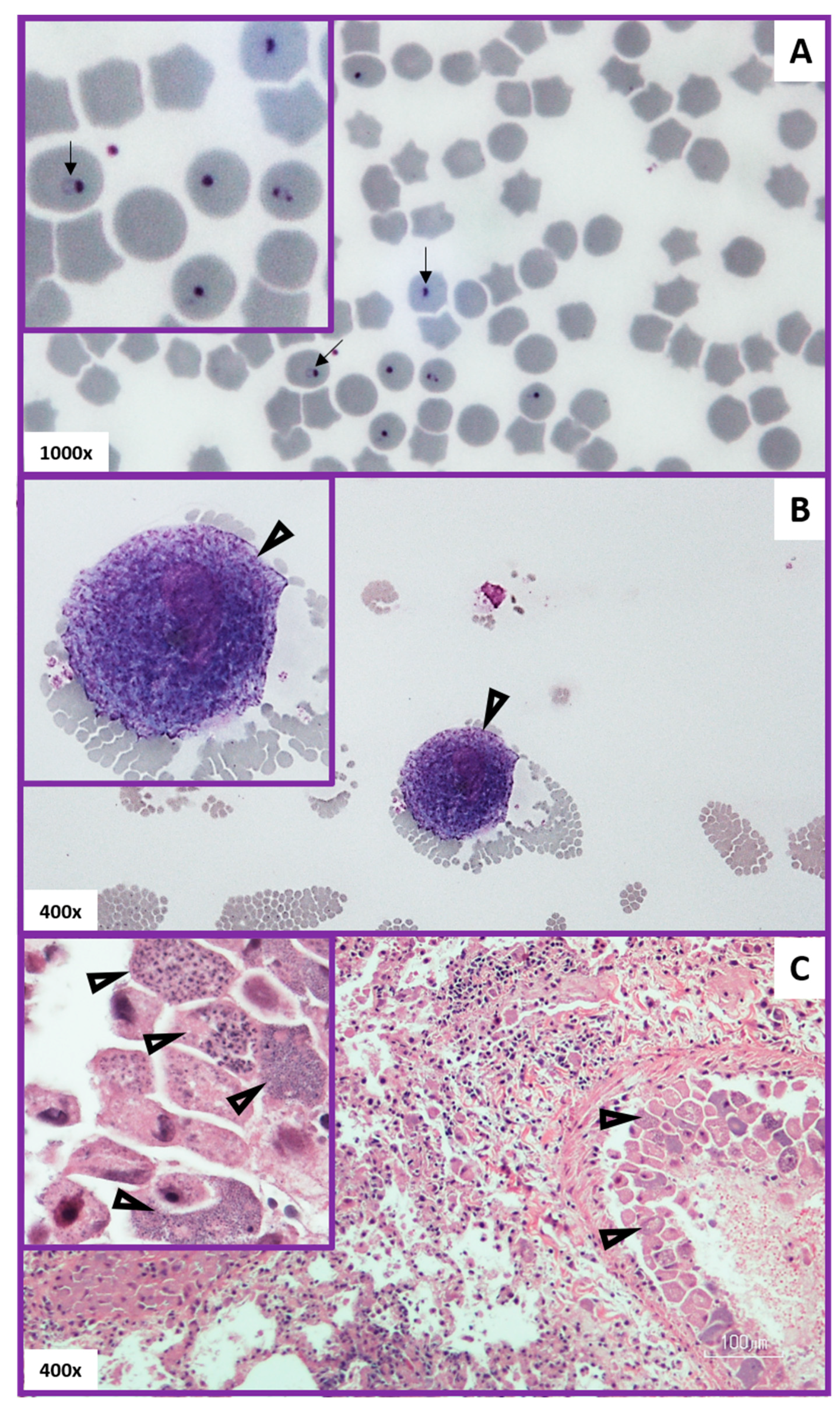
3.4. Evaluation of Method Used to Diagnose Acute Cytauxzoonosis in Domestic Cats from Eastern Kansas
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jalovecká, M.; Hajdusek, O.; Sojka, D.; Kopacek, P.; Malandrin, L. The complexity of piroplasms life cycles. Front. Cell. Infect. Microbiol. 2018, 8, 248. [Google Scholar] [CrossRef] [PubMed]
- Reichard, M.V.; Meinkoth, J.H.; Edwards, A.C.; Snider, T.A.; Kocan, K.M.; Blouin, E.F.; Little, S.E. Transmission of Cytauxzoon felis to a domestic cat by Amblyomma americanum. Vet. Parasitol. 2009, 161, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.E.; Ohmes, C.M.; Payton, M.E.; Hostetler, J.A.; Reichard, M.V. Minimum transmission time of Cytauxzoon felis by Amblyomma americanum to domestic cats in relation to duration of infestation, and investigation of ingestion of infected ticks as a potential route of transmission. J. Feline Med. Surg. 2018, 20, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Allen, K.E.; Thomas, J.E.; Wohltjen, M.L.; Reichard, M.V. Transmission of Cytauxzoon felis to domestic cats by Amblyomma americanum nymphs. Parasites Vectors 2019, 12, 28. [Google Scholar] [CrossRef] [PubMed]
- Little, S.E.; Barrett, A.W.; Nagamori, Y.; Herrin, B.H.; Normile, D.; Heaney, K.; Armstrong, R. Ticks from cats in the United States: Patterns of infestation and infection with pathogens. Vet. Parasitol. 2018, 257, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.-H.; Kaufman, P.E. Common Name: American Dog Tick Scientific Name: Dermacentor variabilis (Say) (Arachnida: Ixodida: Ixodidae). Available online: http://entnemdept.ufl.edu/creatures/urban/medical/american_dog_tick.htm (accessed on 24 March 2020).
- Minigan, J.N.; Hager, H.A.; Peregrine, A.S.; Newman, J.A. Current and potential future distribution of the American dog tick (Dermacentor variabilis, Say) in North America. Ticks Tick-Borne Dis. 2018, 9, 354–362. [Google Scholar] [CrossRef]
- Holderman, C.J.; Kaufman, P.E. Common Name: Lone Star Tick Scientific Name: Amblyomma americanum (Linnaeus) (Acari: Ixodidae). Available online: http://entnemdept.ufl.edu/creatures/urban/medical/lone_star_tick.htm (accessed on 15 March 2020).
- Monzón, J.D.; Atkinson, E.G.; Henn, B.M.; Benach, J.L. Population and evolutionary genomics of Amblyomma americanum, an expanding arthropod disease vector. Genome Biol. Evol. 2016, 8, 1351–1360. [Google Scholar] [CrossRef]
- Wikander, Y.M.; Anantatat, T.; Kang, Q.; Reif, K.E. Prevalence of Cytauxzoon felis infection-carriers in eastern Kansas domestic cats. Pathogens 2020, 9, 854. [Google Scholar] [CrossRef]
- Reichard, M.V.; Baum, K.A.; Cadenhead, S.C.; Snider, T.A. Temporal occurrence and environmental risk factors associated with cytauxzoonosis in domestic cats. Vet. Parasitol. 2008, 152, 314–320. [Google Scholar] [CrossRef]
- Ferris, D. A progress report on the status of a new disease of American cats: Cytauxzoonosis. Comp. Immunol. Microbiol. Infect. Dis. 1979, 1, 269–276. [Google Scholar] [CrossRef]
- Wagner, J.E. A fatal cytauxzoonosis-like disease in cats. J. Am. Vet. Med Assoc. 1976, 168, 585–588. [Google Scholar] [PubMed]
- Hoover, J.P.; Walker, D.B.; Hedges, J.D. Cytauxzoonosis in cats: Eight cases (1985–1992). J. Am. Vet. Med. Assoc. 1994, 205, 455–460. [Google Scholar] [PubMed]
- Meinkoth, J.; Kocan, A.A.; Whitworth, L.; Murphy, G.; Fox, J.C.; Woods, J.P. Cats surviving natural infection with Cytauxzoon felis: 18 cases (1997–1998). J. Vet. Intern. Med. 2000, 14, 521–525. [Google Scholar] [CrossRef]
- Birkenheuer, A.J.; Le, J.A.; Valenzisi, A.M.; Tucker, M.D.; Levy, M.G.; Breitschwerdt, E.B. Cytauxzoon felis infection in cats in the mid-Atlantic states: 34 cases (1998–2004). J. Am. Vet. Med. Assoc. 2006, 228, 568–571. [Google Scholar] [CrossRef] [PubMed]
- Jackson, C.B.; Fisher, T. Fatal cytauxzoonosis in a Kentucky cat (Felis domesticus). Vet. Parasitol. 2006, 139, 192–195. [Google Scholar] [CrossRef] [PubMed]
- Snider, T.A.; Confer, A.W.; Payton, M.E. Pulmonary Histopathology of Cytauxzoon felis infections in the cat. Vet. Pathol. 2010, 47, 698–702. [Google Scholar] [CrossRef] [PubMed]
- Frontera-Acevedo, K. Feline Immune Response to Infection with Cytauxzoon felis and the Role of CD18 in the Pathogenesis of Cytauxzoonosis. Ph.D. Thesis, University of Georgia, Athens, GA, USA, 2013. [Google Scholar]
- Conner, B.J.; Hanel, R.M.; Brooks, M.B.; Cohn, L.A.; Birkenheuer, A.J. Coagulation abnormalities in 5 cats with naturally occurring cytauxzoonosis. J. Vet. Emerg. Crit. Care 2015, 25, 538–545. [Google Scholar] [CrossRef]
- Cohn, L.A.; Birkenheuer, A.; Brunker, J.; Ratcliff, E.; Craig, A. Efficacy of atovaquone and azithromycin or imidocarb dipropionate in cats with acute cytauxzoonosis. J. Vet. Intern. Med. 2010, 25, 55–60. [Google Scholar] [CrossRef]
- Wang, J.-L.; Li, T.-T.; Liu, G.-H.; Zhu, X.-Q.; Yao, C.-Q. Two tales of Cytauxzoon felis infections in domestic cats. Clin. Microbiol. Rev. 2017, 30, 861–885. [Google Scholar] [CrossRef]
- Zou, F.-C.; Li, Z.; Yang, J.-F.; Chang, J.-Y.; Liu, G.-H.; Lv, Y.; Zhu, X.-Q. Cytauxzoon felis infection in domestic cats, Yunnan Province, China, 2016. Emerg. Infect. Dis. 2019, 25, 353–354. [Google Scholar] [CrossRef]
- Furtado, M.M.; Taniwaki, S.A.; Metzger, B.; Paduan, K.D.S.; O’Dwyer, H.L.; Jácomo, A.T.D.A.; Porfírio, G.E.; Silveira, L.; Sollmann, R.; Tôrres, N.M.; et al. Is the free-ranging jaguar (Panthera onca) a reservoir for Cytauxzoon felis in Brazil? Ticks Tick-Borne Dis. 2017, 8, 470–476. [Google Scholar] [CrossRef] [PubMed]
- Legroux, J.-P.; Halos, L.; René-Martellet, M.; Servonnet, M.; Pingret, J.-L.; Bourdoiseau, G.; Baneth, G.; Chabanne, L. First clinical case report of Cytauxzoon sp. infection in a domestic cat in France. BMC Vet. Res. 2017, 13, 81. [Google Scholar] [CrossRef]
- Veronesi, F.; Ravagnan, S.; Cerquetella, M.; Carli, E.; Olivieri, E.; Santoro, A.; Pesaro, S.; Berardi, S.; Rossi, G.; Ragni, B.; et al. First detection of Cytauxzoon spp. infection in European wildcats (Felis silvestris silvestris) of Italy. Ticks Tick-Borne Dis. 2016, 7, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.; Davis, C. Increasing frequency of feline cytauxzoonosis cases diagnosed in western Kentucky from 2001 to 2011. Vet. Parasitol. 2013, 198, 205–208. [Google Scholar] [CrossRef] [PubMed]
- Raghavan, R.K.; Almes, K.M.; Goodin, D.G.; Harrington, J.J.A.; Stackhouse, J.P.W. Spatially heterogeneous land cover/land use and climatic risk factors of tick-borne feline cytauxzoonosis. Vector-Borne Zoonotic Dis. 2014, 14, 486–495. [Google Scholar] [CrossRef] [PubMed]
- Pets by the Numbers: U.S. Pet Ownership, Community Cat and Shelter Population Sstimates. Available online: https://www.humanesociety.org/resources/pets-numbers (accessed on 10 August 2020).
- Pets by the Numbers: Data and Statistics on Pet Ownership, Community Cat, and Shelter Population in the United States. Available online: https://www.animalsheltering.org/page/pets-by-the-numbers (accessed on 10 August 2020).
- Gedeon, J. Special Report: States with the Most and Least Cat Owners. Available online: https://247wallst.com/special-report/2017/07/19/states-with-the-most-and-least-cat-owners/ (accessed on 10 August 2020).
- History of National Feral Cat Day. Available online: https://nationaltoday.com/national-feral-cat-day/ (accessed on 10 August 2020).
- Saleh, M.N.; Sundstrom, K.D.; Duncan, K.T.; Ientile, M.M.; Jordy, J.; Ghosh, P.; Little, S.E. Show us your ticks: A survey of ticks infesting dogs and cats across the USA. Parasites Vectors 2019, 12, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Schreeg, M.E.; Marr, H.S.; Tarigo, J.; Cohn, L.A.; Levy, M.G.; Birkenheuer, A.J. Pharmacogenomics of Cytauxzoon felis cytochrome b: Implications for atovaquone and azithromycin therapy in domestic cats with cytauxzoonosis. J. Clin. Microbiol. 2013, 51, 3066–3069. [Google Scholar] [CrossRef] [PubMed]
- Brown, H.M.; Modaresi, S.M.; Cook, J.L.; Latimer, K.S.; Peterson, D.S. Genetic variability of archived Cytauxzoon felis histologic specimens from domestic cats in Georgia, 1995–2007. J. Vet. Diagn. Investig. 2009, 21, 493–498. [Google Scholar] [CrossRef]
- Shock, B.C.; Birkenheuer, A.J.; Patton, L.L.; Olfenbuttel, C.; Beringer, J.; Grove, D.M.; Peek, M.; Butfiloski, J.W.; Hughes, D.W.; Lockhart, J.M.; et al. Variation in the ITS-1 and ITS-2 rRNA genomic regions of Cytauxzoon felis from bobcats and pumas in the eastern United States and comparison with sequences from domestic cats. Vet. Parasitol. 2012, 190, 29–35. [Google Scholar] [CrossRef]
- The Territory of Outdoor Cats. Available online: https://www.knowyourcat.info/info/teritory.htm (accessed on 31 August 2020).
- Meek, P. Home range of house cats Felis catus living within a National Park. Aust. Mammal. 2003, 25, 51–60. [Google Scholar] [CrossRef]
- Horn, J.A.; Mateus-Pinilla, N.; Warner, R.E.; Heske, E.J. Home range, habitat use, and activity patterns of free-roaming domestic cats. J. Wildl. Manag. 2011, 75, 1177–1185. [Google Scholar] [CrossRef]
- Hanmer, H.J.; Thomas, R.L.; Fellowes, M.D.E. Urbanization influences range size of the domestic cat (Felis catus): Consequences for conservation. J. Urban Ecol. 2017, 3, 1–11. [Google Scholar] [CrossRef]
- Sykes, J. Feline hemotropic mycoplasmas. Vet. Clin. North Am. Small Anim. Pr. 2010, 40, 1157–1170. [Google Scholar] [CrossRef] [PubMed]
- Penzhorn, B.L.; Oosthuizen, M.C. Babesia species of domestic cats: Molecular characterization has opened Pandora’s box. Front. Vet. Sci. 2020, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]

| 1 of Incidence per Year | Ratio to (Adj. p Value of Testing for Ratio ≠1) | |||
|---|---|---|---|---|
| Year Block | Mean | SE 2 | 2010–2014 | 2015–2019 |
| 2005~2009 | 13.3 | 1.8 | 1.14 (0.764) | 1.12 (0.813) |
| 2010~2014 | 11.6 | 1.5 | - | 0.98 (0.995) |
| 2015~2019 | 11.8 | 1.5 | - | - |
| Case | Control | |||||
|---|---|---|---|---|---|---|
| Effect | Season | Count | Percent | Count | Percent | p Value of Exact Chi-Square Test |
| Season | Spring | 52 | 30.6% | 21 | 17.1% | <0.001 |
| Summer | 92 | 54.1% | 46 | 37.4% | ||
| Fall | 26 | 15.3% | 47 | 38.2% | ||
| Winter | 0 | 0.0% | 9 | 7.3% | ||
| Case | Control | |||||
|---|---|---|---|---|---|---|
| Effect | Age Group 1 | Count 2 | Percent | Count | Percent | p Value of Chi-Square Test |
| Age | <1 | 19 | 12.2% | 47 | 47.0% | <0.001 |
| 1–3 | 63 | 40.4% | 24 | 24.0% | ||
| 3–5 | 34 | 21.8% | 18 | 18.0% | ||
| ≥5 | 40 | 25.6% | 11 | 11.0% | ||
| Case | |||||
|---|---|---|---|---|---|
| Effect | Sex | Count 1 | Percent | Percent Tested against | p Value of Chi-Square Test |
| Sex | F | 48 | 29.6% | 50.0% | <0.001 |
| M | 114 | 70.4% | 50.0% | ||
| Case | |||||
|---|---|---|---|---|---|
| Effect | Lifestyle | Count | Percent | Percent Tested against | p Value of Exact Chi-Square Test |
| Lifestyle | feral | 4 | 2.4% | 3.0% | <0.001 |
| owned | 164 | 96.5% | 47.1% | ||
| rescue | 2 | 1.2% | 49.9% | ||
| Case | |||||
|---|---|---|---|---|---|
| Effect | Group | Count | Percent | Percent Tested against | p Value of Chi-Square Test |
| Method of Diagnosis | blood smear | 96 | 56.5% | 50% | 0.092 |
| necropsy | 74 | 43.5% | 50% | ||
| Blood smear rings vs. schizonts | rings | 49 | 51% | 50% | 0.838 |
| schizonts | 47 | 49% | 50% | ||
| Relative number of rings observed | frequent | 30 | 61.2% | 50% | 0.116 |
| occasional | 19 | 38.8% | 50% | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wikander, Y.M.; Kang, Q.; Reif, K.E. Acute Cytauxzoon felis Cases in Domestic Cats from Eastern Kansas, a Retrospective Case-Control Study (2006–2019). Vet. Sci. 2020, 7, 205. https://doi.org/10.3390/vetsci7040205
Wikander YM, Kang Q, Reif KE. Acute Cytauxzoon felis Cases in Domestic Cats from Eastern Kansas, a Retrospective Case-Control Study (2006–2019). Veterinary Sciences. 2020; 7(4):205. https://doi.org/10.3390/vetsci7040205
Chicago/Turabian StyleWikander, Yvonne M., Qing Kang, and Kathryn E. Reif. 2020. "Acute Cytauxzoon felis Cases in Domestic Cats from Eastern Kansas, a Retrospective Case-Control Study (2006–2019)" Veterinary Sciences 7, no. 4: 205. https://doi.org/10.3390/vetsci7040205
APA StyleWikander, Y. M., Kang, Q., & Reif, K. E. (2020). Acute Cytauxzoon felis Cases in Domestic Cats from Eastern Kansas, a Retrospective Case-Control Study (2006–2019). Veterinary Sciences, 7(4), 205. https://doi.org/10.3390/vetsci7040205







